Abstract
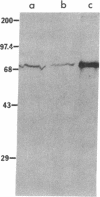
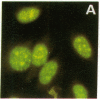
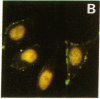
The Ku complex, a heterodimer of 86- and 70-kD proteins, is a nuclear DNA-binding autoantigen. However, hydrophobicity analysis of the deduced amino acid sequence of the 70-kD protein had strongly suggested that this might also be a membrane protein. In the present study, using antibodies to synthetic peptides and a polyclonal antiserum to the purified protein, we show that the 70-kD protein of the Ku complex is present in isolated plasma membranes of human cells. By indirect immunofluorescence microscopy and fluorescein-activated cell sorting, we demonstrate that this autoantigen is exposed on the cell surface. In addition, we have identified several domains of the protein that are exposed. Our study provides one of the first demonstrations of a eukaryotic, nuclear DNA-binding protein that is also on the cell membrane. Moreover, our results might help explain how autoantibodies to the Ku autoantigen could target cells for an autoimmune attack.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allaway G. P., Vivino A. A., Kohn L. D., Notkins A. L., Prabhakar B. S. Characterization of the 70KDA component of the human Ku autoantigen expressed in insect cell nuclei using a recombinant baculovirus vector. Biochem Biophys Res Commun. 1990 Apr 30;168(2):747–755. doi: 10.1016/0006-291x(90)92385-d. [DOI] [PubMed] [Google Scholar]
- Bression D., Michard M., Le Dafniet M., Pagesy P., Peillon F. Evidence for a specific estradiol binding site on rat pituitary membranes. Endocrinology. 1986 Sep;119(3):1048–1051. doi: 10.1210/endo-119-3-1048. [DOI] [PubMed] [Google Scholar]
- Butel J. S., Jarvis D. L. The plasma-membrane-associated form of SV40 large tumor antigen: biochemical and biological properties. Biochim Biophys Acta. 1986 Oct 28;865(2):171–195. doi: 10.1016/0304-419x(86)90027-2. [DOI] [PubMed] [Google Scholar]
- Chan J. Y., Lerman M. I., Prabhakar B. S., Isozaki O., Santisteban P., Kuppers R. C., Oates E. L., Notkins A. L., Kohn L. D. Cloning and characterization of a cDNA that encodes a 70-kDa novel human thyroid autoantigen. J Biol Chem. 1989 Mar 5;264(7):3651–3654. [PubMed] [Google Scholar]
- Gametchu B. Glucocorticoid receptor-like antigen in lymphoma cell membranes: correlation to cell lysis. Science. 1987 Apr 24;236(4800):456–461. doi: 10.1126/science.3563523. [DOI] [PubMed] [Google Scholar]
- Gruenstein E., Rich A., Weihing R. R. Actin associated with membranes from 3T3 mouse fibroblast and HeLa cells. J Cell Biol. 1975 Jan;64(1):223–234. doi: 10.1083/jcb.64.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffan J. J., Firshein W. Origin-specific DNA-binding membrane-associated protein may be involved in repression of initiation of DNA replication in Bacillus subtilis. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7452–7456. doi: 10.1073/pnas.85.20.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori T., Akizuki M., Yamagata H., Inada S., Yoshida S., Homma M. Characterization of a high molecular weight acidic nuclear protein recognized by autoantibodies in sera from patients with polymyositis-scleroderma overlap. J Clin Invest. 1981 Sep;68(3):611–620. doi: 10.1172/JCI110295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori T., Hardin J. A. Mechanism of interaction between Ku protein and DNA. J Biol Chem. 1986 Aug 5;261(22):10375–10379. [PubMed] [Google Scholar]
- Mimori T., Hardin J. A., Steitz J. A. Characterization of the DNA-binding protein antigen Ku recognized by autoantibodies from patients with rheumatic disorders. J Biol Chem. 1986 Feb 15;261(5):2274–2278. [PubMed] [Google Scholar]
- Peck E. J., Jr, Burgner J., Clark J. H. Estrophilic binding sites of the uterus. Relation to uptake and retention of estradiol in vitro. Biochemistry. 1973 Nov 6;12(23):4596–4603. doi: 10.1021/bi00747a009. [DOI] [PubMed] [Google Scholar]
- Reeves W. H., Sthoeger Z. M. Molecular cloning of cDNA encoding the p70 (Ku) lupus autoantigen. J Biol Chem. 1989 Mar 25;264(9):5047–5052. [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Weihing R. R. Purification of a HeLa cell high molecular weight action binding protein and its identification in HeLa cell plasma membrane ghosts and intact HeLa cells. Biochemistry. 1983 Apr 12;22(8):1839–1847. doi: 10.1021/bi00277a015. [DOI] [PubMed] [Google Scholar]
- Yaneva M., Arnett F. C. Antibodies against Ku protein in sera from patients with autoimmune diseases. Clin Exp Immunol. 1989 Jun;76(3):366–372. [PMC free article] [PubMed] [Google Scholar]
- Yaneva M., Busch H. A 10S particle released from deoxyribonuclease-sensitive regions of HeLa cell nuclei contains the 86-kilodalton-70-kilodalton protein complex. Biochemistry. 1986 Sep 9;25(18):5057–5063. doi: 10.1021/bi00366a013. [DOI] [PubMed] [Google Scholar]
- Yaneva M., Ochs R., McRorie D. K., Zweig S., Busch H. Purification of an 86-70 kDa nuclear DNA-associated protein complex. Biochim Biophys Acta. 1985 Jul 26;841(1):22–29. doi: 10.1016/0304-4165(85)90270-3. [DOI] [PubMed] [Google Scholar]