Abstract
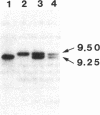
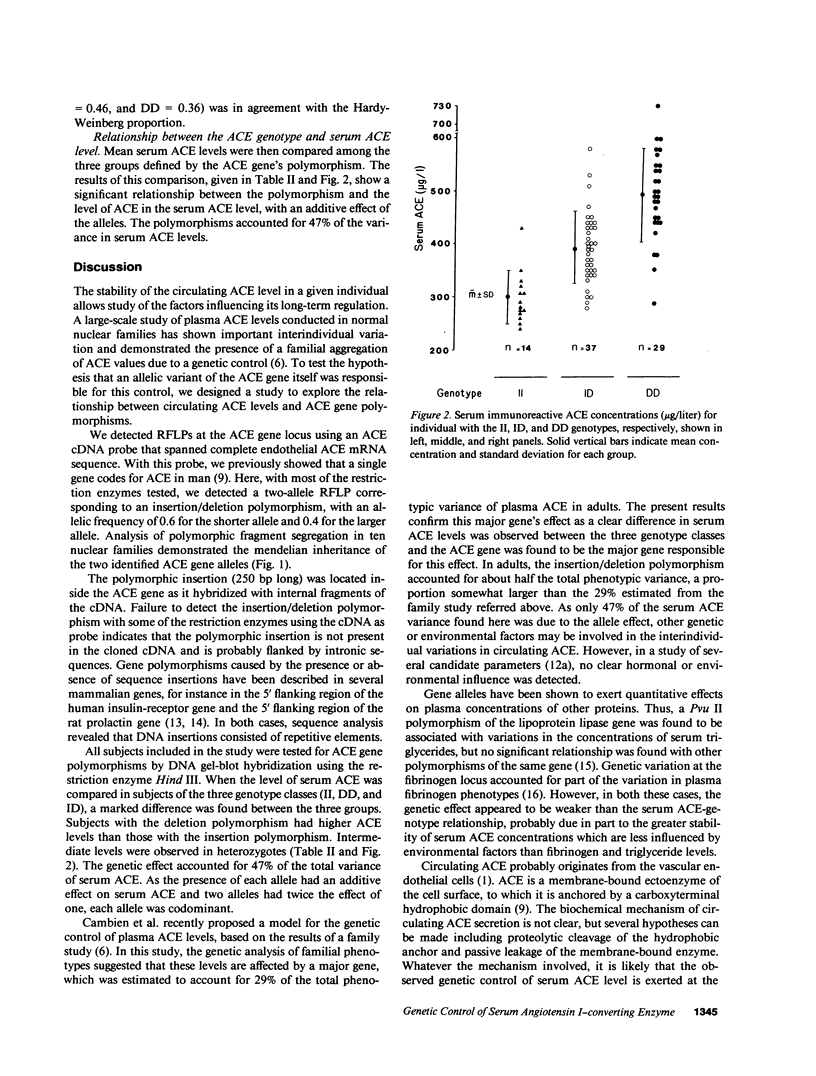
A polymorphism consisting of the presence or absence of a 250-bp DNA fragment was detected within the angiotensin I-converting enzyme gene (ACE) using the endothelial ACE cDNA probe. This polymorphism was used as a marker genotype in a study involving 80 healthy subjects, whose serum ACE levels were concomitantly measured. Allele frequencies were 0.6 for the shorter allele and 0.4 for the longer allele. A marked difference in serum ACE levels was observed between subjects in each of the three ACE genotype classes. Serum immunoreactive ACE concentrations were, respectively, 299.3 +/- 49, 392.6 +/- 66.8, and 494.1 +/- 88.3 micrograms/liter, for homozygotes with the longer allele (n = 14), and heterozygotes (n = 37) and homozygotes (n = 29) with the shorter allele. The insertion/deletion polymorphism accounted for 47% of the total phenotypic variance of serum ACE, showing that the ACE gene locus is the major locus that determines serum ACE concentration. Concomitant determination of the ACE genotype will improve discrimination between normal and abnormal serum ACE values by allowing comparison with a more appropriate reference interval.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhenc-Gelas F., Weare J. A., Johnson R. L., Jr, Erdös E. G. Measurement of human converting enzyme level by direct radioimmunoassay. J Lab Clin Med. 1983 Jan;101(1):83–96. [PubMed] [Google Scholar]
- Boerwinkle E., Sing C. F. Bias of the contribution of single-locus effects to the variance of a quantitative trait. Am J Hum Genet. 1986 Jul;39(1):137–144. [PMC free article] [PubMed] [Google Scholar]
- Cambien F., Alhenc-Gelas F., Herbeth B., Andre J. L., Rakotovao R., Gonzales M. F., Allegrini J., Bloch C. Familial resemblance of plasma angiotensin-converting enzyme level: the Nancy Study. Am J Hum Genet. 1988 Nov;43(5):774–780. [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. C., Thorn J. A., Oka K., Galton D. J., Stocks J. DNA polymorphisms at the lipoprotein lipase gene: associations in normal and hypertriglyceridaemic subjects. Atherosclerosis. 1989 Sep;79(1):85–91. doi: 10.1016/0021-9150(89)90037-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Qasba P. K. Alkaline transfer of DNA to plastic membrane. Biochem Biophys Res Commun. 1984 Jul 18;122(1):340–344. doi: 10.1016/0006-291x(84)90480-7. [DOI] [PubMed] [Google Scholar]
- Das M., Hartley J. L., Soffers R. L. Serum angiotensin-converting enzyme. Isolation and relationship to the pulmonary enzyme. J Biol Chem. 1977 Feb 25;252(4):1316–1319. [PubMed] [Google Scholar]
- Ehlers M. R., Riordan J. F. Angiotensin-converting enzyme: new concepts concerning its biological role. Biochemistry. 1989 Jun 27;28(13):5311–5318. doi: 10.1021/bi00439a001. [DOI] [PubMed] [Google Scholar]
- Elbein S. C. Molecular and clinical characterization of an insertional polymorphism of the insulin-receptor gene. Diabetes. 1989 Jun;38(6):737–743. doi: 10.2337/diab.38.6.737. [DOI] [PubMed] [Google Scholar]
- Erdös E. G., Skidgel R. A. The angiotensin I-converting enzyme. Lab Invest. 1987 Apr;56(4):345–348. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fogarty Y., Fraser C. G., Browning M. C. Intra- and inter-individual variation of serum angiotensin converting enzyme: clinical implications. Ann Clin Biochem. 1989 Mar;26(Pt 2):201–202. doi: 10.1177/000456328902600224. [DOI] [PubMed] [Google Scholar]
- Humphries S. E., Cook M., Dubowitz M., Stirling Y., Meade T. W. Role of genetic variation at the fibrinogen locus in determination of plasma fibrinogen concentrations. Lancet. 1987 Jun 27;1(8548):1452–1455. doi: 10.1016/s0140-6736(87)92205-7. [DOI] [PubMed] [Google Scholar]
- Humphries S. E. DNA polymorphisms of the apolipoprotein genes--their use in the investigation of the genetic component of hyperlipidaemia and atherosclerosis. Atherosclerosis. 1988 Aug;72(2-3):89–108. doi: 10.1016/0021-9150(88)90069-x. [DOI] [PubMed] [Google Scholar]
- Lieberman J. Elevation of serum angiotensin-converting-enzyme (ACE) level in sarcoidosis. Am J Med. 1975 Sep;59(3):365–372. doi: 10.1016/0002-9343(75)90395-2. [DOI] [PubMed] [Google Scholar]
- Okabe T., Yamagata K., Fujisawa M., Watanabe J., Takaku F., Lanzillo J. J., Fanburg B. L. Increased angiotensin-converting enzyme in peripheral blood monocytes from patients with sarcoidosis. J Clin Invest. 1985 Mar;75(3):911–914. doi: 10.1172/JCI111791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler L. A., Weber J. L., Gorski J. Polymorphism near the rat prolactin gene caused by insertion of an Alu-like element. Nature. 1983 Sep 8;305(5930):159–160. doi: 10.1038/305159a0. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Porro E. B., Patton J. G., Nadal-Ginard B. Scanning from an independently specified branch point defines the 3' splice site of mammalian introns. Nature. 1989 Nov 16;342(6247):243–247. doi: 10.1038/342243a0. [DOI] [PubMed] [Google Scholar]
- Soubrier F., Alhenc-Gelas F., Hubert C., Allegrini J., John M., Tregear G., Corvol P. Two putative active centers in human angiotensin I-converting enzyme revealed by molecular cloning. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9386–9390. doi: 10.1073/pnas.85.24.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvikis S., Chan L., Siest G., Drouin P., Boerwinkle E. An insertion deletion polymorphism in the signal peptide of the human apolipoprotein B gene. Hum Genet. 1990 Mar;84(4):373–375. doi: 10.1007/BF00196239. [DOI] [PubMed] [Google Scholar]