Abstract
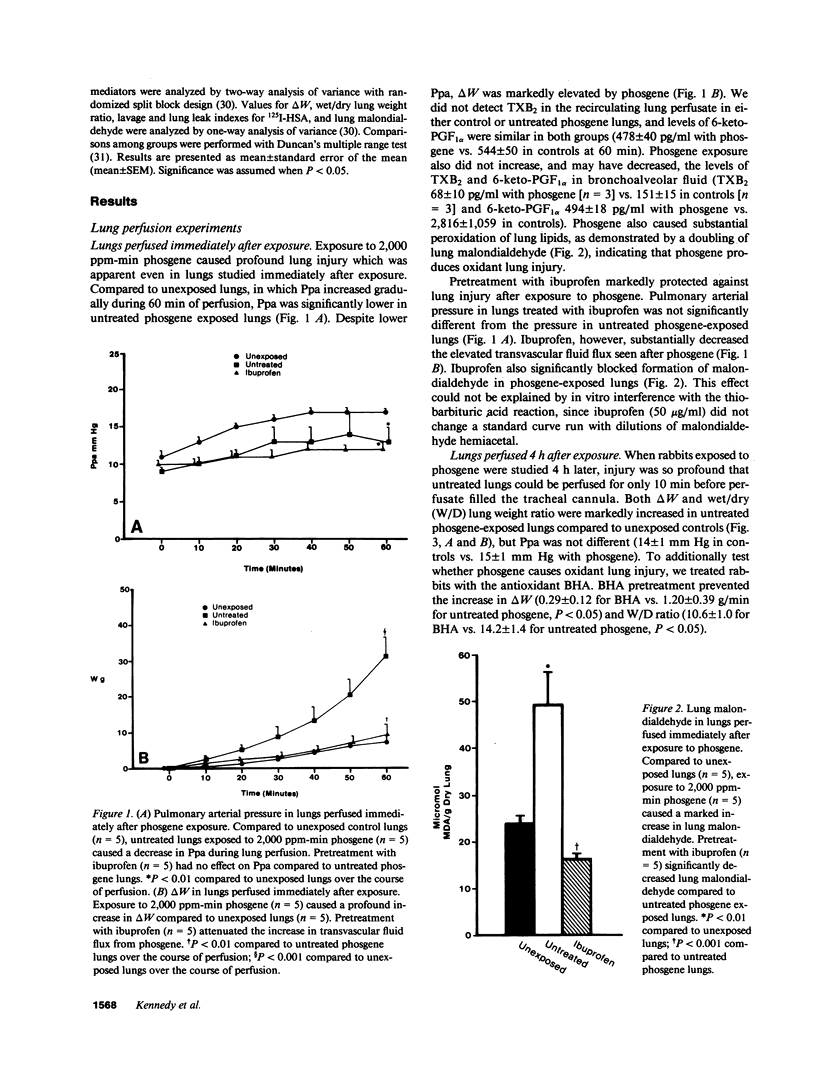
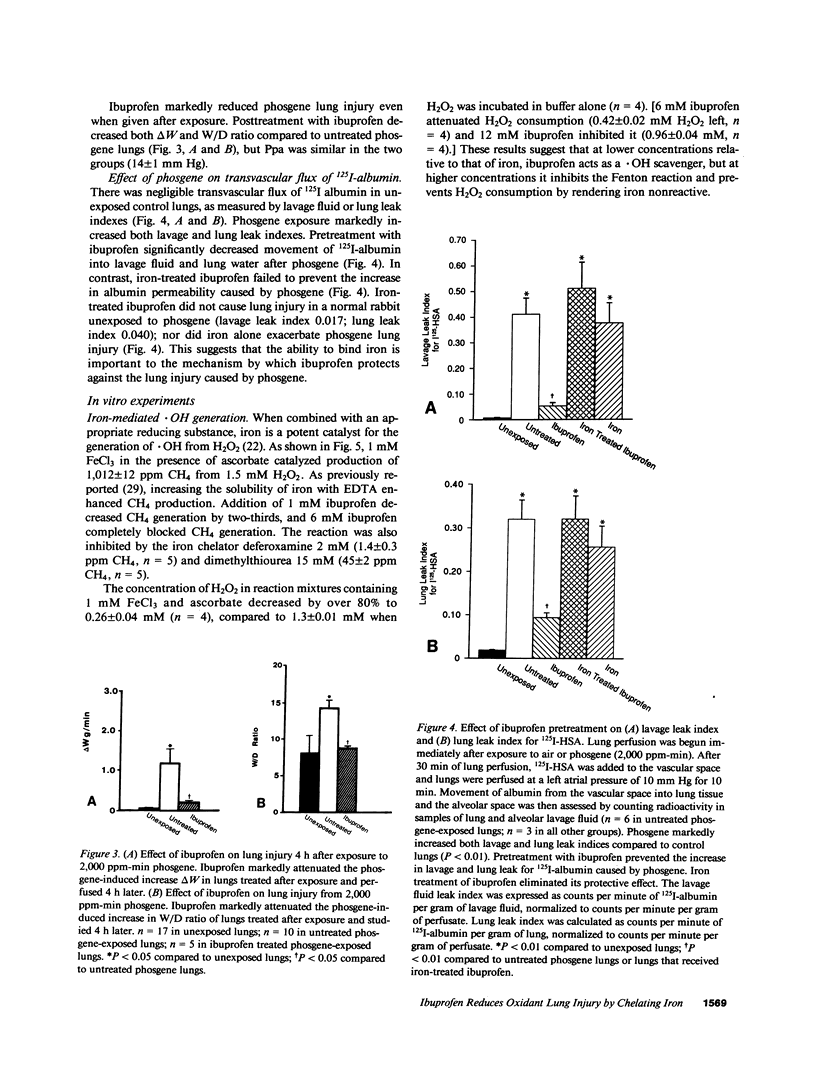
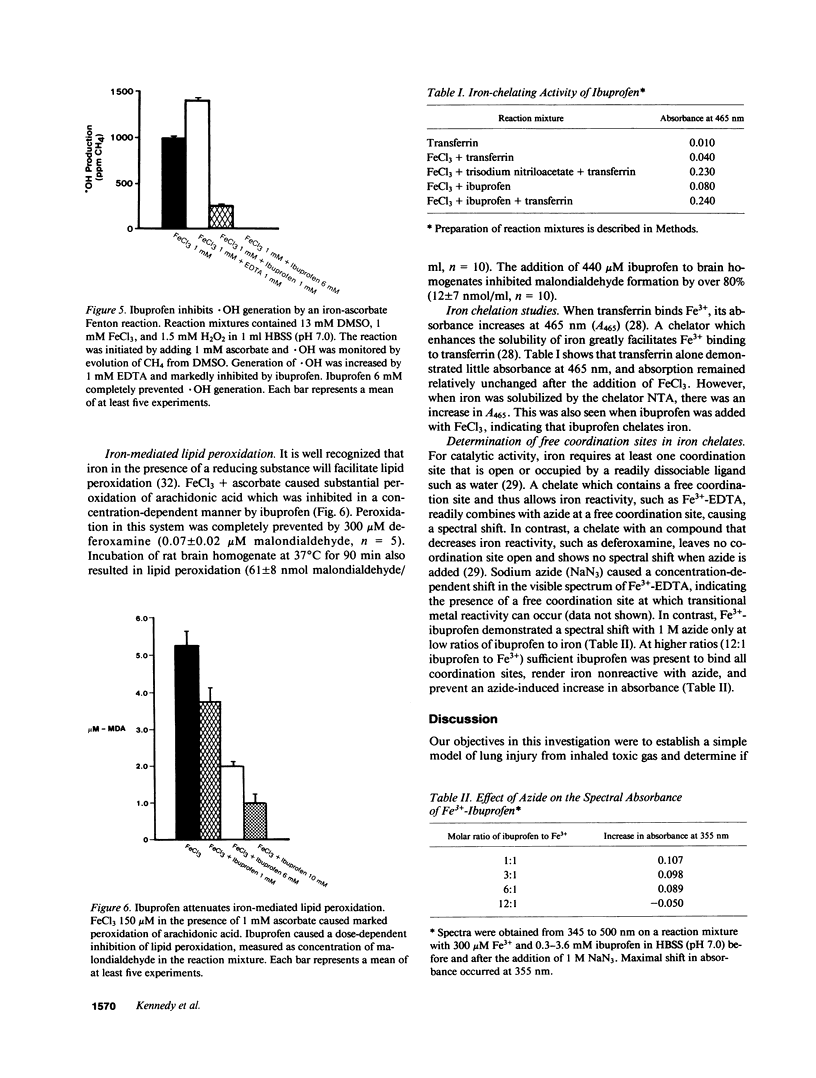
Because ibuprofen protects from septic lung injury, we studied the effect of ibuprofen in oxidant lung injury from phosgene. Lungs from rabbits exposed to 2,000 ppm-min phosgene were perfused with Krebs-Henseleit buffer at 50 ml/min for 60 min. Phosgene caused no increase in lung generation of cyclooxygenase metabolites and no elevation in pulmonary arterial pressure, but markedly increased transvascular fluid flux (delta W = 31 +/- 5 phosgene vs. 8 +/- 1 g unexposed, P less than 0.001), permeability to albumin (125I-HSA) lung leak index 0.274 +/- 0.035 phosgene vs. 0.019 +/- 0.001 unexposed, P less than 0.01; 125I-HSA lavage leak index 0.352 +/- 0.073 phosgene vs. 0.008 +/- 0.001 unexposed, P less than 0.01), and lung malondialdehyde (50 +/- 7 phosgene vs. 24 +/- 0.7 mumol/g dry lung unexposed, P less than 0.01). Ibuprofen protected lungs from phosgene (delta W = 10 +/- 2 g; lung leak index 0.095 +/- 0.013; lavage leak index 0.052 +/- 0.013; and malondialdehyde 16 +/- 3 mumol/g dry lung, P less than 0.01). Because iron-treated ibuprofen failed to protect, we studied the effect of ibuprofen in several iron-mediated reactions in vitro. Ibuprofen attenuated generation of .OH by a Fenton reaction and peroxidation of arachidonic acid by FeCl3 and ascorbate. Ibuprofen also formed iron chelates that lack the free coordination site required for iron to be reactive. Thus, ibuprofen may prevent iron-mediated generation of oxidants or iron-mediated lipid peroxidation after phosgene exposure. This suggests a new mechanism for ibuprofen's action.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almqvist P. M., Kuenzig M., Schwartz S. I. Treatment of experimental canine endotoxin shock with ibuprofen, a cyclooxygenase inhibitor. Circ Shock. 1984;13(3):227–232. [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B. The iron-binding and hydroxyl radical scavenging action of anti-inflammatory drugs. Xenobiotica. 1988 Apr;18(4):459–470. doi: 10.3109/00498258809041682. [DOI] [PubMed] [Google Scholar]
- Baldwin S. R., Simon R. H., Boxer L. A., Till G. O., Kunkel R. G. Attenuation by 2,3-dihydroxybenzoic acid of acute lung injury induced by cobra venom factor in the rat. Am Rev Respir Dis. 1985 Dec;132(6):1288–1293. doi: 10.1164/arrd.1985.132.6.1288. [DOI] [PubMed] [Google Scholar]
- Bates G. W., Billups C., Saltman P. The kinetics and mechanism of iron (3) exchange between chelates and transferrin. I. The complexes of citrate and nitrilotriacetic acid. J Biol Chem. 1967 Jun 25;242(12):2810–2815. [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Blake D. R., Hall N. D., Bacon P. A., Dieppe P. A., Halliwell B., Gutteridge J. M. Effect of a specific iron chelating agent on animal models of inflammation. Ann Rheum Dis. 1983 Feb;42(1):89–93. doi: 10.1136/ard.42.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham K. L., Meyrick B., Berry L. C., Jr, Repine J. E. Antioxidants protect cultured bovine lung endothelial cells from injury by endotoxin. J Appl Physiol (1985) 1987 Aug;63(2):840–850. doi: 10.1152/jappl.1987.63.2.840. [DOI] [PubMed] [Google Scholar]
- Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Currie W. D., Hatch G. E., Frosolono M. F. Pulmonary alterations in rats due to acute phosgene inhalation. Fundam Appl Toxicol. 1987 Jan;8(1):107–114. doi: 10.1016/0272-0590(87)90106-0. [DOI] [PubMed] [Google Scholar]
- Diller W. F. Medical phosgene problems and their possible solution. J Occup Med. 1978 Mar;20(3):189–193. doi: 10.1097/00043764-197803000-00007. [DOI] [PubMed] [Google Scholar]
- Farrukh I. S., Gurtner G. H., Michael J. R. Pharmacological modification of pulmonary vascular injury: possible role of cAMP. J Appl Physiol (1985) 1987 Jan;62(1):47–54. doi: 10.1152/jappl.1987.62.1.47. [DOI] [PubMed] [Google Scholar]
- Farrukh I. S., Michael J. R., Peters S. P., Sciuto A. M., Adkinson N. F., Jr, Freeland H. S., Paky A., Spannhake E. W., Summer W. R., Gurtner G. H. The role of cyclooxygenase and lipoxygenase mediators in oxidant-induced lung injury. Am Rev Respir Dis. 1988 Jun;137(6):1343–1349. doi: 10.1164/ajrccm/137.6.1343. [DOI] [PubMed] [Google Scholar]
- Farrukh I. S., Michael J. R., Summer W. R., Adkinson N. F., Jr, Gurtner G. H. Thromboxane-induced pulmonary vasoconstriction: involvement of calcium. J Appl Physiol (1985) 1985 Jan;58(1):34–44. doi: 10.1152/jappl.1985.58.1.34. [DOI] [PubMed] [Google Scholar]
- Gannon D. E., Varani J., Phan S. H., Ward J. H., Kaplan J., Till G. O., Simon R. H., Ryan U. S., Ward P. A. Source of iron in neutrophil-mediated killing of endothelial cells. Lab Invest. 1987 Jul;57(1):37–44. [PubMed] [Google Scholar]
- Gnidec A. G., Sibbald W. J., Cheung H., Metz C. A. Ibuprofen reduces the progression of permeability edema in an animal model of hyperdynamic sepsis. J Appl Physiol (1985) 1988 Sep;65(3):1024–1032. doi: 10.1152/jappl.1988.65.3.1024. [DOI] [PubMed] [Google Scholar]
- Graf E., Empson K. L., Eaton J. W. Phytic acid. A natural antioxidant. J Biol Chem. 1987 Aug 25;262(24):11647–11650. [PubMed] [Google Scholar]
- Graf E., Mahoney J. R., Bryant R. G., Eaton J. W. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem. 1984 Mar 25;259(6):3620–3624. [PubMed] [Google Scholar]
- Gurtner G. H., Michael J. R., Farrukh I. S., Sciuto A. M., Adkinson N. F. Mechanism of hyperoxia-induced pulmonary vascular paralysis: effect of antioxidant pretreatment. J Appl Physiol (1985) 1985 Sep;59(3):953–958. doi: 10.1152/jappl.1985.59.3.953. [DOI] [PubMed] [Google Scholar]
- Hiller K. O., Wilson R. L. Hydroxyl-free radicals and anti-inflammatory drugs: biological inactivation studies and reaction rate constants. Biochem Pharmacol. 1983 Jul 1;32(13):2109–2111. doi: 10.1016/0006-2952(83)90434-3. [DOI] [PubMed] [Google Scholar]
- Jacobs E. R., Soulsby M. E., Bone R. C., Wilson F. J., Jr, Hiller F. C. Ibuprofen in canine endotoxin shock. J Clin Invest. 1982 Sep;70(3):536–541. doi: 10.1172/JCI110645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L. J., Lalonde C., Demling R. H. Lung dysfunction after thermal injury in relation to prostanoid and oxygen radical release. J Appl Physiol (1985) 1986 Jul;61(1):103–112. doi: 10.1152/jappl.1986.61.1.103. [DOI] [PubMed] [Google Scholar]
- Johnson A., Malik A. B. Pulmonary transvascular fluid and protein exchange after thrombin-induced microembolism. Differential effects of cyclooxygenase inhibitors. Am Rev Respir Dis. 1985 Jul;132(1):70–76. doi: 10.1164/arrd.1985.132.1.70. [DOI] [PubMed] [Google Scholar]
- Kaplan H. B., Edelson H. S., Korchak H. M., Given W. P., Abramson S., Weissmann G. Effects of non-steroidal anti-inflammatory agents on human neutrophil functions in vitro and in vivo. Biochem Pharmacol. 1984 Feb 1;33(3):371–378. doi: 10.1016/0006-2952(84)90228-4. [DOI] [PubMed] [Google Scholar]
- Kennedy T. P., Gordon G. B., Paky A., McShane A., Adkinson N. F., Jr, Peters S. P., Friday K., Jackman W., Sciuto A. M., Gurtner G. H. Amiodarone causes acute oxidant lung injury in ventilated and perfused rabbit lungs. J Cardiovasc Pharmacol. 1988 Jul;12(1):23–36. doi: 10.1097/00005344-198807000-00004. [DOI] [PubMed] [Google Scholar]
- Kennedy T. P., Michael J. R., Hoidal J. R., Hasty D., Sciuto A. M., Hopkins C., Lazar R., Bysani G. K., Tolley E., Gurtner G. H. Dibutyryl cAMP, aminophylline, and beta-adrenergic agonists protect against pulmonary edema caused by phosgene. J Appl Physiol (1985) 1989 Dec;67(6):2542–2552. doi: 10.1152/jappl.1989.67.6.2542. [DOI] [PubMed] [Google Scholar]
- Lindenschmidt R. C., Patterson C. E., Forney R. B., Rhoades R. A. Selective action of prostaglandin F2 alpha during paraquat-induced pulmonary edema in the perfused lung. Toxicol Appl Pharmacol. 1983 Aug;70(1):105–114. doi: 10.1016/0041-008x(83)90183-7. [DOI] [PubMed] [Google Scholar]
- Noweir M. H., Pfitzer E. A. An improved method for determination of phosgene in air. Am Ind Hyg Assoc J. 1971 Mar;32(3):163–169. doi: 10.1080/0002889718506431. [DOI] [PubMed] [Google Scholar]
- Okusawa S., Gelfand J. A., Ikejima T., Connolly R. J., Dinarello C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988 Apr;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacht E. R., Davis W. B. Role of transferrin and ceruloplasmin in antioxidant activity of lung epithelial lining fluid. J Appl Physiol (1985) 1988 May;64(5):2092–2099. doi: 10.1152/jappl.1988.64.5.2092. [DOI] [PubMed] [Google Scholar]
- Perkowski S. Z., Havill A. M., Flynn J. T., Gee M. H. Role of intrapulmonary release of eicosanoids and superoxide anion as mediators of pulmonary dysfunction and endothelial injury in sheep with intermittent complement activation. Circ Res. 1983 Nov;53(5):574–583. doi: 10.1161/01.res.53.5.574. [DOI] [PubMed] [Google Scholar]
- Perlman M. B., Johnson A., Malik A. B. Ibuprofen prevents thrombin-induced lung vascular injury: mechanism of effect. Am J Physiol. 1987 Mar;252(3 Pt 2):H605–H614. doi: 10.1152/ajpheart.1987.252.3.H605. [DOI] [PubMed] [Google Scholar]
- Peterson D. A., Gerrard J. M., Rao G. H., White J. G. Inhibition of ferrous iron induced oxidation of arachidonic acid by indomethacin. Prostaglandins Med. 1979 Feb;2(2):97–108. doi: 10.1016/0161-4630(79)90044-2. [DOI] [PubMed] [Google Scholar]
- RINEHART W. E., HATCH T. CONCENTRATION-TIME PRODUCT (CT) AS AN EXPRESSION OF DOSE IN SUBLETHAL EXPOSURES TO PHOSGENE. Am Ind Hyg Assoc J. 1964 Nov-Dec;25:545–553. doi: 10.1080/00028896409342641. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romson J. L., Hook B. G., Rigot V. H., Schork M. A., Swanson D. P., Lucchesi B. R. The effect of ibuprofen on accumulation of indium-111-labeled platelets and leukocytes in experimental myocardial infarction. Circulation. 1982 Nov;66(5):1002–1011. doi: 10.1161/01.cir.66.5.1002. [DOI] [PubMed] [Google Scholar]
- Schulman E. S., Newball H. H., Demers L. M., Fitzpatrick F. A., Adkinson N. F., Jr Anaphylactic release of thromboxane A2, prostaglandin D2, and prostacyclin from human lung parenchyma. Am Rev Respir Dis. 1981 Oct;124(4):402–406. doi: 10.1164/arrd.1981.124.4.402. [DOI] [PubMed] [Google Scholar]
- Sedgwick A. D., Blake D. R., Winwood P., Moore A. R., Al-Duaij A., Willoughby D. A. Studies into the effects of the iron chelator desferrioxamine on the inflammatory process. Eur J Rheumatol Inflamm. 1984;7(3):87–94. [PubMed] [Google Scholar]
- Shinozawa Y., Hales C., Jung W., Burke J. Ibuprofen prevents synthetic smoke-induced pulmonary edema. Am Rev Respir Dis. 1986 Dec;134(6):1145–1148. doi: 10.1164/arrd.1986.134.6.1145. [DOI] [PubMed] [Google Scholar]
- Stocks J., Gutteridge J. M., Sharp R. J., Dormandy T. L. Assay using brain homogenate for measuring the antioxidant activity of biological fluids. Clin Sci Mol Med. 1974 Sep;47(3):215–222. doi: 10.1042/cs0470215. [DOI] [PubMed] [Google Scholar]
- Tate R. M., Vanbenthuysen K. M., Shasby D. M., McMurtry I. F., Repine J. E. Oxygen-radical-mediated permeability edema and vasoconstriction in isolated perfused rabbit lungs. Am Rev Respir Dis. 1982 Nov;126(5):802–806. doi: 10.1164/arrd.1982.126.5.802. [DOI] [PubMed] [Google Scholar]
- Thurman R. G., Ley H. G., Scholz R. Hepatic microsomal ethanol oxidation. Hydrogen peroxide formation and the role of catalase. Eur J Biochem. 1972 Feb;25(3):420–430. doi: 10.1111/j.1432-1033.1972.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Traber D. L., Adams T., Jr, Henriksen N., Traber L. D. Ibuprofen and diphenhydramine reduce the lung lesion of endotoxemia in sheep. J Trauma. 1984 Sep;24(9):835–840. doi: 10.1097/00005373-198409000-00010. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Venezio F. R., DiVincenzo C., Pearlman F., Phair J. P. Effects of the newer nonsteroidal anti-inflammatory agents, ibuprofen, fenoprofen, and sulindac, on neutrophil adherence. J Infect Dis. 1985 Oct;152(4):690–694. doi: 10.1093/infdis/152.4.690. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Till G. O., Kunkel R., Beauchamp C. Evidence for role of hydroxyl radical in complement and neutrophil-dependent tissue injury. J Clin Invest. 1983 Sep;72(3):789–801. doi: 10.1172/JCI111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi N., Kuriyama H., Watanabe N., Neda H., Maeda M., Niitsu Y. Intracellular hydroxyl radical production induced by recombinant human tumor necrosis factor and its implication in the killing of tumor cells in vitro. Cancer Res. 1989 Apr 1;49(7):1671–1675. [PubMed] [Google Scholar]


