Abstract
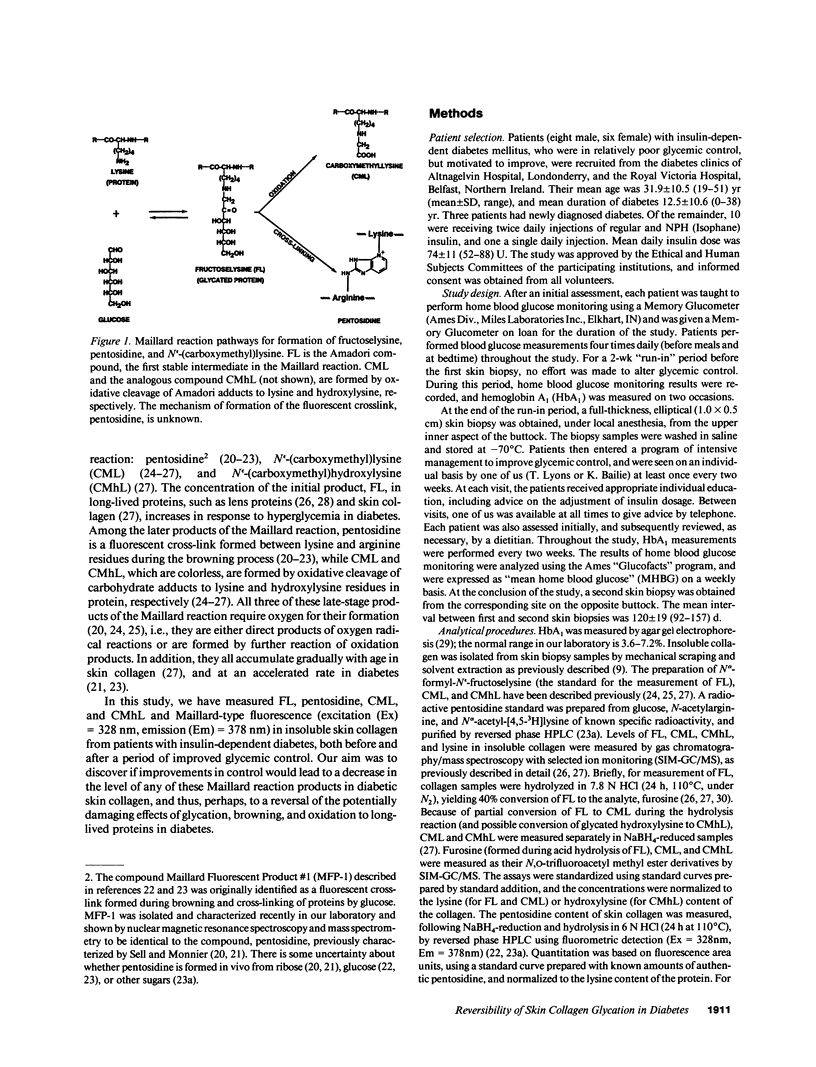
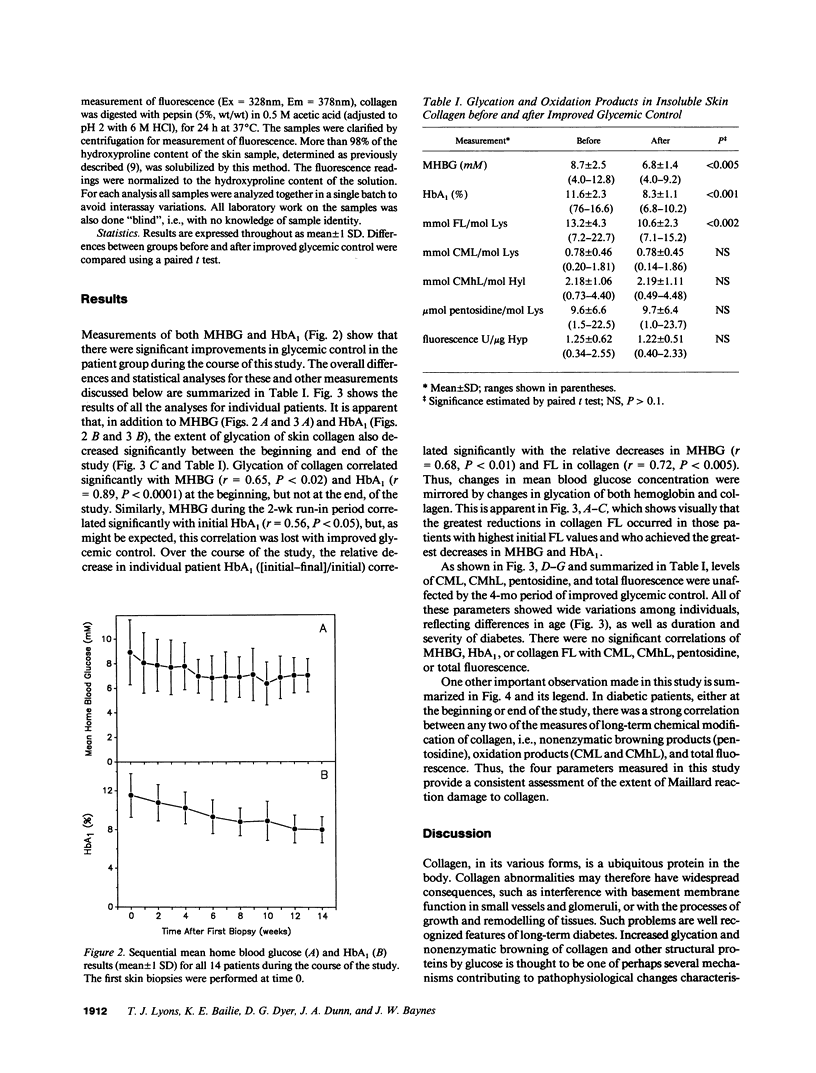
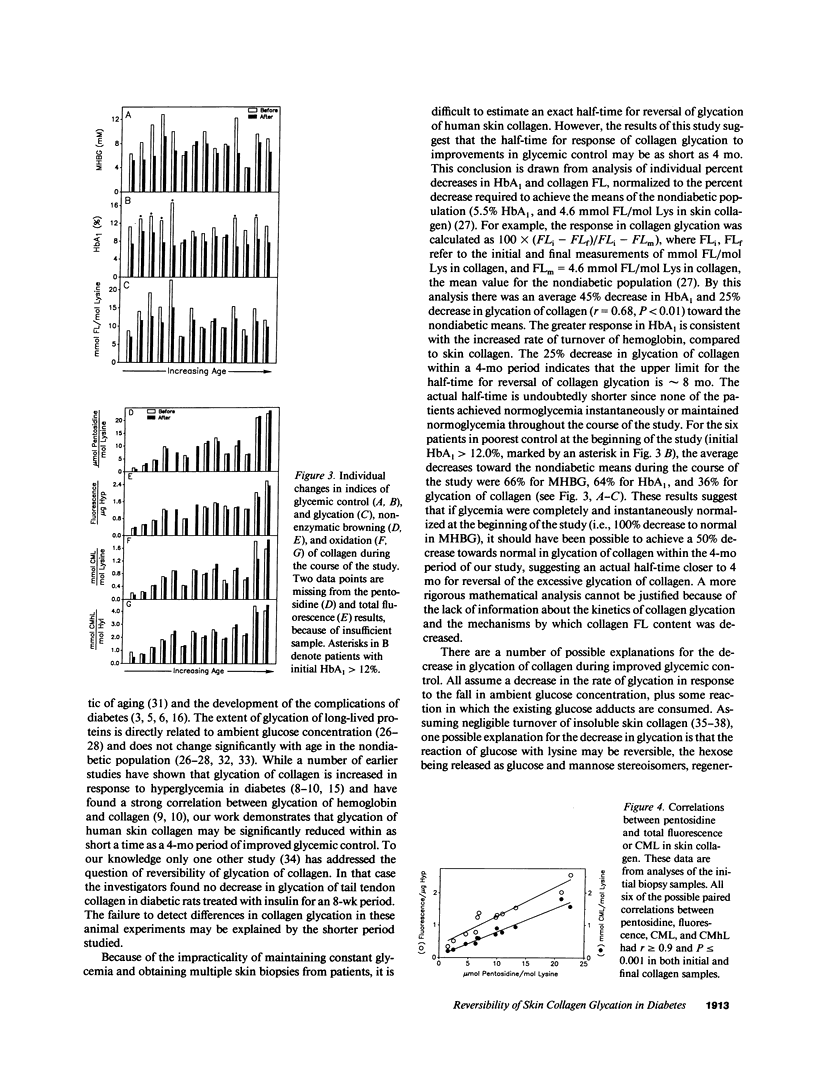
Glycation, oxidation, and nonenzymatic browning of protein have all been implicated in the development of diabetic complications. The initial product of glycation of protein, fructoselysine (FL), undergoes further reactions, yielding a complex mixture of browning products, including the fluorescent lysine-arginine cross-link, pentosidine. Alternatively, FL may be cleaved oxidatively to form N(epsilon)-(carboxymethyl)lysine (CML), while glycated hydroxylysine, an amino-acid unique to collagen, may yield N(epsilon)-(carboxymethyl)hydroxylysine (CMhL). We have measured FL, pentosidine, fluorescence (excitation = 328 nm, emission = 378 nm), CML, and CMhL in insoluble skin collagen from 14 insulin-dependent diabetic patients before and after a 4-mo period of intensive therapy to improve glycemic control. Mean home blood glucose fell from 8.7 +/- 2.5 (mean +/- 1 SD) to 6.8 +/- 1.4 mM (P less than 0.005), and mean glycated hemoglobin (HbA1) from 11.6 +/- 2.3% to 8.3 +/- 1.1% (P less than 0.001). These changes were accompanied by a significant decrease in glycation of skin collagen, from 13.2 +/- 4.3 to 10.6 +/- 2.3 mmol FL/mol lysine (P less than 0.002). However, levels of browning and oxidation products (pentosidine, CML, and CMhL) and fluorescence were unchanged. These results show that the glycation of long-lived proteins can be decreased by improved glycemic control, but suggest that once cumulative damage to collagen by browning and oxidation reactions has occurred, it may not be readily reversed. Thus, in diabetic patients, institution and maintenance of good glycemic control at any time could potentially limit the extent of subsequent long-term damage to proteins by glycation and oxidation reactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M. U., Dunn J. A., Walla M. D., Thorpe S. R., Baynes J. W. Oxidative degradation of glucose adducts to protein. Formation of 3-(N epsilon-lysino)-lactic acid from model compounds and glycated proteins. J Biol Chem. 1988 Jun 25;263(18):8816–8821. [PubMed] [Google Scholar]
- Ahmed M. U., Thorpe S. R., Baynes J. W. Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J Biol Chem. 1986 Apr 15;261(11):4889–4894. [PubMed] [Google Scholar]
- BALLOU J. E., THOMPSON R. C. Studies of metabolic turnover with tritium as a tracer. V. The predominantly non-dynamic state of body constituents in the rat. J Biol Chem. 1956 Dec;223(2):795–809. [PubMed] [Google Scholar]
- Baynes J. W., Watkins N. G., Fisher C. I., Hull C. J., Patrick J. S., Ahmed M. U., Dunn J. A., Thorpe S. R. The Amadori product on protein: structure and reactions. Prog Clin Biol Res. 1989;304:43–67. [PubMed] [Google Scholar]
- Brownlee M., Vlassara H., Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Intern Med. 1984 Oct;101(4):527–537. doi: 10.7326/0003-4819-101-4-527. [DOI] [PubMed] [Google Scholar]
- Dominiczak M. H., Bell J., Cox N. H., McCruden D. C., Jones S. K., Finlay A. Y., Percy-Robb I. W., Frier B. M. Increased collagen-linked fluorescence in skin of young patients with type I diabetes mellitus. Diabetes Care. 1990 May;13(5):468–472. doi: 10.2337/diacare.13.5.468. [DOI] [PubMed] [Google Scholar]
- Dunn J. A., McCance D. R., Thorpe S. R., Lyons T. J., Baynes J. W. Age-dependent accumulation of N epsilon-(carboxymethyl)lysine and N epsilon-(carboxymethyl)hydroxylysine in human skin collagen. Biochemistry. 1991 Feb 5;30(5):1205–1210. doi: 10.1021/bi00219a007. [DOI] [PubMed] [Google Scholar]
- Dunn J. A., Patrick J. S., Thorpe S. R., Baynes J. W. Oxidation of glycated proteins: age-dependent accumulation of N epsilon-(carboxymethyl)lysine in lens proteins. Biochemistry. 1989 Nov 28;28(24):9464–9468. doi: 10.1021/bi00450a033. [DOI] [PubMed] [Google Scholar]
- Garlick R. L., Bunn H. F., Spiro R. G. Nonenzymatic glycation of basement membranes from human glomeruli and bovine sources. Effect of diabetes and age. Diabetes. 1988 Aug;37(8):1144–1150. doi: 10.2337/diab.37.8.1144. [DOI] [PubMed] [Google Scholar]
- Hamlin C. R., Kohn R. R., Luschin J. H. Apparent accelerated aging of human collagen in diabetes mellitus. Diabetes. 1975 Oct;24(10):902–904. doi: 10.2337/diab.24.10.902. [DOI] [PubMed] [Google Scholar]
- Jones A. F., Lunec J. Protein fluorescence and its relationship to free radical activity. Br J Cancer Suppl. 1987 Jun;8:60–65. [PMC free article] [PubMed] [Google Scholar]
- Kennedy L., Baynes J. W. Non-enzymatic glycosylation and the chronic complications of diabetes: an overview. Diabetologia. 1984 Feb;26(2):93–98. doi: 10.1007/BF00281113. [DOI] [PubMed] [Google Scholar]
- Lyons T. J., Kennedy L. Non-enzymatic glycosylation of skin collagen in patients with type 1 (insulin-dependent) diabetes mellitus and limited joint mobility. Diabetologia. 1985 Jan;28(1):2–5. doi: 10.1007/BF00276991. [DOI] [PubMed] [Google Scholar]
- McLennan S., Yue D. K., Marsh M., Swanson B., Delbridge L., Reeve T., Turtle J. R. The prevention and reversibility of tissue non-enzymatic glycosylation in diabetes. Diabet Med. 1986 Mar;3(2):141–146. doi: 10.1111/j.1464-5491.1986.tb00725.x. [DOI] [PubMed] [Google Scholar]
- Menard L., Dempsey M. E., Blankstein L. A., Aleyassine H., Wacks M., Soeldner J. S. Quantitiative determination of glycosylated hemoglobin A1 by agar gel electrophoresis. Clin Chem. 1980 Oct;26(11):1598–1602. [PubMed] [Google Scholar]
- Molnar J. A., Alpert N. M., Wagner D. A., Miyatani S., Burke J. F., Young V. R. Synthesis and degradation of collagens in skin of healthy and protein-malnourished rats in vivo, studied by 18O2 labelling. Biochem J. 1988 Feb 15;250(1):71–76. doi: 10.1042/bj2500071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar J. A., Alpert N., Burke J. F., Young V. R. Synthesis and degradation rates of collagens in vivo in whole skin of rats, studied with 1802 labelling. Biochem J. 1986 Dec 1;240(2):431–435. doi: 10.1042/bj2400431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier V. M., Kohn R. R., Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci U S A. 1984 Jan;81(2):583–587. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier V. M. Nonenzymatic glycosylation, the Maillard reaction and the aging process. J Gerontol. 1990 Jul;45(4):B105–B111. doi: 10.1093/geronj/45.4.b105. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Sell D. R., Abdul-Karim F. W., Emancipator S. N. Collagen browning and cross-linking are increased in chronic experimental hyperglycemia. Relevance to diabetes and aging. Diabetes. 1988 Jul;37(7):867–872. doi: 10.2337/diab.37.7.867. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Vishwanath V., Frank K. E., Elmets C. A., Dauchot P., Kohn R. R. Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N Engl J Med. 1986 Feb 13;314(7):403–408. doi: 10.1056/NEJM198602133140702. [DOI] [PubMed] [Google Scholar]
- Schleicher E., Wieland O. H. Kinetic analysis of glycation as a tool for assessing the half-life of proteins. Biochim Biophys Acta. 1986 Oct 29;884(1):199–205. doi: 10.1016/0304-4165(86)90244-8. [DOI] [PubMed] [Google Scholar]
- Schnider S. L., Kohn R. R. Effects of age and diabetes mellitus on the solubility and nonenzymatic glucosylation of human skin collagen. J Clin Invest. 1981 Jun;67(6):1630–1635. doi: 10.1172/JCI110198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell D. R., Monnier V. M. End-stage renal disease and diabetes catalyze the formation of a pentose-derived crosslink from aging human collagen. J Clin Invest. 1990 Feb;85(2):380–384. doi: 10.1172/JCI114449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell D. R., Monnier V. M. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989 Dec 25;264(36):21597–21602. [PubMed] [Google Scholar]
- Vishwanath V., Frank K. E., Elmets C. A., Dauchot P. J., Monnier V. M. Glycation of skin collagen in type I diabetes mellitus. Correlation with long-term complications. Diabetes. 1986 Aug;35(8):916–921. doi: 10.2337/diab.35.8.916. [DOI] [PubMed] [Google Scholar]
- Yue D. K., McLennan S., Delbridge L., Handelsman D. J., Reeve T., Turtle J. R. The thermal stability of collagen in diabetic rats: correlation with severity of diabetes and non-enzymatic glycosylation. Diabetologia. 1983 Apr;24(4):282–285. doi: 10.1007/BF00282714. [DOI] [PubMed] [Google Scholar]



