Abstract
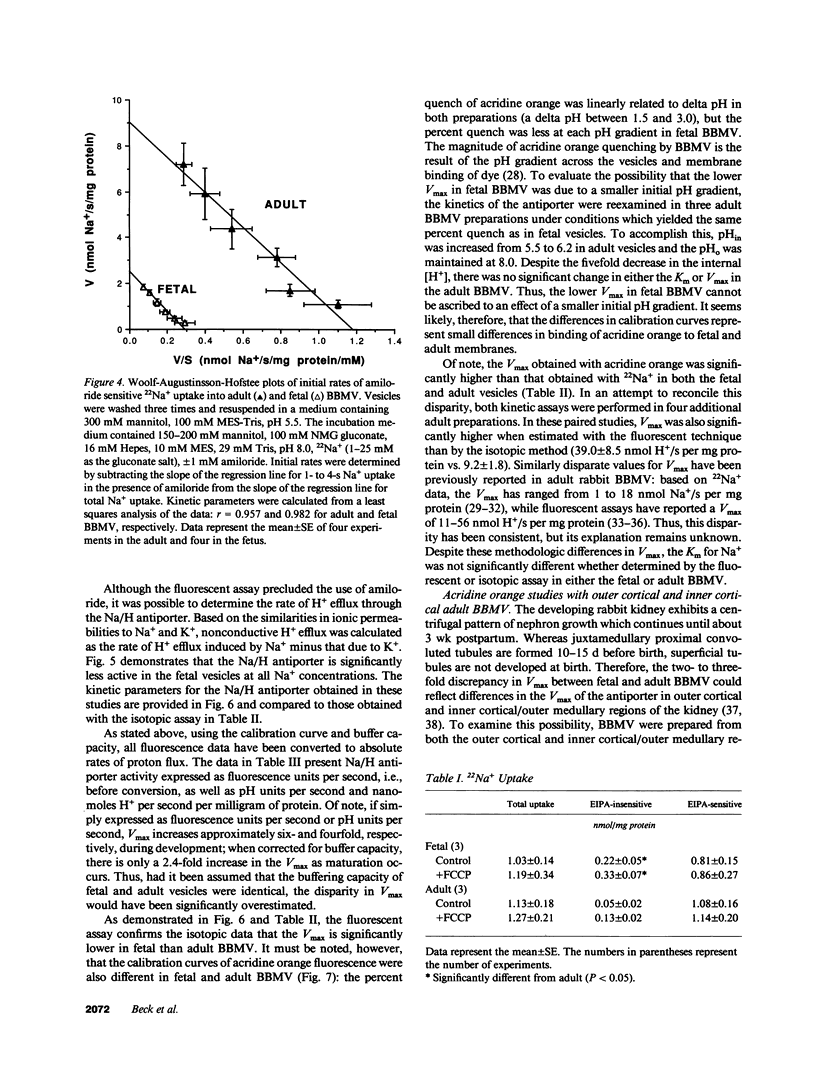
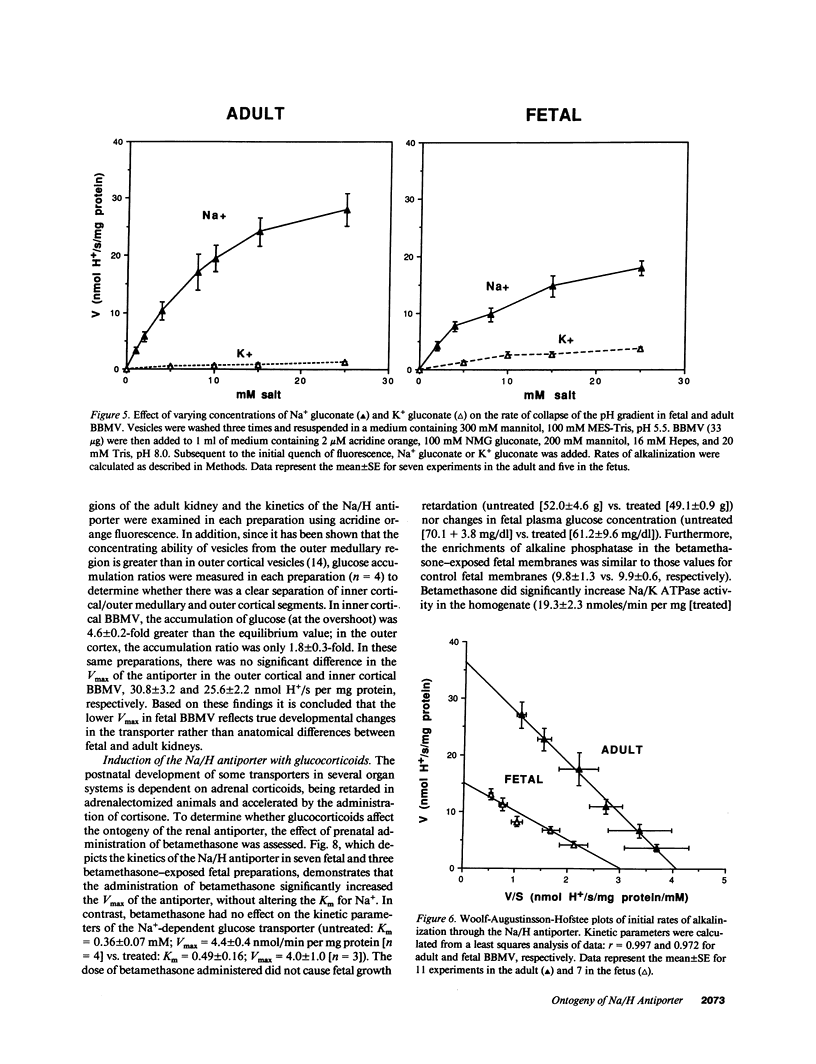
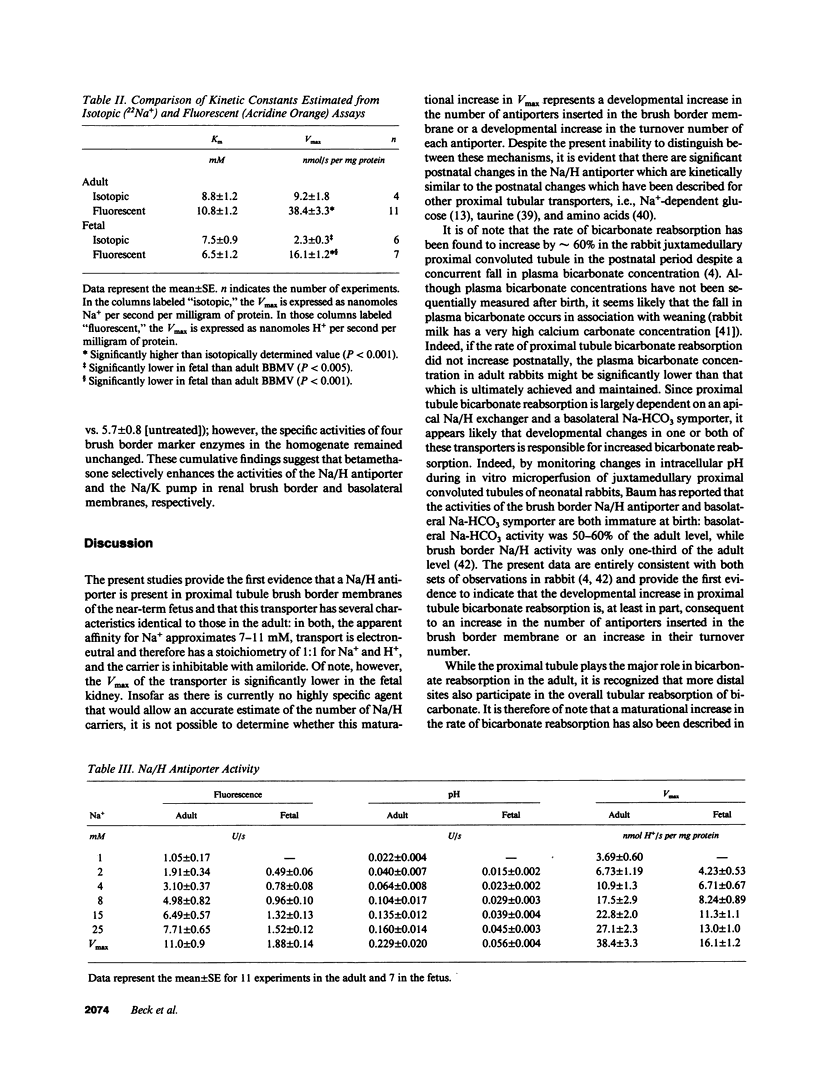
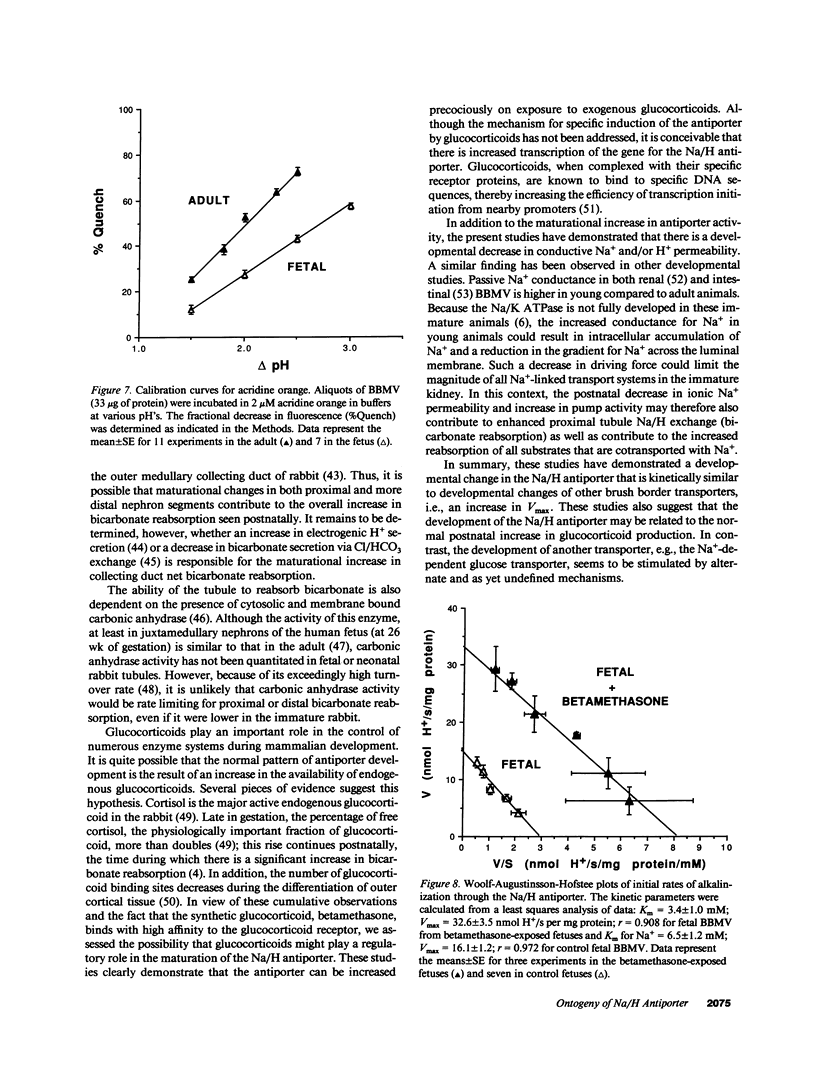
The development of the Na/H antiporter was studied in renal brush border membrane vesicles (BBMV) from fetal and adult rabbits using isotopic and fluorescent techniques. The kinetics of the antiporter studied by 22Na+ uptake revealed that the Vmax was only 25% of that in the adult; however, the Km's for Na+ were not significantly different. These data were confirmed by a fluorescent assay using the pH-sensitive probe, acridine orange: the Vmax was significantly lower in the fetal BBMV. Conductive Na+ movement was estimated from amiloride-insensitive 22Na+ uptake and the rate of alkalinization induced by K+, an ion whose relative conductance was found to be similar to that of Na+. Although relative Na+ conductance was significantly greater in fetal BBMV, the lower Vmax in fetal vesicles could not be ascribed to this factor. Maternal administration of betamethasone (50 micrograms/kg intramuscularly) for 2 d before delivery significantly increased the Vmax of the antiporter to levels observed in the adult; Km was unaffected. Na/K ATPase activity increased fourfold after betamethasone, but the specific activities of four brush border marker enzymes and the kinetics of Na(+)-glucose cotransport were unchanged. These data indicate that there is a developmental increase in brush border Na/H exchange which is the result of an increase in the number and/or the turnover number of the carriers. Further, these data suggest that the postnatal increase in antiporter activity may be related to the surge in glucocorticoid concentration that occurs perinatally.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AHMED K., JUDAH J. D. PREPARATION OF LIPOPROTEINS CONTAINING CATION-DEPENDENT ATPASE. Biochim Biophys Acta. 1964 Dec 9;93:603–613. doi: 10.1016/0304-4165(64)90343-5. [DOI] [PubMed] [Google Scholar]
- Aperia A., Haldosén L. A., Larsson L., Gustafsson J. A. Ontogeny of triamcinolone-acetonide binding sites in outer cortical tissue from rat kidneys. Am J Physiol. 1985 Dec;249(6 Pt 2):F891–F897. doi: 10.1152/ajprenal.1985.249.6.F891. [DOI] [PubMed] [Google Scholar]
- Aperia A., Larsson L., Zetterström R. Hormonal induction of Na-K-ATPase in developing proximal tubular cells. Am J Physiol. 1981 Oct;241(4):F356–F360. doi: 10.1152/ajprenal.1981.241.4.F356. [DOI] [PubMed] [Google Scholar]
- Aronson P. S. Energy-dependence of phlorizin binding to isolated renal microvillus membranes. Evidence concerning the mechanism of coupling between the electrochemical Na+ gradient the sugar transport. J Membr Biol. 1978 Jul 21;42(1):81–98. doi: 10.1007/BF01870395. [DOI] [PubMed] [Google Scholar]
- Aronson P. S., Sacktor B. The Na+ gradient-dependent transport of D-glucose in renal brush border membranes. J Biol Chem. 1975 Aug 10;250(15):6032–6039. [PubMed] [Google Scholar]
- Aronson P. S., Suhm M. A., Nee J. Interaction of external H+ with the Na+-H+ exchanger in renal microvillus membrane vesicles. J Biol Chem. 1983 Jun 10;258(11):6767–6771. [PubMed] [Google Scholar]
- Baum M. Neonatal rabbit juxtamedullary proximal convoluted tubule acidification. J Clin Invest. 1990 Feb;85(2):499–506. doi: 10.1172/JCI114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J. C., Lipkowitz M. S., Abramson R. G. Characterization of the fetal glucose transporter in rabbit kidney. Comparison with the adult brush border electrogenic Na+-glucose symporter. J Clin Invest. 1988 Aug;82(2):379–387. doi: 10.1172/JCI113609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Hirsch S., Gluck S. Localization of a proton-pumping ATPase in rat kidney. J Clin Invest. 1988 Dec;82(6):2114–2126. doi: 10.1172/JCI113833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney R. W., Gusowski N., Zeilkovic I., Padilla M. Developmental aspects of renal beta-amino acid transport. V: Brush border membrane transport in nursing animals--effect of age and diet. Pediatr Res. 1986 Sep;20(9):890–894. doi: 10.1203/00006450-198609000-00017. [DOI] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Bidani A. Kinetics of CO2 exchange in the kidney. Annu Rev Physiol. 1988;50:653–667. doi: 10.1146/annurev.ph.50.030188.003253. [DOI] [PubMed] [Google Scholar]
- Edelmann C. M., Soriano J. R., Boichis H., Gruskin A. B., Acosta M. I. Renal bicarbonate reabsorption and hydrogen ion excretion in normal infants. J Clin Invest. 1967 Aug;46(8):1309–1317. doi: 10.1172/JCI105623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. W., Medow M. S., Bovee K. C., Segal S. Developmental aspects of cystine transport in the dog. Pediatr Res. 1986 Jul;20(7):593–597. doi: 10.1203/00006450-198607000-00003. [DOI] [PubMed] [Google Scholar]
- Freiberg J. M., Kinsella J., Sacktor B. Glucocorticoids increase the Na+-H+ exchange and decrease the Na+ gradient-dependent phosphate-uptake systems in renal brush border membrane vesicles. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4932–4936. doi: 10.1073/pnas.79.16.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich T., Sablotni J., Burckhardt G. Species differences between rat and rabbit renal Na+/H+ exchangers. Biochem Biophys Res Commun. 1987 Apr 29;144(2):869–875. doi: 10.1016/s0006-291x(87)80045-1. [DOI] [PubMed] [Google Scholar]
- Ghishan F. K., Wilson F. A. Developmental maturation of D-glucose transport by rat jejunal brush-border membrane vesicles. Am J Physiol. 1985 Jan;248(1 Pt 1):G87–G92. doi: 10.1152/ajpgi.1985.248.1.G87. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Renal ammonia and acid excretion in infant rats. Am J Physiol. 1970 May;218(5):1394–1398. doi: 10.1152/ajplegacy.1970.218.5.1394. [DOI] [PubMed] [Google Scholar]
- Henning S. J. Postnatal development: coordination of feeding, digestion, and metabolism. Am J Physiol. 1981 Sep;241(3):G199–G214. doi: 10.1152/ajpgi.1981.241.3.G199. [DOI] [PubMed] [Google Scholar]
- Hilden S. A., Johns C. A., Madias N. E. Adaptation of rabbit renal cortical Na+-H+ exchange activity in chronic hypocapnia. Am J Physiol. 1989 Oct;257(4 Pt 2):F615–F622. doi: 10.1152/ajprenal.1989.257.4.F615. [DOI] [PubMed] [Google Scholar]
- Holmberg C., Tisher C. C., Jacobson H. R., Kokko J. P. Na to Cl permeability in newborn rabbit superficial and juxtamedullary proximal convoluted tubules. Miner Electrolyte Metab. 1985;11(4):215–222. [PubMed] [Google Scholar]
- Holmberg E. G., Verkman A. S., Dix J. A. Mechanism of acridine orange interaction with phospholipids and proteins in renal microvillus vesicles. Biophys Chem. 1989 Jul;33(3):245–256. doi: 10.1016/0301-4622(89)80026-2. [DOI] [PubMed] [Google Scholar]
- Hümmelink R., Ballard P. L. Endogenous corticoids and lung development in the fetal rabbit. Endocrinology. 1986 Apr;118(4):1622–1629. doi: 10.1210/endo-118-4-1622. [DOI] [PubMed] [Google Scholar]
- Ives H. E. Proton/hydroxyl permeability of proximal tubule brush border vesicles. Am J Physiol. 1985 Jan;248(1 Pt 2):F78–F86. doi: 10.1152/ajprenal.1985.248.1.F78. [DOI] [PubMed] [Google Scholar]
- Jacobsen C., Kragh-Hansen U., Sheikh M. I. Na+-H+ exchange in luminal-membrane vesicles from rabbit proximal convoluted and straight tubules in response to metabolic acidosis. Biochem J. 1986 Oct 15;239(2):411–416. doi: 10.1042/bj2390411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella J. L., Freiberg J. M., Sacktor B. Glucocorticoid activation of Na+/H+ exchange in renal brush border vesicles: kinetic effects. Am J Physiol. 1985 Feb;248(2 Pt 2):F233–F239. doi: 10.1152/ajprenal.1985.248.2.F233. [DOI] [PubMed] [Google Scholar]
- Kurtz I. Apical Na+/H+ antiporter and glycolysis-dependent H+-ATPase regulate intracellular pH in the rabbit S3 proximal tubule. J Clin Invest. 1987 Oct;80(4):928–935. doi: 10.1172/JCI113184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lipkowitz M. S., Abramson R. G. Ionic permeabilities of rat renal cortical brush-border membrane vesicles. Am J Physiol. 1987 Apr;252(4 Pt 2):F700–F711. doi: 10.1152/ajprenal.1987.252.4.F700. [DOI] [PubMed] [Google Scholar]
- Lönnerholm G., Wistrand P. J. Carbonic anhydrase in the human fetal kidney. Pediatr Res. 1983 May;17(5):390–397. doi: 10.1203/00006450-198305000-00015. [DOI] [PubMed] [Google Scholar]
- Medow M. S., Roth K. S., Goldmann D. R., Ginkinger K., Hsu B. Y., Segal S. Developmental aspects of proline transport in rat renal brush border membranes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7561–7564. doi: 10.1073/pnas.83.19.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E. S., Fine B. P., Satrasook S. S., Vergel Z. M., Edelmann C. M., Jr Renal reabsorption of bicarbonate in puppies: effect of extracellular volume contraction on the renal threshold for bicarbonate. Pediatr Res. 1972 Dec;6(12):859–867. doi: 10.1203/00006450-197212000-00002. [DOI] [PubMed] [Google Scholar]
- Nord E. P., Hafezi A., Kaunitz J. D., Trizna W., Fine L. G. pH gradient-dependent increased Na+-H+ antiport capacity of the rabbit remnant kidney. Am J Physiol. 1985 Jul;249(1 Pt 2):F90–F98. doi: 10.1152/ajprenal.1985.249.1.F90. [DOI] [PubMed] [Google Scholar]
- Nord E. P., Hafezi A., Wright E. M., Fine L. G. Mechanisms of Na+ uptake into renal brush border membrane vesicles. Am J Physiol. 1984 Oct;247(4 Pt 2):F548–F554. doi: 10.1152/ajprenal.1984.247.4.F548. [DOI] [PubMed] [Google Scholar]
- Ruiz O. S., Arruda J. A., Talor Z. Na-HCO3 cotransport and Na-H antiporter in chronic respiratory acidosis and alkalosis. Am J Physiol. 1989 Mar;256(3 Pt 2):F414–F420. doi: 10.1152/ajprenal.1989.256.3.F414. [DOI] [PubMed] [Google Scholar]
- Schmidt U., Horster M. Na-K-activated ATPase: activity maturation in rabbit nephron segments dissected in vitro. Am J Physiol. 1977 Jul;233(1):F55–F60. doi: 10.1152/ajprenal.1977.233.1.F55. [DOI] [PubMed] [Google Scholar]
- Schuster V. L. Cyclic adenosine monophosphate-stimulated bicarbonate secretion in rabbit cortical collecting tubules. J Clin Invest. 1985 Jun;75(6):2056–2064. doi: 10.1172/JCI111925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. J., Evan A. P. Development of solute transport in rabbit proximal tubule. I. HCO-3 and glucose absorption. Am J Physiol. 1983 Sep;245(3):F382–F390. doi: 10.1152/ajprenal.1983.245.3.F382. [DOI] [PubMed] [Google Scholar]
- Schwartz G. J., Evan A. P. Development of solute transport in rabbit proximal tubule. III. Na-K-ATPase activity. Am J Physiol. 1984 Jun;246(6 Pt 2):F845–F852. doi: 10.1152/ajprenal.1984.246.6.F845. [DOI] [PubMed] [Google Scholar]
- Schwartz M. J., Kokko J. P. Urinary concentrating defect of adrenal insufficiency. Permissive role of adrenal steroids on the hydroosmotic response across the rabbit cortical collecting tubule. J Clin Invest. 1980 Aug;66(2):234–242. doi: 10.1172/JCI109849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talor Z., Yang W. C., Shuffield J., Sack E., Arruda J. A. Chronic hypercapnia enhances Vmax of Na-H antiporter of renal brush-border membranes. Am J Physiol. 1987 Sep;253(3 Pt 2):F394–F400. doi: 10.1152/ajprenal.1987.253.3.F394. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Moran A. Heterogeneity of sodium-dependent D-glucose transport sites along the proximal tubule: evidence from vesicle studies. Am J Physiol. 1982 Apr;242(4):F406–F414. doi: 10.1152/ajprenal.1982.242.4.F406. [DOI] [PubMed] [Google Scholar]
- Warnock D. G., Reenstra W. W., Yee V. J. Na+/H+ antiporter of brush border vesicles: studies with acridine orange uptake. Am J Physiol. 1982 Jun;242(6):F733–F739. doi: 10.1152/ajprenal.1982.242.6.F733. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Yang W. C., Arruda J. A., Talor Z. Na+-H+ antiporter in posthypercapnic state. Am J Physiol. 1987 Nov;253(5 Pt 2):F833–F840. doi: 10.1152/ajprenal.1987.253.5.F833. [DOI] [PubMed] [Google Scholar]
- van der Heijden A. J., Guignard J. P. Bicarbonate reabsorption by the kidney of the newborn rabbit. Am J Physiol. 1989 Jan;256(1 Pt 2):F29–F34. doi: 10.1152/ajprenal.1989.256.1.F29. [DOI] [PubMed] [Google Scholar]