Abstract
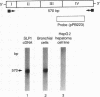
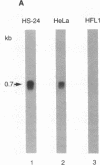
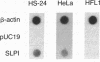
The secretory leukoprotease inhibitor (SLPI) gene codes for a 12-kD protein that within the lung protects the airway epithelium from neutrophil elastase. Screening of 228 alleles in 114 individuals for sequence differences by RNase protection of genomic DNA revealed no detectable polymorphisms in SLPI gene exons II-IV. SLPI gene expression in the lung was demonstrated by identifying SLPI mRNA transcripts in bronchial epithelial cells freshly isolated from normals. Cell lines derived from mucosal surfaces (HS-24 bronchial squamous cell carcinoma, HeLa cervical carcinoma) actively transcribe the SLPI gene and contain SLPI mRNA transcripts, while lung fibroblasts demonstrate no evidence of SLPI gene expression. SLPI mRNA transcripts appear to be relatively stable, with mRNA levels only mildly affected by inhibition of RNA synthesis. Chromatin DNA of HS-24 cells demonstrates two DNase I hypersensitivity sites within the 5' flanking region of exon I of the SLPI gene, whereas fibroblast chromatin has no DNase I accessible sites in the same region. Further analysis of the 5' flanking region demonstrated two contiguous transcription start sites, CAAT and TATA boxes, and several potential regions of known DNA binding proteins. Overall, the SLPI gene appears to be a relatively nonpolymorphic, stable gene that is constitutively expressed at specific tissue sites, but has the potential to be modulated at both the transcriptional and posttranscriptional levels.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Takahashi H., Holmes M. D., Curiel D. T., Crystal R. G. Ribonuclease A cleavage combined with the polymerase chain reaction for detection of the Z mutation of the alpha-1-antitrypsin gene. Am J Respir Cell Mol Biol. 1989 Oct;1(4):329–334. doi: 10.1165/ajrcmb/1.4.329. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Appelhans B., Ender B., Sachse G., Nikiforov T., Appelhans H., Ebert W. Secretion of antileucoprotease from a human lung tumor cell line. FEBS Lett. 1987 Nov 16;224(1):14–18. doi: 10.1016/0014-5793(87)80413-1. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Calzone F. J., Britten R. J., Davidson E. H. Mapping of gene transcripts by nuclease protection assays and cDNA primer extension. Methods Enzymol. 1987;152:611–632. doi: 10.1016/0076-6879(87)52069-9. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crystal R. G. Alpha 1-antitrypsin deficiency, emphysema, and liver disease. Genetic basis and strategies for therapy. J Clin Invest. 1990 May;85(5):1343–1352. doi: 10.1172/JCI114578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal R. G., Brantly M. L., Hubbard R. C., Curiel D. T., States D. J., Holmes M. D. The alpha 1-antitrypsin gene and its mutations. Clinical consequences and strategies for therapy. Chest. 1989 Jan;95(1):196–208. doi: 10.1378/chest.95.1.196. [DOI] [PubMed] [Google Scholar]
- De Water R., Willems L. N., Van Muijen G. N., Franken C., Fransen J. A., Dijkman J. H., Kramps J. A. Ultrastructural localization of bronchial antileukoprotease in central and peripheral human airways by a gold-labeling technique using monoclonal antibodies. Am Rev Respir Dis. 1986 May;133(5):882–890. [PubMed] [Google Scholar]
- Deininger P. L., Jolly D. J., Rubin C. M., Friedmann T., Schmid C. W. Base sequence studies of 300 nucleotide renatured repeated human DNA clones. J Mol Biol. 1981 Sep 5;151(1):17–33. doi: 10.1016/0022-2836(81)90219-9. [DOI] [PubMed] [Google Scholar]
- Eisenberg S. P., Hale K. K., Heimdal P., Thompson R. C. Location of the protease-inhibitory region of secretory leukocyte protease inhibitor. J Biol Chem. 1990 May 15;265(14):7976–7981. [PubMed] [Google Scholar]
- Franken C., Meijer C. J., Dijkman J. H. Tissue distribution of antileukoprotease and lysozyme in humans. J Histochem Cytochem. 1989 Apr;37(4):493–498. doi: 10.1177/37.4.2926127. [DOI] [PubMed] [Google Scholar]
- Fryksmark U., Prellner T., Tegner H., Ohlsson K. Studies on the role of antileukoprotease in respiratory tract diseases. Eur J Respir Dis. 1984 Apr;65(3):201–209. [PubMed] [Google Scholar]
- Gauthier F., Fryksmark U., Ohlsson K., Bieth J. G. Kinetics of the inhibition of leukocyte elastase by the bronchial inhibitor. Biochim Biophys Acta. 1982 Jan 18;700(2):178–183. doi: 10.1016/0167-4838(82)90095-4. [DOI] [PubMed] [Google Scholar]
- Gidoni D., Dynan W. S., Tjian R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. 1984 Nov 29-Dec 5Nature. 312(5993):409–413. doi: 10.1038/312409a0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grütter M. G., Fendrich G., Huber R., Bode W. The 2.5 A X-ray crystal structure of the acid-stable proteinase inhibitor from human mucous secretions analysed in its complex with bovine alpha-chymotrypsin. EMBO J. 1988 Feb;7(2):345–351. doi: 10.1002/j.1460-2075.1988.tb02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck C. M., Rinehart F. P., Schmid C. W. A ubiquitous family of repeated DNA sequences in the human genome. J Mol Biol. 1979 Aug 15;132(3):289–306. doi: 10.1016/0022-2836(79)90261-4. [DOI] [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Ko H. S., Fast P., McBride W., Staudt L. M. A human protein specific for the immunoglobulin octamer DNA motif contains a functional homeobox domain. Cell. 1988 Oct 7;55(1):135–144. doi: 10.1016/0092-8674(88)90016-5. [DOI] [PubMed] [Google Scholar]
- Kramps J. A., Franken C., Dijkman J. H. ELISA for quantitative measurement of low-molecular-weight bronchial protease inhibitor in human sputum. Am Rev Respir Dis. 1984 Jun;129(6):959–963. doi: 10.1164/arrd.1984.129.6.959. [DOI] [PubMed] [Google Scholar]
- Kramps J. A., Franken C., Dijkman J. H. Quantity of anti-leucoprotease relative to alpha 1-proteinase inhibitor in peripheral airspaces of the human lung. Clin Sci (Lond) 1988 Oct;75(4):351–353. doi: 10.1042/cs0750351. [DOI] [PubMed] [Google Scholar]
- Lee W., Haslinger A., Karin M., Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987 Jan 22;325(6102):368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Levinson B., Janco R., Phillips J., 3rd, Gitschier J. A novel missense mutation in the factor VIII gene identified by analysis of amplified hemophilia DNA sequences. Nucleic Acids Res. 1987 Dec 10;15(23):9797–9805. doi: 10.1093/nar/15.23.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Mooren H. W., Kramps J. A., Franken C., Meijer C. J., Dijkman J. A. Localisation of a low-molecular-weight bronchial protease inhibitor in the peripheral human lung. Thorax. 1983 Mar;38(3):180–183. doi: 10.1136/thx.38.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornex J. F., Chytil-Weir A., Martinet Y., Courtney M., LeCocq J. P., Crystal R. G. Expression of the alpha-1-antitrypsin gene in mononuclear phagocytes of normal and alpha-1-antitrypsin-deficient individuals. J Clin Invest. 1986 Jun;77(6):1952–1961. doi: 10.1172/JCI112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Isolation and analysis of nuclear RNA. Methods Enzymol. 1987;152:234–241. doi: 10.1016/0076-6879(87)52025-0. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Daniels J. D., Auerbach H. S., De Schryver-Kecskemeti K., Winter H. S., Alpers D. H. The alpha 1-antitrypsin gene is expressed in a human intestinal epithelial cell line. J Biol Chem. 1989 Jun 5;264(16):9485–9490. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemüller U., Arnhold M., Fritz H., Wiedenmann K., Machleidt W., Heinzel R., Appelhans H., Gassen H. G., Lottspeich F. The acid-stable proteinase inhibitor of human mucous secretions (HUSI-I, antileukoprotease). Complete amino acid sequence as revealed by protein and cDNA sequencing and structural homology to whey proteins and Red Sea turtle proteinase inhibitor. FEBS Lett. 1986 Apr 7;199(1):43–48. doi: 10.1016/0014-5793(86)81220-0. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Stetler G., Brewer M. T., Thompson R. C. Isolation and sequence of a human gene encoding a potent inhibitor of leukocyte proteases. Nucleic Acids Res. 1986 Oct 24;14(20):7883–7896. doi: 10.1093/nar/14.20.7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Nukiwa T., Basset P., Crystal R. G. Myelomonocytic cell lineage expression of the neutrophil elastase gene. J Biol Chem. 1988 Feb 15;263(5):2543–2547. [PubMed] [Google Scholar]
- Thompson R. C., Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6692–6696. doi: 10.1073/pnas.83.18.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelmeier C., Hubbard R. C., Fells G. A., Schnebli H. P., Thompson R. C., Fritz H., Crystal R. G. Anti-neutrophil elastase defense of the normal human respiratory epithelial surface provided by the secretory leukoprotease inhibitor. J Clin Invest. 1991 Feb;87(2):482–488. doi: 10.1172/JCI115021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Wilson T., Treisman R. Removal of poly(A) and consequent degradation of c-fos mRNA facilitated by 3' AU-rich sequences. Nature. 1988 Nov 24;336(6197):396–399. doi: 10.1038/336396a0. [DOI] [PubMed] [Google Scholar]
- Yoshimura K., Nakamura H., Trapnell B. C., Dalemans W., Pavirani A., Lecocq J. P., Crystal R. G. The cystic fibrosis gene has a "housekeeping"-type promoter and is expressed at low levels in cells of epithelial origin. J Biol Chem. 1991 May 15;266(14):9140–9144. [PubMed] [Google Scholar]