Abstract
Aim
Few interventions exist to reduce alcohol and non-injection drug use among people living with HIV/AIDS. This study tested the effects of a coping group intervention for HIV-positive adults with childhood sexual abuse histories on alcohol, cocaine, and marijuana use.
Design
Participants were randomly assigned to the experimental coping group or a time-matched comparison support group. Both interventions were delivered in a group format over 15 weekly 90-minute sessions.
Methods
A diverse sample of 247 HIV-positive men and women with childhood sexual abuse were recruited in New York City. Substance use was assessed pre- and post-intervention and every 4 months during a 1-year follow-up period. Using an intent-to-treat analyses, longitudinal changes in substance use by condition were assessed using generalized estimating equations.
Results
At baseline, 41% of participants drank alcohol, 26% used cocaine, and 26% used marijuana. Relative to participants in the support group, those in the coping group had greater reductions in quantity of alcohol use (Wald χ2(4)= 10.77, p< .05) and any cocaine use (Wald χ2(4)= 9.81, p< .05).
Conclusion
Many HIV patients, particularly those with childhood sexual abuse histories, continue to abuse substances. This group intervention that addressed coping with HIV and sexual trauma was effective in reducing alcohol and cocaine use, with effects sustained at 12-month follow-up. Integrating mental health treatment into HIV prevention may improve outcomes.
Keywords: HIV/AIDS, substance use, coping, sexual abuse, randomized controlled trial
INTRODUCTION
In the United States, over 1 million people are living with HIV/AIDS (PLWHA) [1], and approximately 56,000 are newly infected each year [2]. Improved treatments and access to healthcare have dramatically increased survival and quality of life, and many PLWHA can now lead productive lives [3]. Secondary HIV prevention focusing on reducing HIV transmission risk behavior and optimizing health outcomes, including substance abuse, has become increasingly important [4].
Many PLWHA continue to abuse drugs and alcohol. In the United States, the most frequently used substances are alcohol, cocaine, and marijuana. Across several large, nationally representative samples of PLWHA, 40–45% drank alcohol, 14–24% reported hazardous alcohol use, and approximately 50% used illicit drugs [5–8]. Substance abuse, particularly alcohol and cocaine, is associated with poor HIV clinical outcomes. For example, in a cohort of nearly 3,000 PLWHA, weekly alcohol consumption decreased survival by >3 years for hazardous drinking and >1 year for non-hazardous drinking [9]. In other cohorts, hazardous drinking was associated with decreased viral suppression [6, 10]. Similarly, crack-cocaine was associated with CD4 cell count decline, higher viral loads, faster disease progression, more AIDS-defining illnesses, and death [11–14].
While many risk reduction interventions have targeted injection drug users [15], few have been developed to reduce non-injection drug and alcohol use among PLWHA. In Positive Choices, participants who received a single session of an interactive computer program providing tailored risk reduction had significantly greater reductions in illicit drug use compared to those in a no-treatment control, but there was no change in hazardous drinking in either condition [16]. In the Healthy Living Project, a 15-session case management intervention, participants had significantly greater reductions in alcohol/marijuana, any substance, and illicit drug use compared to no-treatment controls [17]. In the only intervention to date to target hazardous drinkers, an 8-session motivational interviewing and cognitive-behavioral skills-building intervention was compared to a time-matched and content-equivalent educational condition [18]. Participants in both conditions demonstrated significant reductions in number of drinks at follow-up, but there was no difference by condition. These studies provide evidence that behavioral interventions may be effective in reducing substance abuse among PLWHA, though improvements are needed. Furthermore, it is unclear if results generalize to the many PLWHA who present with significant psychological difficulties [19–22]. HIV prevention may need to integrate mental health treatment in order to have sustained effects on risk reduction, including substance use [23, 24].
PLWHA who have childhood sexual abuse (CSA) histories are at particularly high risk for continued substance abuse. CSA is consistently found to be a risk factor for substance abuse in community samples [25–28], and individuals who have experienced CSA are less responsive to HIV risk reduction interventions [29–32]. CSA is common among PLWHA, with rates ranging from 33% to 53% [33–42], and those with CSA are more likely to abuse substances [33, 43]. CSA disrupts normal child development and interferes with the acquisition of self-capacities (e.g., distress tolerance, self-esteem) [44]. Thus, drug and alcohol use may represent maladaptive attempts to counter negative assumptions and self-evaluations, regulate affect, or cope with the psychological sequelae of sexual trauma [45–47]. Of the few interventions that exist to reduce substance abuse among adults with CSA [48, 49], none has addressed CSA and substance use in the context of HIV infection.
Sikkema and colleagues developed "Living in the Face of Trauma" (LIFT), a group coping intervention, to address the unique needs of HIV-positive men and women with CSA histories. This intervention focuses on identifying current stressors associated with HIV and CSA and developing adaptive coping strategies. As reported previously, a randomized controlled trial found that participants in the experimental coping intervention had greater reductions in traumatic stress [50] and sexual risk behavior [51] than those in a therapeutic support group intervention. In the current analysis, we report on the effects of the intervention on substance use. We hypothesized that the coping intervention would lead to greater reductions in substance use, particularly alcohol and cocaine, compared to the support intervention.
METHODS
Participants
Participants were recruited from AIDS service organizations and community health centers in New York City between March 2002 and January 2004. Brochures were distributed at these locations, and providers informed clients of the study. As described in detail elsewhere [51], inclusion criteria were ≥18 years old, HIV-positive serostatus, and sexual abuse as a child or adolescent. Participants who reported acute distress due to sexual revictimization in the past month, impaired mental status, and/or extreme distress that would interfere with group treatment (e.g., suicidal intention, acute psychosis) were excluded and referred to services as appropriate.
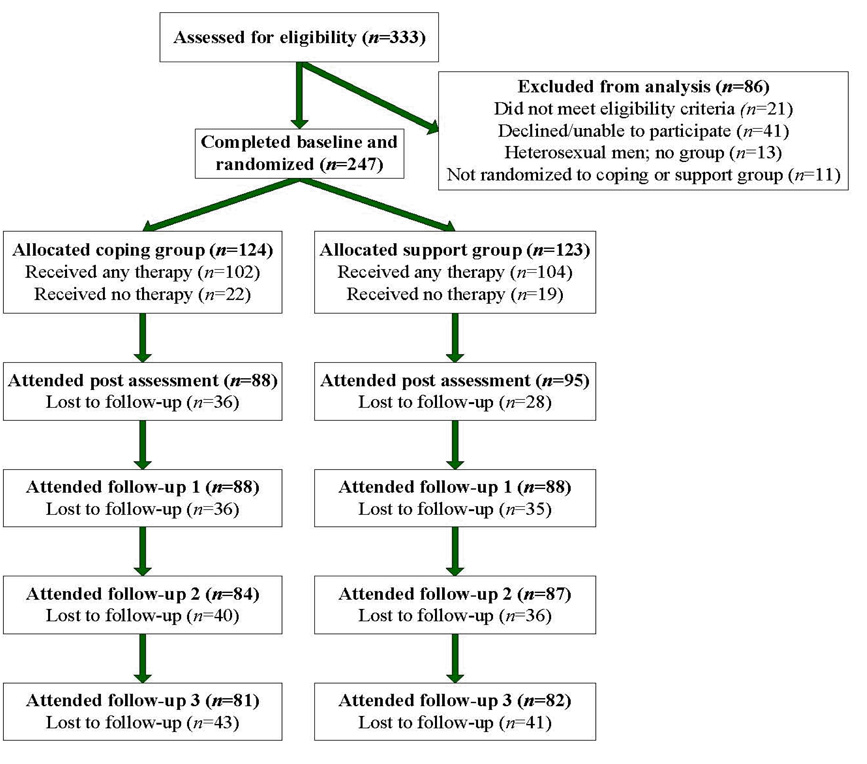
Figure 1 outlines the flow of participants throughout the trial. Adhering to the intent-to-treat principle [52], all participants were followed regardless of intervention exposure. Excluding participants due to noncompliance can severely bias the interpretation of results [53]. Of 247 participants, 76% completed ≥1 follow-up, with no difference by condition.
Figure 1.
Flow of participants through the trial
Procedures
This study was approved by an institutional review board, and all participants provided written informed consent. Assessments occurred at baseline, within 2 weeks post intervention, and every 4 months over a 12-month follow-up period. At each assessment, participants completed a battery of psychosocial and behavioral measures using computer-assisted structured interview technology. They were compensated an average of $40 per assessment.
Randomization
After completing the baseline assessment, participants were randomly assigned either to the experimental coping condition (LIFT) or a time-matched comparison support condition. Randomization occurred in “waves”, with approximately 10 participants allocated to each condition within each wave. Randomization and intervention were conducted separately by gender to account for potential differences experienced in coping and trauma issues. However, the intervention protocol and content remained uniform across gender.
Intervention conditions
The LIFT coping intervention was based on a model that integrates cognitive theory of stress and coping [54, 55] and effective cognitive-behavioral treatment strategies for sexual trauma [32, 48] within a transactional framework for understanding sexual abuse outcomes [56]. Within a group environment that encouraged reciprocal support, feedback, and sharing of experiences, the intervention taught coping skills-building, focusing on appraisal of HIV- and trauma-related stressors and development and application of adaptive coping strategies. It addressed the association between stress experienced and strategies utilized to cope with the interconnected traumas of HIV and CSA. Substance use, in combination with other health behaviors, was addressed explicitly in two intervention sessions and could be identified by participants throughout the intervention as a maladaptive strategy for which alternative coping methods could be developed. The comparison intervention was a support group that provided a therapeutic environment for participants to process any issues related to HIV and CSA, including current life events and relationships.
Both interventions were delivered by co-therapists in a community health center that was centrally located in New York City and easily accessible by public transportation. The therapists were experienced mental health providers with masters or doctoral degrees in social work or psychology. The interventions included 15 weekly 90-minute sessions. The mean number of sessions attended was 8.6 (SD= 5.2, range= 0–15), with no difference by condition [t(245)= 0.43, p= .66]; 83% attended ≥1 session and 49% attended ≥11 sessions.
Measures
The primary outcomes were the three most commonly used substances: cocaine (powder or crack), marijuana, and alcohol. At each assessment, participants reported the frequency of use of each substance in the past month using the following scale: 0= none, 1= 1–2 days, 2= 3–5 days, 3= 6–10 days, 4= 11–20 days, 5= 21–28 days, and 6= everyday. For cocaine and marijuana, a dichotomous variable of any use was created. For alcohol, participants also reported how many drinks they typically consumed on drinking days using the following scale: 0= none, 1= 1 or less, 2= 2–3, 3= 4–5, and 4= 6 or more. An estimate of the number of drinks consumed per month was computed by multiplying frequency of drinking by quantity of drinks (using the lower bound of each range for a conservative estimate). Hazardous drinking was defined per NIAAA guidelines [57]: for women, >3 drinks/occasion or >28 drinks/month; for men, >4 drinks/occasion or >56 drinks/month. Moderate drinking was defined as any alcohol use that did not meet criteria for hazardous drinking. A trichotomous variable describing level of drinking (none, moderate, hazardous) was created.
Data analysis
Longitudinal changes in substance use were examined over the 16-month period from baseline through 12-month follow-up (5 assessment points at 4-month intervals) using generalized estimating equations (GEE). GEE uses all data collected for each participant at each time point. To control for the correlation among repeated assessments on individual participants over time, all models assumed a first-order autoregressive correlation structure. In each model, the effects of time, intervention condition, and time by intervention condition interaction on substance use were examined. For level of alcohol use (none/moderate/hazardous), an ordinal logistic regression model utilizing a multinomial distribution was fit to the repeated trichotomous outcome. For quantity of alcohol use (number of drinks per month), linear regression models utilizing a negative binomial distribution were fit to the repeated continuous outcome. For any cocaine and marijuana use (no/yes), logistic regression models utilizing a binomial distribution were fit to the repeated binary outcomes. For frequency of cocaine and marijuana use (measured on a 0–6 scale), linear regression models utilizing a negative binomial distribution were fit to the repeated continuous outcomes. Analyses included all participants regardless of baseline substance use due to possible fluctuations in substance use over time (i.e., participants who were abstinent at baseline could have used during the course of the study). Overall, women reported less substance use than men, but there were no gender differences in patterns of change. Therefore, only unadjusted results are reported. Analyses were conducted in SPSS 17.0
RESULTS
Sample characteristics
The sample comprised of 130 women and 117 men. All men were gay/had sex with men, and 24% of women were lesbian/bisexual. Participants were primarily low-income (92% earned <$20,000 annually) and ethnically diverse (68% African-American, 17% Hispanic, 10% Caucasian). They had a mean age of 42.3 years (SD= 6.8) and a mean education of 12.2 years (SD= 2.4). The mean time since HIV diagnosis was 10.0 years (SD= 5.8). The mean CD4 cell count at baseline was 454.6 cells/mm3 (SD= 308.7), and 69% were on antiretroviral medications.
The sexual abuse histories of participants were profound, typically occurring repetitively with multiple perpetrators. Penetrative vaginal or anal sex was experienced by 67% during childhood (≤12 years) and 66% during adolescence (13–17 years); 90% experience it during childhood or adolescence. The majority (87%) were sexually revictimized, with 53% reporting unwanted penetrative vaginal/anal sex as adults. At baseline, 40% screened positive for PTSD. There were no differences by intervention condition on these sample characteristics.
Baseline substance abuse
Based on the Personality Assessment Inventory [58], which has been found to reliably and validly identify substance use disorders [59–61], 48% of participants had a substance use disorder. Specifically, 21% had an alcohol use disorder (10% abuse, 11% dependence) and 41% a drug use disorder (30% abuse, 11% dependence). At baseline, 29% of participants were moderate drinkers, 13% were hazardous drinkers, 26% had used cocaine, 26% had used marijuana, and 18% had used other illicit drugs in the past month. Among participants who reported any alcohol use at baseline, the mean number of drinks in the past month was 27.9 (SD= 40.2). Among baseline cocaine and marijuana users, the mean frequency of use was 2.4 (SD= 1.4) for cocaine and 2.5 (SD= 1.8) for marijuana; this translates to approximately 6 days/month. The only group difference was that more participants in the support group had used marijuana (χ2(1)= 6.2, p= 0.009). In the 4 months prior to baseline, 32% reported receiving substance abuse treatment. Thus, at baseline, 74% of the sample had indications of substance abuse problems based on substance use disorder diagnoses, substance abuse treatment, hazardous alcohol use, and/or illicit drug use.
Changes in substance use over time
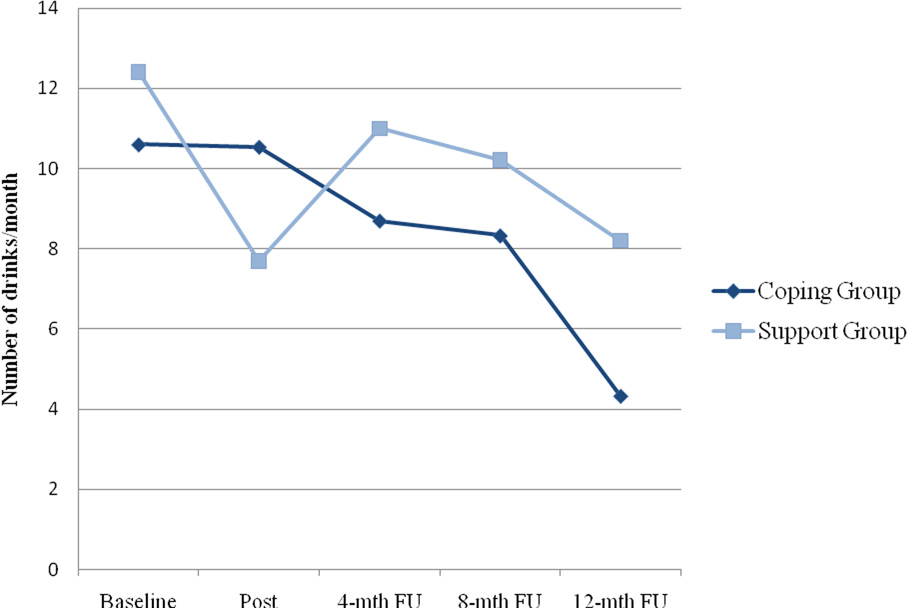
Table 1 presents the results of the GEE models predicting change in substance use over time. For alcohol use, there was a significant time effect for both level (Wald χ2(1)= 13.83, p= .008) and quantity (Wald χ2(1)= 10.67, p= .030), indicating that participants in both conditions reduced their alcohol consumption. There was also a significant time by condition interaction for quantity of alcohol use (Wald χ2(4)= 10.77, p= .029), indicating that participants in the coping intervention had greater reductions than those in the support group. As illustrated in Figure 2, from baseline to the 12-month follow-up, the mean reduction in number of drinks was 4.9 (SD= 2.1) in the coping group compared to 1.6 (SD= 2.9) in the support group.
Table 1.
Results of GEE analyses predicting change in past-month substance use over time by intervention condition (n=247)
| Categorical outcomes | Wald chi-square (df) | p-value | Continuous outcomes | Wald chi-square (df) | p-value |
|---|---|---|---|---|---|
| Level of alcohol use1 | Quantity of alcohol use2 | ||||
| condition | 0.483 (1) | .487 | condition | 0.325 (1) | .568 |
| time | 13.833 (4) | .008 | time | 10.678 (4) | .030 |
| condition × time | 8.769 (4) | .067 | condition × time | 10.774 (4) | .029 |
| Any cocaine use | Frequency of cocaine use3 | ||||
| condition | 1.491 (1) | .220 | condition | 1.231 (1) | .267 |
| time | 17.773 (4) | .001 | time | 14.324 (4) | .006 |
| condition × time | 9.813 (4) | .044 | condition × time | 8.516 (4) | .006 |
| Any marijuana use | Frequency of marijuana use3 | ||||
| condition | 2.951 (1) | .086 | condition | 0.200 (1) | .655 |
| time | 3.953 (4) | .412 | time | 8.617 (4) | .071 |
| condition × time | 2.507 (4) | .643 | condition × time | 1.233 (4) | .873 |
Categorized as none vs. moderate vs. hazardous
Measured as number of drinks per month
Measured on a 0–6 scale
Figure 2.
Quantity of alcohol use by intervention condition over time
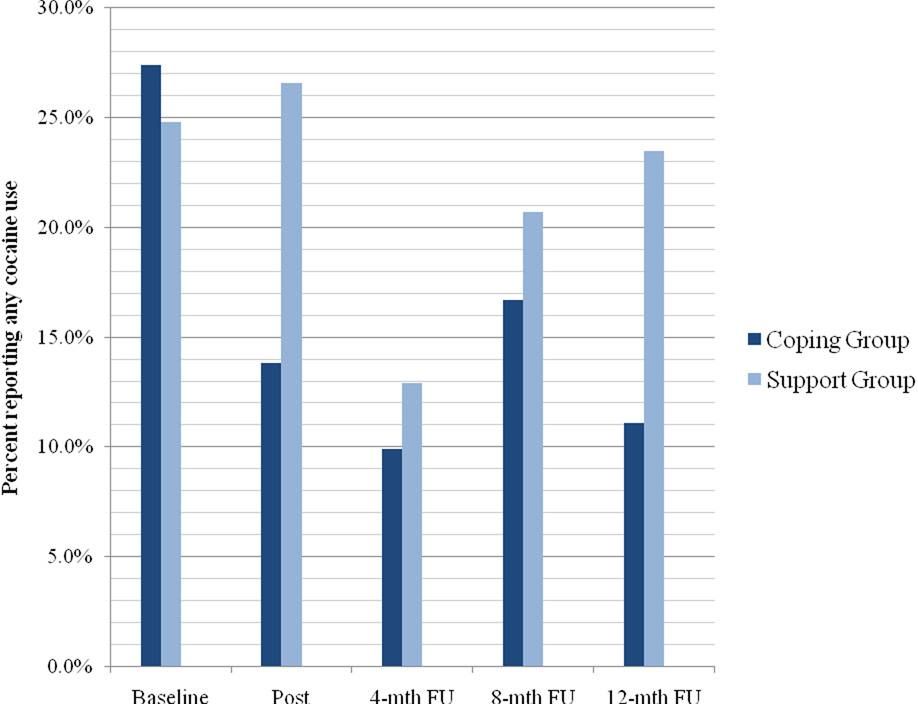
For cocaine use, there was a significant time effect for both any use (Wald χ2(1)= 17.77, p= .001) and frequency of use (Wald χ2(1)= 14.32, p= .006), indicating that participants in both conditions reduced their cocaine use. There was also a significant time by condition interaction for any use (Wald χ2(4)= 9.813, p= 0.044), indicating that participants in the coping group were less likely than those in the support group to use any cocaine over time. As illustrated in Figure 3, the proportion of participants in the coping group who used any cocaine decreased markedly from 27% at baseline to 14% at post intervention and 11% at 12-month follow-up. In contrast, there was little change in the proportion of participants in the support group who used any cocaine over time (25% at baseline, 27% at post intervention, and 24% at 12-month follow-up). In other words, immediately following the intervention, the coping group had a 58% reduction in the odds of any cocaine use compared to a 9% increase in the support group. At 12-month follow-up, the coping group had a 60% reduction in the odds of any cocaine use compared to a 10% reduction in the support group. For frequency of cocaine use, there was no significant condition by time interaction (Wald χ2(1)= 8.52, p= .07). From baseline to 12-month follow-up, the mean reduction in frequency of cocaine use was 0.30 (SD= 0.13) in the coping group compared to 0.05 (SD= 0.16) in the support group.
Figure 3.
Percent of participants reporting any cocaine use by intervention condition over time
For marijuana use, there was no change in any use or frequency of use as a function of condition, time, and condition by time. This suggests that, regardless of intervention condition, there was no change in marijuana use over the course of the study.
DISCUSSION
Despite the high prevalence of alcohol and cocaine use among PLWHA and its deleterious effect on HIV clinical outcomes, there are few risk reduction interventions for PLWHA who abuse alcohol and non-injection drugs. The results of this randomized controlled trial demonstrate that LIFT, a theoretically-grounded coping group intervention designed specifically for men and women with HIV and CSA, is effective in reducing alcohol and cocaine use. As expected, participants in both the experimental coping and comparison support groups demonstrated reductions in alcohol and cocaine use over time. However, relative to the support group, participants in LIFT had significantly greater reductions in quantity of alcohol consumed and greater likelihood of abstaining from cocaine use over time. Reductions in alcohol and cocaine use were evident immediately post-intervention and sustained at 12-month follow-up for the LIFT condition but not the support condition.
The LIFT intervention used a theoretically-grounded, integrated model to innovatively address stress and coping among PLWHA with complex and repetitive trauma histories. Participants learned how to identify specific stressors related to HIV and CSA and develop problem-focused strategies (e.g., effective communication, problem solving) and emotion-focused strategies (e.g., cognitive restructuring) to cope with changeable and unchangeable stressors, respectively. While the majority of the coping intervention was not specific to substance use, it was frequently identified by participants as a maladaptive coping strategy in response to stress. LIFT participants identified strategies to reduce triggers for substance use, such as regulating negative affect, staying away from certain neighborhoods, breaking off ties with former drug-using peers, and attending 12-step and other self-help programs. The group also focused on the development of skills to more effectively cope with stressors related to living with HIV and CSA within the context of a safe and supportive therapeutic environment. Thus, substance abuse was addressed both directly and indirectly throughout the treatment.
Research consistently demonstrates that CSA is associated with negative physical and mental health outcomes [35, 40, 62–65]. Other common sequelae of CSA, such as helplessness, avoidance, and low self-esteem, likely contribute to behavioral risk [44] and may interfere with the effectiveness of risk reduction interventions [29, 30, 44, 66]. Unfortunately, despite the elevated rates of sexual trauma among PLWHA, few empirically-supported interventions are tailored to these issues [67, 68], and none have been found to reduce substance abuse. A common concern among clinicians is that addressing trauma may lead to psychological decompensation and increased substance abuse [48, 69]. The results of the present trial suggest that trauma can be safely and effectively addressed in a group context with improvements across multiple outcomes, including traumatic stress [50], sexual risk [51], and substance use. Based on these findings, it is imperative that future research examine potential mediators of change, such as development of adaptive coping strategies.
It is important to note that LIFT was not designed as a treatment for substance abuse, nor did it target treatment-seeking substance abusers. However, consistent with the greater population of HIV patients, many participants had a history of substance abuse, and a substantial proportion reported current alcohol and illicit drug use. While only two sessions directly addressed substance abuse, the skills taught throughout the intervention were relevant to substance use. Given the positive findings, this intervention should be further tested with PLWHA seeking substance abuse treatment.
Despite the marked reductions in alcohol and cocaine use, there was no change in marijuana use over time in either group. At baseline and throughout the study, approximately one quarter of participants used marijuana. There are a number of possible explanations for this lack of change. While some PLWHA use marijuana primarily to get high, many others use it therapeutically. The purported medicinal benefits of marijuana use are well publicized, and research has established that many PLWHA use marijuana to cope with neuropathy, muscle pain, fatigue, diarrhea, anxiety, and other physical symptoms [70, 71]. In the current trial, we could not distinguish reasons for marijuana use. Furthermore, relative to alcohol, cocaine, and other “hard drugs,” marijuana may not have been conceptualized as a maladaptive behavior by participants. Thus, participants may not have been motivated to reduce their marijuana use. Alternatively, marijuana use may be difficult to treat in HIV patients. Prior secondary HIV preventions trials have not reported on the effects of the intervention on marijuana use at all [16, 18] or independent of other substances [17]. Despite its widespread use as a self-care strategy, marijuana may have deleterious effects on health outcomes, particularly among patients with advanced HIV disease [72]. Further research is needed to identify effective strategies for reducing marijuana use among PLWHA.
This study had a number of noteworthy strengths. First, it utilized a randomized controlled design with an attention-matched, active comparison intervention and had a 12-month follow-up period following the intervention to assess for sustained treatment effects. Second, substance use was measured at each time point, enabling the modeling of change in substance use over time using a rigorous statistical analysis method. Third, we analyzed the effect of LIFT on multiple measures of substance use, thereby providing a robust and comprehensive picture of the patterns of substance use among participants over time.
The study also had several limitations. First, it was open to all PLWHA with CSA histories, regardless of current substance use. As a result, only 13% were hazardous drinkers and 26% cocaine users at baseline, limiting our power to detect treatment effects. Future studies will need to test whether this intervention is effective among PLWHA seeking treatment for drug and alcohol dependence . Second, recruitment efforts failed to enroll a sufficient number of heterosexual men; therefore, results are generalizable only to women and men who have sex with men. Third, results are based on self-reported substance use, which may be underreported. In the context of a trial that used an attention-matched control condition, this source of bias is less troubling because it tends to bias results towards the null, and is unlikely to vary by condition. Future studies might use urine toxicology tests to corroborate self-reports of drug use. Fourth, the increased attrition at the 12-month visit was suboptimal, but follow-up at all other visits was good. Finally, this was a convenience sample of volunteers living in New York City. Results may not generalize to individuals living in other parts of the world or to those who are unwilling to participate in clinical trials.
In conclusion, results of this randomized controlled trial suggest that LIFT, a theory-based group coping intervention, is effective in promoting sustained reductions in risky drinking and cocaine use among PLWHA who have with histories of CSA. Despite the urgent need for secondary HIV prevention interventions [24], there is a dearth of empirically-supported treatments for non-injecting substance abusers. By teaching patients how to identify and implement effective coping strategies to manage stressors related to living with HIV and CSA, LIFT had beneficial effects on multiple outcomes, notably traumatic stress, sexual risk, and substance abuse. This approach could be incorporated into community-based mental health services to improve clinical outcomes and quality of life among HIV patients.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge their community collaboration with the Callen-Lorde Community Health Center in New York City.
This research was supported by grants from the National Institutes of Health R01-MH62965 (Sikkema) and P30-AI064519 (Weinhold). The LIFT intervention manual is available upon request from Dr. Kathleen J. Sikkema.
REFERENCES
- 1.UNAIDS. Report on the Global HIV/AIDS Epidemic Geneva: World Health Organization. 2008
- 2.Hall HI, Song R, Rhodes P, Prejean J, An Q, Lee LM, et al. Estimation of HIV incidence in the United States. JAMA. 2008 Aug 6;300(5):520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima VD, Harrigan R, Bangsberg DR, Hogg RS, Gross R, Yip B, et al. The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. Journal of Acquired Immune Deficiency Syndrome. 2009 Apr;50(5):529–536. doi: 10.1097/QAI.0b013e31819675e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon CM, Stall R, Cheever LW. Prevention interventions with persons living with HIV/AIDS: challenges, progress, and research priorities. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2004 October 1;37 supp2:S53–S57. doi: 10.1097/01.qai.0000142321.27136.8b. [DOI] [PubMed] [Google Scholar]
- 5.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Archives of General Psychiatry. 2001;58(8):721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 6.Chander G, Lau B, Moore RD. Hazardous alcohol use: A risk factor for non-adherence and lack of suppression in HIV infection. Journal of Acquired Immune Deficiency Syndrome. 2006;43(4):411–417. doi: 10.1097/01.qai.0000243121.44659.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook RL, Zhu F, Belnap BH, Weber K, Cook JA, Vlahov D, et al. Longitudinal trends in hazardous alcohol consumption among women with human immunodeficiency virus infection, 1995–2006. American Journal of Epidemiology. 2009 Apr 15;169(8):1025–1032. doi: 10.1093/aje/kwp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chander G, Josephs J, Fleishman JA, Korthuis PT, Gaist P, Hellinger J, et al. Alcohol use among HIV-infected persons in care: results of a multi-site survey. HIV Medicine. 2008 Apr;9(4):196–202. doi: 10.1111/j.1468-1293.2008.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braithwaite RS, Conigliaro J, Roberts MS, Shechter S, Schaefer A, McGinnis K, et al. Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care. 2007 Apr;19(4):459–466. doi: 10.1080/09540120601095734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conigliaro J, Gordon AJ, McGinnis KA, Rabeneck L, Justice AC. Study VAC-S. How harmful is hazardous alcohol use and abuse in HIV infection: do health care providers know who is at risk? Journal of Acquired Immune Deficiency Syndrome. 2003;33(4):521–525. doi: 10.1097/00126334-200308010-00014. [DOI] [PubMed] [Google Scholar]
- 11.Webber MP, Schoenbaum EE, Gourevitch MN, Buono D, Klein RS. A prospective study of HIV disease progression in female and male drug users. AIDS. 1999 Feb 4;13(2):257–262. doi: 10.1097/00002030-199902040-00014. [DOI] [PubMed] [Google Scholar]
- 12.Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. Journal of Acquired Immune Deficiency Syndrome. 2009 Jan 1;50(1):93–99. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- 13.Cook JA, Burke-Miller JK, Cohen MH, Cook RL, Vlahov D, Wilson TE, et al. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS. 2008 Jul 11;22(11):1355–1363. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vittinghoff E, Hessol NA, Bacchetti P, Fusaro RE, Holmberg SD, Buchbinder SP. Cofactors for HIV disease progression in a cohort of homosexual and bisexual men. Journal of Acquired Immune Deficiency Syndrome. 2001 Jul 1;27(3):308–314. doi: 10.1097/00126334-200107010-00015. [DOI] [PubMed] [Google Scholar]
- 15.Des Jarlais DC, Semaan S. HIV prevention for injecting drug users: the first 25 years and counting. Psychosomatic Medicine. 2008;70(5):606–611. doi: 10.1097/PSY.0b013e3181772157. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert P, Ciccarone D, Gansky SA, Bangsberg DR, Clanon K, McPhee SJ, et al. Interactive "Video Doctor" counseling reduces drug and sexual risk behaviors among HIV-positive patients in diverse outpatient settings. PLoS One. 2008;3(4):e1988. doi: 10.1371/journal.pone.0001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong FL, Rotheram-Borus MJ, Lightfoot M, Pequegnat W, Comulada WS, Cumberland W, et al. Effects of behavioral intervention on substance use among people living with HIV: the Healthy Living Project randomized controlled study. Addiction. 2008 Jul;103(7):1206–1214. doi: 10.1111/j.1360-0443.2008.02222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: a randomized controlled trial. Journal of Acquired Immune Deficiency Syndrome. 2007 Dec 1;46(4):443–450. doi: 10.1097/qai.0b013e318158a461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyketsos CG, Hutton H, Fishman M, Schwartz J, Treisman GJ. Psychiatric morbidity on entry to an HIV primary care clinic. AIDS. 1996 Aug;10(9):1033–1039. doi: 10.1097/00002030-199610090-00015. [DOI] [PubMed] [Google Scholar]
- 20.Olley BO, Seedat S, Stein DJ. Persistence of psychiatric disorders in a cohort of HIV/AIDS patients in South Africa: a 6-month follow-up study. Journal of Psychosomatic Research. 2006 Oct;61(4):479–484. doi: 10.1016/j.jpsychores.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Pence BW, Miller WC, Gaynes BN, Eron JJ., Jr Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. Journal of Acquired Immune Deficiency Syndrome. 2007 Feb 1;44(2):159–166. doi: 10.1097/QAI.0b013e31802c2f51. [DOI] [PubMed] [Google Scholar]
- 22.Israelski DM, Prentiss DE, Lubega S, Balmas G, Garcia P, Muhammad M, et al. Psychiatric co-morbidity in vulnerable populations receiving primary care for HIV/AIDS. AIDS Care. 2007 Feb;19(2):220–225. doi: 10.1080/09540120600774230. [DOI] [PubMed] [Google Scholar]
- 23.Grossman CI, Gordon CM. Mental health considerations in secondary HIV prevention. AIDS Behav. 2010 Apr;14(2):263–271. doi: 10.1007/s10461-008-9496-8. [DOI] [PubMed] [Google Scholar]
- 24.Sikkema KJ, Watt MH, Drabkin AS, Meade CS, Hansen NB, Pence BW. Mental health treatment to reduce HIV transmission risk behavior: a positive prevention model. AIDS Behav. 2010 Apr;14(2):252–262. doi: 10.1007/s10461-009-9650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson TL, Miller WR. Concomitance between childhood sexual and physical abuse and substance use problems: A review. Clinical Psychology Review. 2002;22(1):27–77. doi: 10.1016/s0272-7358(00)00088-x. [DOI] [PubMed] [Google Scholar]
- 26.Spak L, Spak F, Allebeck P. Sexual abuse and alcoholism in a female population. Addiction. 1998 Sep;93(9):1365–1373. doi: 10.1046/j.1360-0443.1998.93913657.x. [DOI] [PubMed] [Google Scholar]
- 27.Wilsnack SC, Vogeltanz ND, Klassen AD, Harris TR. Childhood sexual abuse and women's substance abuse: National survey findings. Journal of Studies on Alcohol. 1997;58(3):264–271. doi: 10.15288/jsa.1997.58.264. [DOI] [PubMed] [Google Scholar]
- 28.MacMillan HL, Fleming JE, Streiner DL, Lin E, Boyle MH, Jamieson E, et al. Childhood abuse and lifetime psychopathology in a community sample. American Journal of Psychiatry. 2001;158(11):1878–1883. doi: 10.1176/appi.ajp.158.11.1878. [DOI] [PubMed] [Google Scholar]
- 29.Mimiaga MJ, Noonan E, Donnell D, Safren SA, Koenen KC, Gortmaker S, et al. Childhood sexual abuse is highly associated with HIV risk-taking behavior and infection among MSM in the EXPLORE Study. Journal of Acquired Immune Deficiency Syndrome. 2009 Jul 1;51(3):340–348. doi: 10.1097/QAI.0b013e3181a24b38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalichman SC, Carey MP, Johnson BT. Prevention of sexually transmitted HIV infection: a meta-analytic review of behavioral outcomes. Annuals of Behavioral Medicine. 1996;18(1):6–15. doi: 10.1007/BF02903934. [DOI] [PubMed] [Google Scholar]
- 31.Belcher L, Kalichman S, Topping M, Smith S, Emshoff J, Norris F, et al. A randomized trial of a brief HIV risk reduction counseling intervention for women. Journal of Consulting and Clinical Psychology. 1998 Oct;66(5):856–861. doi: 10.1037//0022-006x.66.5.856. [DOI] [PubMed] [Google Scholar]
- 32.Briere J. Treating adult survivors of severe childhood abuse and neglect: Further development of an integrative model. In: Myers JEB, Berliner L, Briere J, Hendrix CT, Jenny C, Reid T, editors. The APSAC Handbook on Child Maltreatment. 2nd Edition. Newbury Park, CA: Sage Publications; 2002. pp. 175–203. [Google Scholar]
- 33.Kalichman SC, Sikkema KJ, DiFonzo K, Luke W, Austin J. Emotional adjustment in survivors of sexual assault living with HIV/AIDS. Journal of Traumatic Stress. 2002;15(4):189–296. doi: 10.1023/A:1016247727498. [DOI] [PubMed] [Google Scholar]
- 34.Bedimo AL, Kissinger P, Bessinger R. History of sexual abuse among HIV-infected women. International Journal of STD & AIDS. 1997;8(5):332–335. doi: 10.1258/0956462971920046. [DOI] [PubMed] [Google Scholar]
- 35.Liebschutz J, Savetsky JB, Saitz R, Horton NJ, Lloyd-Travaglini C, Samet JH. The relationship between sexual and physical abuse and substance abuse consequences. Journal of Substance Abuse Treatment. 2002;22:121–128. doi: 10.1016/s0740-5472(02)00220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allers CT, Benjack KJ. Connection between childhood abuse and HIV infection. Journal of Counseling and Development. 1991;70:309–313. [Google Scholar]
- 37.Koenig LJ, Clark H. Sexual abuse of girls and HIV infection among women: Are they related? In: Koenig LJ, Doll LS, O'Leary A, Pequegnat W, editors. From Child Sexual Abuse to Adult Sexual Risk: Trauma, Revictimization, and Intervention. Washington, DC: American Psychological Association; 2004. pp. 69–92. [Google Scholar]
- 38.Cohen M, Deamant C, Barkan S, Richardson J, Young M, Holman S, et al. Domestic violence and childhood sexual abuse in HIV-infected women and women at risk for HIV. American Journal of Public Health. 2000;90(4):560–565. doi: 10.2105/ajph.90.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wyatt GE, Myers HF, Williams JK, Kitchen CR, Loeb T, Carmone JV, et al. Does a history of trauma contribute to HIV risk for women of color? Implications for prevention and policy. American Journal of Public Health. 2002 April;92(4):660–665. doi: 10.2105/ajph.92.4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liebschutz JM, Feinman G, Sullivan L, Stein M, Samet J. Physical and sexual abuse in women infected with the human immunodeficiency virus: Increased illness and health care utilization. Archives of Internal Medicine. 2000 June 12;160(11):1659–1664. doi: 10.1001/archinte.160.11.1659. [DOI] [PubMed] [Google Scholar]
- 41.O'Leary A, Purcell D, Remien RH, Gomez C. Childhood sexual abuse and sexual transmission risk behavior among HIV-positive men who have sex with men. AIDS Care. 2003;15(1):17–26. doi: 10.1080/0954012021000039725. [DOI] [PubMed] [Google Scholar]
- 42.Mugavero M, Ostermann J, Whetten K, Leserman J, Swartz M, Stangl D, et al. Barriers to antiretroviral adherence: the importance of depression, abuse, and other traumatic events. AIDS Patient Care and STDs. 2006;20(6):418–428. doi: 10.1089/apc.2006.20.418. [DOI] [PubMed] [Google Scholar]
- 43.Holmes WC. Association between a history of childhood sexual abuse and subsequent, adolescent psychoactive substance use disorder in a sample of HIV seropositive men. Journal of Adolescent Health. 1997 Jun;20(6):414–419. doi: 10.1016/S1054-139X(96)00278-9. [DOI] [PubMed] [Google Scholar]
- 44.Briere J. Integrating HIV/AIDS prevention activities into psychotherapy for sexual abuse survivors. In: Koenig LJ, Doll LS, O'Leary A, Pequegnat W, editors. From Child Sexual Abuse to Adult Sexual Risk: Trauma, Revictimization, and Intervention. Washington,Dc: American Psychological Association; 2004. [Google Scholar]
- 45.Molnar BE, Buka SL, Kessler RC. Child sexual abuse and subsequent psychopathology: Results from the National Comorbidity Survey. American Journal of Public Health. 2001;91(5):753–760. doi: 10.2105/ajph.91.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Study. Archives of General Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 47.Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: Results from the adverse childhood experiences study. American Journal of Psychiatry. 2003;160(8):1453–1460. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- 48.Najavits L. Seeking Safety: A Treatment Manual for PTSD and Substance Abuse. New York: Guilford Press; 2002. [Google Scholar]
- 49.Hein DA, Campbell ANC, Killeen T, Hu M, Hansen C, Jiang H, et al. The impact of trauma-focused group therapy upon sexual risk behaviors in the NIDA Clinical Trials Network “Women and Trauma” multi-site study. AIDS Behav. doi: 10.1007/s10461-009-9573-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sikkema KJ, Hansen NB, Kochman A, Tarakeshwar N, Neufeld S, Meade CS, et al. Outcomes from a group intervention for coping with HIV/AIDS and childhood sexual abuse: Reductions in traumatic stress. AIDS Behav. 2007;11(1):49–90. doi: 10.1007/s10461-006-9149-8. [DOI] [PubMed] [Google Scholar]
- 51.Sikkema KJ, Wilson PD, Hansen NB, Kochman A, Neufeld S, Ghebremichael MS, et al. Effects of a coping intervention on transmission risk behavior among people living with HIV and a history of childhood sexual abuse. Journal of Acquired Immune Deficiency Syndrome. 2008;47(4):506–513. doi: 10.1097/QAI.0b013e318160d727. [DOI] [PubMed] [Google Scholar]
- 52.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976 Dec;34(6):585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeMets DL. Statistical issues in interpreting clinical trials. J Intern Med. 2004 May;255(5):529–537. doi: 10.1111/j.1365-2796.2004.01320.x. [DOI] [PubMed] [Google Scholar]
- 54.Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York: Springer Publishing Company; 1984. [Google Scholar]
- 55.Folkman S, Chesney M, Mckusick L, Ironson G, Johnson DS, Coates TJ. Translating Coping Theory into Intervention. In: Eckenrode J, editor. The Social Context of Coping. New York: Plenum Press; 1991. pp. 239–260. [Google Scholar]
- 56.Spaccarelli S. Stress, Appraisal, and Coping in Child Sexual Abuse: A Theoretical and Empirical Review. Psychological Bulletin. 1994;116(2):340–362. doi: 10.1037/0033-2909.116.2.340. [DOI] [PubMed] [Google Scholar]
- 57.NIAAA. Alcoholism: The Physician's Guide to Helping Patients with Alcohol Problems. Washington, DC: National Institutes of Health; 1995. [Google Scholar]
- 58.Morey LC. The Personality Assessment Inventory Professional Manual. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- 59.Kellogg SH, Ho A, Bell K, Schluger RP, McHugh PF, McClary KA, et al. The Personality Assessment Inventory Drug Problems Scale: a validity analysis. Journal of Personality Assessment. 2002 Aug;79(1):73–84. doi: 10.1207/S15327752JPA7901_05. [DOI] [PubMed] [Google Scholar]
- 60.Parker JD, Daleiden EL, Simpson CA. Personality Assessment Inventory substance-use scales: convergent and discriminant validity with the Addiction Severity Index in a residential chemical dependence treatment setting. Psychological Assessment. 1999;11(4):507–513. [Google Scholar]
- 61.Ruiz MA, Dickinson KA, Pincus AL. Concurrent validity of the Personality Assessment Inventory Alcohol Problems (ALC) Scale in a college student sample. Assessment. 2002 Sep;9(3):261–270. doi: 10.1177/1073191102009003005. [DOI] [PubMed] [Google Scholar]
- 62.Arriola KRJ, Louden T, Doldren MA, Fortenberry RM. A meta-analysis of the relationship of child sexual abuse to HIV risk behavior among women Child Abuse and Neglect. 2005;29(6):725–746. doi: 10.1016/j.chiabu.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 63.Briere J, Elliot DM. Prevalence and psychological sequelae of self-reported childhood physical and sexual abuse in a general population sample of men and women Child Abuse and Neglect. 2003;27(10):1205–1222. doi: 10.1016/j.chiabu.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 64.Senn TE, Carey MP, Vanable PA, Coury-Doniger P, Urban MA. Childhood sexual abuse and sexual risk behavior among men and women attending sexually transmitted disease clinic. Journal of Consulting and Clinical Psychology. 2006;74(4):720–731. doi: 10.1037/0022-006X.74.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ullman SE, Brecklin LR. Sexual assault history and health-related outcomes in a national sample of women. Psychology of Women Quarterly. 2003;27:46–57. [Google Scholar]
- 66.Belcher L, Kalichman S, Topping M, Smith S, Emshoff J, Norris F, et al. A randomized trial of a brief HIV risk reduction counseling intervention for women. Journal of Consuling and Clinical Psychology. 1998;66(5):856–861. doi: 10.1037//0022-006x.66.5.856. [DOI] [PubMed] [Google Scholar]
- 67.Wyatt GE, Longshore D, Chin D, Carmona JV, Loeb T, Myers HF, et al. The efficacy of an integrated risk reduction intervention for HIV-positive women with child sexual abuse histories. AIDS Behav. 2004;8(4):453–462. doi: 10.1007/s10461-004-7329-y. [DOI] [PubMed] [Google Scholar]
- 68.Williams JK, Wyatt GE, Rivkin I, Ramamurthi HC, Li X, Liu H. Risk reduction for HIV-positive African American and Latino men with histories of childhood sexual abuse. Archives of Sexual Behavior. 2008 Oct;37(5):763–772. doi: 10.1007/s10508-008-9366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ruzek JI, Polusny MA, Abueg FR. Assessment and treatment of concurrent posttraumatic stress disorder and substance abuse. In: Follette VM, Ruzek JI, Abueg FR, editors. Cognitive-Behavioral Therapies for Trauma. New York: Guilford; 1998. pp. 226–255. [Google Scholar]
- 70.Woolridge E, Barton S, Samuel J, Osorio J, Dougherty A, Holdcroft A. Cannabis use in HIV for pain and other medical symptoms. Journal of Pain Symptom Management. 2005 Apr;29(4):358–367. doi: 10.1016/j.jpainsymman.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 71.Corless IB, Lindgren T, Holzemer W, Robinson L, Moezzi S, Kirksey K, et al. Marijuana effectiveness as an HIV self-care strategy. Clin Nurs Res. 2009 May;18(2):172–193. doi: 10.1177/1054773809334958. [DOI] [PubMed] [Google Scholar]
- 72.Cristiani SA, Pukay-Martin ND, Bornstein RA. Marijuana use and cognitive function in HIV-infected people. J Neuropsychiatry Clin Neurosci. 2004 Summer;16(3):330–335. doi: 10.1176/jnp.16.3.330. [DOI] [PubMed] [Google Scholar]