Abstract
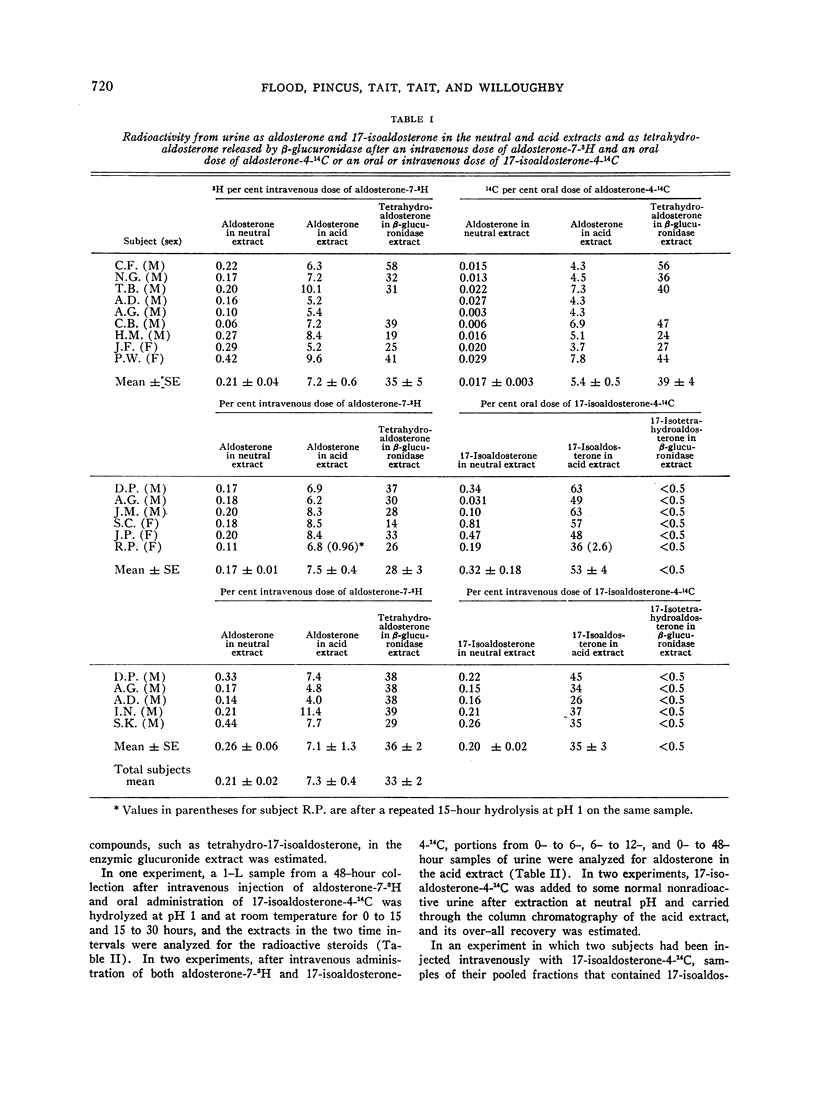
After intravenous and oral administration of radioactive aldosterone to normal subjects, 7.3 ± 0.4 (SE) and 5.4 ± 0.5 (SE)%, respectively, of the dose was recovered from a 48-hour collection of urine as aldosterone released by mild acid hydrolysis (from aldosterone 18-glucuronide), and 35 ± 5 (SE) and 39 ± 4 (SE)%, respectively, was recovered as tetrahydroaldosterone after incubation with β-glucuronidase.
After intravenous and oral administration of 17-isoaldosterone-4-14C to a similar group of subjects, 35 ± 3 (SE) and 53 ± 4 (SE)%, respectively, of the dose was recovered as 17-isoaldosterone released by acid and less than 5% as total metabolites after incubation with β-glucuronidase. No detectable radioactivity (< 0.5%) could be recovered as tetrahydroaldosterone or as a compound with the expected chromatographic properties of tetrahydro-17-isoaldosterone.
The total radioactivity in the neutral extracts was also relatively small (< 2%) after administration of either labeled aldosterone or 17-isoaldosterone. The radioactivity as aldosterone in the neutral extract was much lower after oral [0.017 ± 0.003 (SE)%] than after intravenous [0.21 ± 0.04 (SE)%] administration of labeled aldosterone. The radioactivity as 17-isoaldosterone in the neutral extract was similar after intravenous [0.20 ± 0.02 (SE)%] and after oral [0.38 ± 0.18 (SE)%] administration of 17-isoaldosterone.
These results indicated that, due to lack of A-ring reduction of the molecule and the consequent slowing of hepatic clearance, 17-isoaldosterone is converted to an acid-labile conjugate (presumably 17-isoaldosterone 18-glucuronide) as the major metabolite. 17-Isoaldosterone was not secreted or converted to aldosterone to any significant extent in the normal subjects investigated.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUSH I. E. Methods of paper chromatography of steroids applicable to the study of steroids in mammalian blood and tissues. Biochem J. 1952 Jan;50(3):370–378. doi: 10.1042/bj0500370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe T., Liddle G. W., Riondel A., Island D. P., Bloomfield D., Sinclair-Smith B. Comparative fates of intravenously and orally administered aldosterone: evidence for extrahepatic formation of acid-hydrolyzable conjugate in man. J Clin Invest. 1966 Feb;45(2):264–269. doi: 10.1172/JCI105339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COPPAGE W. S., Jr, ISLAND D. P., COONER A. E., LIDDLE G. W. The metabolism of aldosterone in normal subjects and in patients with hepatic cirrhosis. J Clin Invest. 1962 Aug;41:1672–1680. doi: 10.1172/JCI104624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheville R. A., Luetscher J. A., Hancock E. W., Dowdy A. J., Nokes G. W. Distribution, conjugation, and excretion of labeled aldosterone in congestive heart failure and in controls with normal circulation: development and testing of a model with an analog computer. J Clin Invest. 1966 Aug;45(8):1302–1316. doi: 10.1172/JCI105437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm K., Breuer H. Partial purification of a soluble UDPglucuronyltransferase from human intestine. Biochim Biophys Acta. 1966 Feb 14;113(2):404–406. doi: 10.1016/s0926-6593(66)80082-6. [DOI] [PubMed] [Google Scholar]
- FLOOD C., LAYNE D. S., RAMCHARAN S., ROSSIPAL E., TAIT J. F., TAIT S. A. An investigation of the urinary metabolites and secretion rates of aldosterone and cortisol in man and a description of methods for their measurement. Acta Endocrinol (Copenh) 1961 Feb;36:237–264. doi: 10.1530/acta.0.0360237. [DOI] [PubMed] [Google Scholar]
- HURTER R., NABARRO J. D. Aldosterone metabolism in liver disease. Acta Endocrinol (Copenh) 1960 Feb;33:168–174. doi: 10.1530/acta.0.xxxiii0168. [DOI] [PubMed] [Google Scholar]
- JONES K. M., LLOYD-JONES R., RIONDEL A., TAIT J. F., TAIT S. A., BULBROOK R. D., GREENWOOD F. C. Aldosterone secretion and metabolism in normal men and women and in pregnancy. Acta Endocrinol (Copenh) 1959 Mar;30(3):321–342. doi: 10.1530/acta.0.0300321. [DOI] [PubMed] [Google Scholar]
- Pasqualini J. R., Uhrich F., Jayle M. F. Contribution to the study of the structure of the aldosterone-conjugate isolated from human urine. Biochim Biophys Acta. 1965 Jul 8;104(2):515–523. doi: 10.1016/0304-4165(65)90357-0. [DOI] [PubMed] [Google Scholar]
- SIEGENTHALER W. E., DOWDY A., LUETSCHER J. A. Determination of the secretion rate of aldosterone in normal man by use of 7-H3-d-aldosterone and acid hydrolysis of urine. J Clin Endocrinol Metab. 1962 Feb;22:172–177. doi: 10.1210/jcem-22-2-172. [DOI] [PubMed] [Google Scholar]
- ULICK S., VETTER K. K. SIMULTANEOUS MEASUREMENT OF SECRETORY RATES OF ALDOSTERONE AND 18-HYDROXYCORTICOSTERONE. J Clin Endocrinol Metab. 1965 Aug;25:1015–1026. doi: 10.1210/jcem-25-8-1015. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD R. H., FLOOD C. A., TAIT S. A., TAIT J. F. A comparison of methods for the acid hydrolysis of a urinary conjugate of aldosterone. J Clin Endocrinol Metab. 1961 Sep;21:1092–1098. doi: 10.1210/jcem-21-9-1092. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD R. H., TAIT J. F. PURIFICATION, PARTIAL CHARACTERIZATION AND METABOLISM OF AN ACID LABILE CONJUGATE OF ALDOSTERONE. J Clin Endocrinol Metab. 1964 Nov;24:1110–1124. doi: 10.1210/jcem-24-11-1110. [DOI] [PubMed] [Google Scholar]


