Abstract
Regulatory T cells (Tregs) play an essential role in maintaining the homeostatic balance of immune responses. Asthma is an inflammatory condition of the airways that is driven by dysregulated immune responses toward normally innocuous antigens. Individuals with asthma have fewer and less functional Tregs, which may lead to uncontrolled effector cell responses and promote proasthmatic responses of T helper type 2, T helper 17, natural killer T, antigen-presenting, and B cells. Tregs have the capacity to either directly or indirectly suppress these responses. Hence, the induced expansion of functional Tregs in predisposed or individuals with asthma is a potential approach for the prevention and treatment of asthma. Infection by a number of micro-organisms has been associated with reduced prevalence of asthma, and many infectious agents have been shown to induce Tregs and reduce allergic airways disease in mouse models. The translation of the regulatory and therapeutic properties of infectious agents for use in asthma requires the identification of key modulatory components and the development and trial of effective immunoregulatory therapies. Further translational and clinical research is required for the induction of Tregs to be harnessed as a therapeutic strategy for asthma.
Keywords: asthma, regulatory T cell, forkhead box p3, immunoregulatory therapy
CLINICAL RELEVANCE.
This review highlights a central role for regulatory T cells (Tregs) in suppressing the dysregulated immune responses involved in the pathogenesis of asthma. The development of an immunoregulatory therapy that induces Tregs offers a novel therapeutic strategy for asthma.
Asthma has dramatically increased in developed countries over the past 3 decades. It is a common chronic inflammatory disease of the airways characterized by episodes of breathlessness, coughing, wheezing and airway hyperresponsiveness (AHR). The causes are complex and multifactorial, and are therefore difficult to target therapeutically. Current treatment strategies only suppress the symptoms, rather than inhibiting the underlying mechanisms, and fail to control the disease in a significant proportion of individuals with asthma.
A variety of different cell types are involved in promoting the inflammatory component of asthma. The prevailing paradigm is that T helper (Th) type 2 lymphocytes drive inflammation through the secretion of cytokines, such as IL-4, IL-5, and IL-13, which induce the recruitment and activation of eosinophils, pulmonary inflammation, mucus hypersecretion, B cell isotype switching, and AHR. Over time, lung function declines as a result of airway remodeling, which leads to increased susceptibility to exacerbations of disease.
Th17 cells are a recently recognized member of the T cell family, and are important in modifying immune responses in the airways. Bronchial biopsies from patients during acute episodes of severe asthma are infiltrated with Th17 cells (1). Furthermore, studies using animal models have established that Th17 cells and their cytokines are major inducers of neutrophilic, eosinophilic, and steroid-resistant airway inflammation (2, 3).
Natural killer T (NKT) cells are another unique subset of T cells, which respond to glycolipids and secrete large amounts of Th2 cytokines (4). NKT cells have been detected at higher levels in the sinus mucosa and sputum of individuals with asthma compared with healthy individuals (5, 6). Furthermore, animal studies have identified a potential requirement for NKT cells in asthma, particularly in the induction of AHR that is independent of Th2 cell responses (7).
Antigen-presenting cells (APCs), such as dendritic cells (DCs), have crucial roles in antigen presentation, initiation, and maintenance of allergic disease. Both myeloid and plasmacytoid DCs are increased in the airways of patients with asthma after allergen challenge, highlighting their role in allergic inflammation (8).
Th2 cell–driven B cell secretion of IgE, subsequent crosslinking on mast cells, and release of inflammatory mediators also contributes substantially to the allergic response. Anti-IgE therapy (Omalizumab) has proven effective for allergic disease when administered in conjunction with steroids (9).
Together, the dysregulation of these cellular aspects in asthma highlights the multifactorial processes that contribute to the development and maintenance of disease. Although there have been many attempts, the targeting of individual factors by direct therapeutic intervention has not led to effective therapies to date. This highlights the need for the development of therapeutic strategies that have multifactorial suppressive effects on the causes of asthma.
ASTHMA: THE REGULATORY T CELL DEFICIENCY
In healthy individuals, regulatory T cells (Tregs) play an essential role in modulating and regulating immune responses by promoting tolerance, counterbalancing aggressive inflammatory reactions, and maintaining homeostasis. Several independent studies have shown that the number and function of Tregs is impaired or altered in allergic patients compared with healthy individuals.
Reduced numbers of Tregs are observed in blood and/or induced sputum from patients with severe eczema, elevated IgE levels, eosinophilia, food allergy, and asthma, and, during exacerbations, individuals with asthma have an even greater deficiency of Tregs (10, 11). By contrast, some studies have detected an increase in the number of Tregs in severe disease (12, 13). It is likely that, in severe cases, Tregs are induced to moderate inflammation; however, they are not induced to a sufficient extent to overcome the aggressive inflammatory responses involved. However, the interpretation of these studies may be confounded by the characterization of Tregs with CD4 and CD25 positivity alone, as some of these cells may represent activated effector T cells. Furthermore, the assessment of Treg number in blood does not account for Tregs that may have migrated to the site of inflammation.
As well as reductions in numbers, Tregs from individual atopy have a significantly reduced capacity to suppress effector T cells and Th2 cytokines (14, 15). This may be due to differences in the proportions of Treg subsets in healthy and allergic individuals (16). Chemotactic signals for Tregs, such as those in the CCL-1 pathway, may also be defective in individuals with asthma (17). Furthermore, the forkhead box (Fox) p3 locus is subject to epigenetic modification, which may result in alterations in the suppressive capacity of Tregs (18). The role of epigenetic modifications in Treg function in individuals with asthma requires further investigation.
Evidence that supports the requirement for Tregs in the control of asthma has been provided by the use of mouse models of allergic airways disease. Adoptive transfer of CD4+CD25+ Tregs into sensitized mice before antigen challenge suppresses the development of allergic disease (19, 20). In addition, adoptive transfer of Tregs after the onset of disease attenuates established inflammation (21).
Glucocorticoid treatment of asthma is effective in suppressing inflammation and symptoms. These agents induce a short-term up-regulation of Foxp3 expression and Tregs in patients with asthma (22). However, animal models suggest that, in the long term, corticosteroids may also prevent the development of Tregs and exacerbate Th2 immune responses (23).
Together, these observations provide strong evidence that an effective regulatory response, which is controlled by Tregs, is required to prevent the development and progression of asthma.
TREG CHARACTERIZATION
Tregs are characterized by many phenotypic and functional markers that distinguish them from conventional T cells. CD4, CD25, and Foxp3 are the three markers that have been classically used to characterize Tregs. The transcription factor, Foxp3, is essential for the suppressive activity, survival, and stability of Tregs, and CD25 is found on the vast majority of Foxp3+ T cells.
Tregs may develop as two distinct populations, termed natural or induced Tregs. Lineage commitment into natural Tregs is instructed by self-antigens in the thymus. Induced Tregs have a more “plastic” phenotype. They are derived from the naive CD4 precursor pool in peripheral lymphoid tissue after foreign antigen encounter and are generated under the influence of IL-2 and transforming growth factor (TGF)–β (24). Notably, induced Tregs comprise both Foxp3+ and Foxp3− populations (25).
Two subtypes of Tregs that release soluble factors have also been identified, which are Treg type (Tr) 1 cells that secrete high levels of IL-10 with or without TGF-β production, and Tr3 cells that release TGF-β (26). However, the continual reporting of additional suppressive mechanisms and markers, which are associated with Tregs, indicates that numerous other subtypes are likely to exist.
TREG-SUPPRESSIVE MECHANISMS
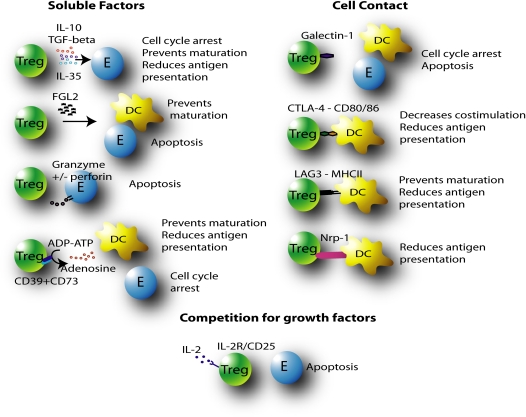
The mechanisms of suppression of immune responses that are employed by Tregs remain controversial, which largely stems from discrepancies between in vivo and in vitro studies. The widely recognized mechanisms of suppression include the secretion of suppressive soluble factors, cell contact–mediated suppression, and competition for growth factors (Figure 1). The regulatory effects of Tregs on effector cell responses include: cell cycle arrest and inhibition of proliferation; induction of apoptosis; and suppression of cytokine release, DC maturation, or antigen presentation and costimulation.
Figure 1.
Regulatory T cell (Treg)–mediated suppression of immune responses may occur through soluble factors (IL-10, transforming growth factor [TGF]–β, IL-35, fibrinogen-like protein (FGL) 2, granzyme+/− perforin, and adenosine), cell contact–dependent mechanisms (galectin-1, cytotoxic T-lymphocyte–associated protein [CTLA]–4, lymphocyte-activation gene (LAG)-3, neuropilin [Nrp]-1), or competition for growth factors (IL-2). Suppression may be the result of cell cycle arrest, apoptosis, prevention of dendritic cell (DC) maturation or antigen presentation, or reduced costimulation of effector cells (E: T or B cells).
Soluble Factors
The immunosuppressive cytokines, IL-10 and TGF-β, were the first factors considered to be involved in mediating suppression by Tregs, and the roles of these cytokines in suppression have been discussed extensively elsewhere (27, 28). In summary, IL-10 release from Tregs prevents the synthesis of proinflammatory cytokines, and down-regulates the expression of effector T cell cytokines and antigen presentation and costimulatory properties of APCs. TGF-β directly prevents T cell proliferation and differentiation by inhibiting the release of many cytokines, including IL-1 and IL-2, and their receptors. TGF-β also inhibits B cell proliferation and apoptosis, and macrophage proliferation and function, including the release of reactive oxygen species. Furthermore, TGF-β maintains Treg function and promotes the differentiation of adaptive Tregs. Nevertheless, the roles and contribution of IL-10 and TGF-β to Treg-mediated immunosuppression are controversial. In vivo studies have demonstrated that both IL-10– and TGF-β–dependent mechanisms exist (29–31). By contrast, neutralizing antibodies against IL-10 and TGF-β fail to abrogate suppression in vitro and in vivo, and supernatants from cell-suppression assays do not attenuate effector T cell responses (19, 32, 33). In addition, the suppressive function of Tregs from mice deficient in these cytokines is not affected (34, 35).
More recently, IL-35 has been identified as an important cytokine released by Tregs to target effector cells directly (36). Epstein-Barr virus–induced gene 3 and IL-12α form the heterodimeric structure of IL-35, which is highly expressed by Foxp3+ cells, but not resting CD4+ cells. Furthermore, Epstein-Barr virus–induced gene 3−/− and IL-12a−/− mice have Tregs with reduced suppressive capacity, which confirms the importance of IL-35 in Treg-mediated suppression.
Fibrinogen-like protein 2 (FGL2) is also highly expressed by Tregs (37). FGL2 down-regulates DC function, limits activation of naive T cells, and induces apoptosis of B cells. Subsequently, a role for this suppressive factor has been confirmed, because anti-FGL2 blocks the suppressive activity of Tregs, and Tregs from Fgl2−/− mice are less effective.
The release of cytotoxic molecules, in close proximity of target cells to induce their apoptosis, has also been implicated as a mechanism of suppression. Tregs can express granzyme A and/or granzyme B and apoptosis may be mediated in a perforin-dependent or -independent manner (38–40). In addition, granzyme B–deficient Tregs have reduced suppressive function.
Tregs preferentially express CD39 and CD73, which convert ADP and ATP to AMP, which is rapidly degraded to adenosine. Adenosine binds the A2A receptor on effector cells to suppress their function, and may reduce DC function and the expression of costimulatory markers (41). Indeed, CD39-deficient Tregs are dysfunctional and less effective at suppressing effector T cell responses.
Cell Contact–Mediated Suppression
The existence and importance of cell contact–mediated suppression is controversial. Transwell experiments have shown that, in some instances, Tregs require cell contact to suppress target cells, and, in others, cell contact is not required (42, 43).
Galectin-1 is a β-galactoside–binding protein that is preferentially expressed on Tregs, and binds glycoproteins (44). Galactin-1 binding to effector cells leads to cell cycle arrest or apoptosis. Furthermore, blocking of Galactin-1 reduces the inhibitory effects of Treg cells and Galactin-1−/− mice have reduced Treg function.
To inhibit the priming and differentiation of effector T cells, Tregs are known to target APCs. Tregs are the only lymphocytes that express cytotoxic T-lymphocyte–associated protein (CTLA)–4, which closely resembles the T cell costimulatory molecule, CD28, but has higher ligand-binding affinity. CD28 ligation with CD80/86 on DCs is essential for T cell activation, and CTLA-4 ligation inhibits CD28 ligation and results in a higher proportion of anergic T cells. CTLA-4 may also block or down-regulate the expression of CD80/86, resulting in reduced priming of naive T cells. Furthermore, Treg CTLA-4–mediated ligation of CD80/CD86 can stimulate the production of indoleamine 2,3-dioxygenase and therefore condition DCs to become more immuno-suppressive (45). Indoleamine 2,3-dioxygenase is the rate-limiting enzyme for the degradation of tryptophan. Tryptophan depletion results in APC immunosuppressive activity by inducing the production of proapoptotic factors. Anti–CTLA-4 treatment reverses Treg suppression of effector T cell responses, and CTLA-4–deficient mice have defective Tregs (46, 47).
Tregs may also express lymphocyte-activation gene (LAG)-3 (CD223), a homolog of the major histocompatibility complex (MHC) II coreceptor, CD4, but with higher binding affinity. The direct interaction of LAG-3 with MHCII maintains the immaturity of DCs by reducing MHCII–peptide presentation to naive T cells (48). The control of APCs by Tregs at different stages of the immune response provides fine modulatory control.
Neuropilin-1 (Nrp-1) is also expressed by Tregs, and prolongs the interaction with DCs and reduces antigen presentation to naive T cells. The role of murine Nrp-1 in suppression was confirmed when anti–Nrp-1 was used to abrogate Treg-mediated suppressive activity (49). However, Nrp-1 cannot be used as a marker of human Foxp3+ Tregs, because Nrp-1 is not only expressed on human Foxp3+ Tregs, and occurs on other CD4+ cells (50). This study also demonstrated that Nrp-1 expression can be induced by stimulation of peripheral blood T cells, and may, in fact, be a novel marker of T cell activation. This suggests that anti–Nrp-1 may abrogate Treg-mediated suppression by interfering with cell activation rather than Treg function. The identification of factors that are important in initiating Treg suppression and are separate from contact-dependent suppression events requires further study.
Recently, Collison and colleagues (51) showed that Treg/effector cell contact increased the expression of IL-35. They also found that conventional T cell activation was required for heightened Treg function. They proposed that the function of Tregs is not contact dependent; however, the induction of suppression by T cell receptor (TCR) activation is. Elucidation of the requirements for the initiation of suppression will further the understanding of the contextual importance of each mechanism.
Deprivation of Growth Factors
Tregs may also attenuate effector responses by competing with effector cells for essential growth factors. IL-2 is essential for both Treg and effector cell function (52). Tregs compete with effector T cells for secreted IL-2; subsequently, effector T cells are deprived of stimulation, and this leads to B cell lymphoma-2-interacting mediator (Bim)-mediated apoptosis (53).
Other Mechanisms of Suppression
Immune modulation by Tregs may also occur via nonspecific “bystander suppression” or outgrowth of a new population of Tregs, known as “infectious tolerance.” This is supported by data showing that Tregs do not require TCR recognition to suppress effector T cells (54). In this study, Tregs were shown to require activation via their TCR to become suppressive; however, their function was antigen nonspecific. This was demonstrated in vitro by culture of transgenic Tregs, which have TCRs specific for one antigen, with transgenic T cells in the presence of either specific or nonspecific antigen. Transgenic Tregs suppressed proliferation of transgenic T cells, regardless of the antigen used. Hence, Tregs possess constitutive activity, and suppression can occur in the absence of MHC–peptide recognition and in the absence of APCs.
It is possible that, depending on the nature of immune response, eliciting agent, immunological make-up of the host, and site of suppression, certain mechanisms of Treg-mediated suppression prevail. Furthermore, in the absence of one suppressive mechanism, Tregs may employ alternative suppressor functions. Therefore, multiple Treg cell functions may act alone or synergistically, directly or indirectly, at the site of antigen presentation to suppress immune responses.
OTHER MARKERS OF TREGS
A number of additional markers have been associated with Tregs (Table 1). These markers have enabled the further delineation of the subtypes, activation, and function of Tregs that may be important in different disease states.
TABLE 1.
ADDITIONAL MARKERS AND THEIR ASSOCIATION WITH REGULATORY T CELLS
| Marker | Associated with: | Ref. No. |
|---|---|---|
| CD69 | A unique subset | (55) |
| CD103 | Increased activation status | (56) |
| TNFR2 | Increased activation status | (57) |
| CD101 | Increased activation status | (58) |
| CD45RB | Increased activation status | (59) |
| GITR | Clonal expansion | (60) |
| ICOS | Clonal expansion | (61) |
| Activin A | Clonal expansion | (62) |
| IL-9 | Enhanced suppressive function | (63) |
| HO-1 | Generation of Tregs | (64) |
| GPR83 | Generation of Tregs | (65) |
| Retinoic acid | Homing and differentiation | (66) |
| CD62L | Homing state | (67) |
| LFA-1 | Induction and function | (68) |
| OX40/CD134 | Inhibition of suppression | (69) |
| PD-1 | Inhibition of suppression | (70) |
| CD127 | Low expression on Tregs | (71) |
| CD137/4-1BB | Survival | (72) |
Definition of abbreviations: GITR, glucocorticoid-induced TNFR-related protein; GPR, G-protein coupled receptor; HO, heme oxygenase; ICOS, inducible T-cell co-stimulator; LFA, lymphocyte function-associated antigen; PD, programmed death; TNFR, tumour necrosis factor receptor; Tregs, regulatory T cells.
TREG SUPPRESSION OF EFFECTOR TARGETS IN ASTHMA
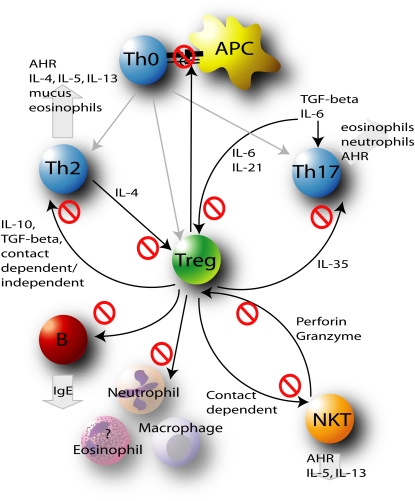
Defective Treg responses may play central roles in mediating dysregulated cellular responses and have been implicated at different stages in the development, progression and exacerbation of asthma (Figure 2). Both natural and induced Tregs are key players in the maintenance of immune homeostasis.
Figure 2.
Treg-mediated immunoregulation is crucial in preventing the dysregulated immune responses that drive the initiation, progression, and exacerbation of asthma. By regulating Th2, Th17, and natural killer T (NKT) cells, antigen-presenting cells (APCs), B cells, and inflammatory cells, Tregs prevent the development of allergic inflammation, IgE release, mucus hypersecretion, and airway hyperresponsiveness (AHR).
Recently, antigen presentation by alveolar epithelial cells has been shown to promote TGF-β–dependent induction of Foxp3 expression (73). In addition, alveolar macrophages may direct the induction of Treg differentiation, and a role for these cells in the suppression of allergic airways disease has been proposed (74). These observations highlight the important interplay between Tregs and APCs in mediating the control of immune responses in allergic airways disease.
During the initiation stage of an immune response, Tregs may attenuate the establishment of stable contacts between APCs and naive T cells, inhibit APC activity, or promote suppressive factors that prevent effector T cell development.
Emerging evidence suggests that there is also an important interplay between Tregs and Th17 cells during the early stage of naive T cell differentiation, which is currently the subject of intense research (75). Differentiation of naive T cells in the presence of IL-6 and TGF-β results in the development of Th17 cells. However, in the absence of IL-6, Tregs arise. IL-21 is also known to contribute to the induction of Th17 differentiation and suppresses Foxp3. Given their role in asthma, the prevention or suppression of Th17 cells by Tregs facilitates the maintenance of immune homeostasis.
In addition to modulating the priming of immune responses, Tregs also directly suppress fully differentiated effector cells. Indeed, the investigation of the suppressive effects of Tregs on Th2 cells has identified an array of suppressive mechanisms. Direct suppression of Th2 cells results in attenuated Th2 cytokine release, leading to reduced cellular inflammation, B cell isotype switching, and hallmark features of asthma.
The emerging involvement of multiple effector cell types in asthma pathogenesis, in addition to Th2 cells, indicates a much broader role for Tregs in counteracting dysregulated immune response in asthma.
Human Tregs suppress the proliferation, cytokine release, and cytotoxic effects of NKT cells in a cell contact–dependent manner (76). Interestingly, however, NKT cells from individuals with asthma, but not healthy control subjects, have the ability to be cytotoxic toward Tregs (39). This supports studies that show a reduced number of Tregs in individuals with asthma, and suggests that Tregs in individuals with asthma may be more vulnerable to destruction. Conversely, NKT cells may also provide proliferative help to Tregs through the secretion of IL-2 (77). This interplay highlights an important relationship between Tregs and NKT cells in individuals with asthma, which is not completely understood.
Effector B cells may also be directly suppressed by Tregs, which provides a secondary mechanism of immune attenuation after the suppression of Th2 function. Activated CD4+CD25+ T cells selectively kill B lymphocytes through close contact–mediated release of granzyme and perforin, in the absence of suppression of Th2 cells (78, 79). Hence, Tregs can specifically prevent IgE release and subsequent mast cell–mediated inflammation. Recently, immunosuppressive IL-10–producing B regulatory cells have been identified, and these cells may also control T cell–mediated inflammation (80). Furthermore, IL-10 induces IgG4 isotypes that are protective against the development of IgE and allergic disease in healthy individuals (81). Interestingly, B cells may contribute to the control of peripheral development of CD4+CD25+ cells, possibly by inducing the expansion or prolonging the survival of these cells (82).
Innate inflammatory cells in the lung are also potential targets for Tregs. Neutrophil function and survival are inhibited by Tregs in response to LPS exposure in vitro (83). Macrophage function and proinflammatory cytokine release are also attenuated in vitro and in vivo (84). These and other studies suggest that Tregs have the capacity to target innate immune responses directly. The potential for Tregs to suppress neutrophil, macrophage, and eosinophil responses directly, in the context of asthma, has not been assessed.
The capacity of Tregs to suppress the initial priming of an allergic response, and the multiple effector cells involved in the pathogenesis of asthma, indicates the potential for the induction of Tregs as a multifactorial immunoregulatory therapeutic approach for asthma.
INDUCTION OF TREGS BY INFECTIOUS AGENTS AND THEIR COMPONENTS
It has been widely proposed that a lack of infection-induced tolerance promotes the development of asthma, and may be responsible for the current asthma epidemic. This lack of tolerance may be mediated by reductions in infection-induced Tregs, which results in maladaptive immune responses that drive the development of allergy and asthma. This indicates the potential of harnessing the induction of Tregs by infectious agents or their components to target the development and effector-phase responses of allergic disease, including those that occur at the site of inflammation.
An inverse association between a number of infectious agents and the prevalence of asthma has been reported. These observations have initiated the elucidation of the mechanisms of induction and protection against asthma with animal models. As a result, a number of infections have been shown to induce Tregs and suppress allergic responses in mouse models of allergic airways disease (Table 2). The majority of infections appear to promote IL-10 or TGF-β–mediated suppression of effector responses; however, additional mechanisms of suppression have not been widely explored. Identification of the microbial components that are involved in the induction of Tregs and subsequent suppression of allergic airways disease is necessary before these effects can be harnessed for therapeutic application.
TABLE 2.
INFECTIOUS MICRO-ORGANISMS THAT MAY INDUCE REGULATORY T CELLS–MEDIATED SUPPRESSION OF ALLERGIC AIRWAYS DISEASE
| Organism | Suppressive Mechanism | Ref. No. |
|---|---|---|
| Bifidobacterium lactis | Associated increase in TGF-β | (85) |
| Heligmosomoides polygyrus | Involves/dependent on IL-10 | (86, 87) |
| Lactobacillus reuteri | Unknown, conflicting results; no change or increased IL-10 | (88) |
| Lactobacilus rhamnosus | Associated increase in TGF-β | (85) |
| Litomosoides sigmodontis | Associated increase in TGF-β, however, blocking had no effect suppression of IL-10 | (89) |
| Mycobacterium vaccae | Dependent on IL-10 and TGF-β | (90) |
| Nippostrongylus brasiliensis products | Independent of TLR-2, TLR-4, IFN-γ, and IL-10 | (91) |
| Nippostrongylus brasiliensis | Involved IL-10 | (92) |
| Schistosoma japonicum | Associated increase in IL-10 | (93) |
| Schistosoma mansoni | Independent of IL-10, likely to be cell contact mediated | (94) |
| Streptococcus pneumoniae | Unknown, not IL-10 or TGF-β mediated, likely to be cell contact mediated | (95) and unpublished data |
| Toxiplasma gondii | Associated increase in IL-10 | (96) |
Definition of abbreviations: TGF, transforming growth factor; TLR, Toll-like receptor.
FUTURE DIRECTIONS
Numerous therapeutic strategies for asthma have been developed (97); however, their specificity toward particular factors limits their success. This is not surprising, because asthma is a multifactorial disease, and allergic airways disease continues to develop in the absence of Th2 cells, IgE, or eosinophilic inflammation in mouse models (98, 99). Immunoregulatory therapies that initiate a shift from Th2 to Th1 responses have also been explored; however, these approaches have had limited success in clinical trials (100, 101).
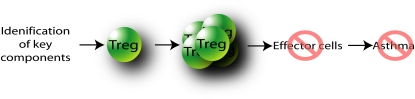
The multitargeting nature of Tregs allows for the regulation of a number of different effector arms of the immune response involved in asthma. The induction of Tregs to target effector responses may be the most holistic approach to modulate the underlying cause of disease. Although this would seem a straightforward approach, there are numerous issues that need to be addressed. Key components of infectious agents need to be identified and developed into immunoregulatory therapies, and administration regimes (dose, timing route) would need to be optimized (Figure 3). Furthermore, a successful immunoregulatory therapy needs to overcome the existing Treg pool within an individual with asthma, and the expansion of Tregs with normal function and chemotactic properties is required. Nevertheless, this approach of inducing Tregs to suppress the numerous allergic inflammatory responses in asthma would seem to be the most logical approach for the development of effective therapies.
Figure 3.
Identification of key components that expand the Treg pool and suppress effector cells involved in the pathogenesis of asthma have the potential to guide the development of successful immunoregulatory therapies.
Current and ongoing studies of harnessing the induction of Tregs by infectious agents for application to allergic disease have been based on the conversion of empirical data into effective therapies. Helminth infection with Litomosoides sigmodontis, Nippostrongylus brasiliensis, Schistosoma japonicum, and Schistosoma mansoni has been shown to induce Tregs and suppress allergic airways disease (89, 92–94). One study has extended these observations and identified helminth-derived products that inhibit allergic responses (91). However, these helminth products have not yet been extensively tested in animal models of allergic disease or in clinical trials. Treatment with probiotic bacteria has been shown to prevent the development of allergic airways disease in both adult and neonatal mouse models (85, 88). Numerous studies have investigated the potential of probiotic treatment for asthma and allergic rhinitis in humans, but have produced conflicting results, and are inconclusive at this stage. These studies have been recently reviewed (102). Mycobacterium vaccae administration has protective effects on allergic airways disease in mouse models, and there have been several attempts to translate these observations in clinical trials. Early studies showed that treatment with heat-killed M. vaccae reduced allergen-induced responses in atopic dermatitis; however, more recent studies that assessed the effect on asthma and atopic dermatitis showed a lack of efficacy (100, 103–105). The full potential of M. vaccae–based therapy for allergic disease remains to be determined, and is the subject of ongoing clinical trials.
Other promising therapeutic strategies for allergic disease, which involve increasing the numbers or function of Tregs, have been recently reviewed (106). Allergen immunotherapy is particularly effective, and involves the administration of increasing doses of a specific allergen. Therapy promotes the development of antigen-specific Tregs that release IL-10 or TGF-β and inhibit allergen-specific Th2 responses (107, 108). However, this strategy requires treatment that is tailored to the specific allergen, constant patient monitoring, and has been associated with serious side effects, including anaphylaxis. Further investigations are underway to improve safety and efficacy of this approach (109). Glucocorticoid administration in conjunction with the active form of vitamin D (1α,25-dihydroxyvitamin D3 or calcitrol) has also been shown to promote the induction of Tregs that release IL-10 (110). Importantly, this strategy is effective in patients that are refractory to steroid treatment, and further studies are refining this strategy (111).
In addition to the development of an immunoregulatory therapy for asthma, in vivo models that involve the induction of Tregs and suppression of allergic airways disease may provide valuable tools to further our understanding of the characteristics, mechanisms, and function of Tregs. These models may facilitate the delineation of “real–time” events that are important in the induction and enhanced suppressive function of Tregs.
New therapeutics, based on our understanding of Treg function and the pathophysiology of asthma, could have profound benefits for the care of individuals with asthma. Hence, it is not surprising that the potential to harness the power of Tregs as an immunoregulatory therapeutic is of great interest. Through Tregs, we have a multifactorial approach to a multifactorial disease, if only we can develop their potential into therapy.
This work was supported by Asthma Foundation of New South Wales, CRC for Asthma and Airways, The University of Newcastle and the National Health and Medical Research Council project grants 401,238 and 569,219.
Originally Published in Press as DOI: 10.1165/rcmb.2009-0342TR on January 22, 2010
Author Disclosure: P.M.H. received patents from Newcastle Innovation for novel treatments for asthma and from Cortecs/Provalis for novel vaccine targets. A.N.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Pene J, Chevalier S, Preisser L, Venereau E, Guilleux M-H, Ghannam S, Moles J-P, Danger Y, Ravon E, Lesaux S, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol 2008;180:7423–7430. [DOI] [PubMed] [Google Scholar]
- 2.Wakashin H, Hirose K, Maezawa Y, Kagami S-i, Suto A, Watanabe N, Saito Y, Hatano M, Tokuhisa T, Iwakura Y, et al. IL-23 and Th17 cells enhance Th2-cell–mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med 2008;178:1023–1032. [DOI] [PubMed] [Google Scholar]
- 3.McKinley L, Alcorn JF, Peterson A, DuPont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, et al. Th17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol 2008;181:4089–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med 2003;9:582. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto H, Okamoto Y, Horiguchi S, Kunii N, Yonekura S, Nakayama T. Detection of natural killer T cells in the sinus mucosa from asthmatics with chronic sinusitis. Allergy 2007;62:1451–1455. [DOI] [PubMed] [Google Scholar]
- 6.Hamzaoui A, Rouhou SC, Graïri H, Abid H, Ammar J, Chelbi H, Hamzaoui K. NKT cells in the induced sputum of severe asthmatics. Mediators Inflamm 2006;2:71214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, DeKruyff RH, Umetsu DT. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci USA 2006;103:2782–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bratke K, Lommatzsch M, Julius P, Kuepper M, Kleine H, Luttmann W, Christian Virchow J. Dendritic cell subsets in human bronchoalveolar lavage fluid after segmental allergen challenge. Thorax 2007;62:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies JM. Altered immunoglobulin E diversity and regulation of allergic inflammation in asthma. Clin Exp Allergy 2009;39:455–457. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Yu HH, Wang LC, Yang YH, Lin YT, Chiang BL. The levels of CD4+CD25+ regulatory T cells in paediatric patients with allergic rhinitis and bronchial asthma. Clin Exp Immunol 2007;148:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mamessier E, Nieves A, Lorec A, Dupuy P, Pinot D, Pinet C, Vervloet D, Magnan A. T-cell activation during exacerbations: a longitudinal study in refractory asthma. Allergy 2008;63:1202–1210. [DOI] [PubMed] [Google Scholar]
- 12.Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol 2007;119:1258. [DOI] [PubMed] [Google Scholar]
- 13.Lin Y-L, Shieh CC, Wang JY. The functional insufficiency of human CD4+CD25high T-regulatory cells in allergic asthma is subjected to TNF-alpha modulation. Allergy 2008;63:67–74. [DOI] [PubMed] [Google Scholar]
- 14.Ling E, Smith T, Nguyen X, Pridgeon C, Dallman M, Arbery J, Carr V, Robinson D. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet 2004;363:608–615. [DOI] [PubMed] [Google Scholar]
- 15.Grindebacke H, Wing K, Andersson A, Suri-Payer E, Rak S, Rudin A. Defective suppression of Th2 cytokines by CD4+CD25+ regulatory T cells in birch allergics during birch pollen season. Clin Exp Allergy 2004;34:1364–1372. [DOI] [PubMed] [Google Scholar]
- 16.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med 2004;199:1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen KD, Vanichsarn C, Fohner A, Nadeau KC. Selective deregulation in chemokine signaling pathways of CD4+CD25hiCD127lo/− regulatory T cells in human allergic asthma. J Allergy Clin Immunol 2009;123:933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lal G, Bromberg JS. Epigenetic mechanisms of regulation of Foxp3 expression. Blood 2009;114:3727–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med 2005;202:1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strickland DH, Stumbles PA, Zosky GR, Subrata LS, Thomas JA. Reversal of airway hyperresponsiveness by induction of airway mucosal CD4+CD25+ regulatory T cells. J Exp Med 2006;203:2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kearley J, Robinson DS, Lloyd CM. CD4+CD25+ regulatory T cells reverse established allergic airway inflammation and prevent airway remodeling. J Allergy Clin Immunol 2008;122:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karagiannidis C, Akdis M, Holopainen P, Woolley NJ, Hense G, Ruckert B, Mantel P-Y, Menz G, Akdis CA, Blaser K, et al. Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J Allergy Clin Immunol 2004;114:1425–1433. [DOI] [PubMed] [Google Scholar]
- 23.Stock P, Akbari O, DeKruyff RH, Umetsu DT. Respiratory tolerance is inhibited by the administration of corticosteroids. J Immunol 2005;175:7380–7387. [DOI] [PubMed] [Google Scholar]
- 24.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 Is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol 2007;178:2018–2027. [DOI] [PubMed] [Google Scholar]
- 25.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10–secreting type 1 regulatory T cells in rodents and humans. Immunol Rev 2006;212:28–50. [DOI] [PubMed] [Google Scholar]
- 26.Faria AM, Weiner HL. Oral tolerance. Immunol Rev 2005;206:232–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med 1996;184:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity 2008;28:468–476. [DOI] [PubMed] [Google Scholar]
- 29.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 2002;420:502–507. [DOI] [PubMed] [Google Scholar]
- 30.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ TR cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med 2003;197:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li MO, Wan YY, Flavell RA. T cell–produced transforming growth factor–beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity 2007;26:579–591. [DOI] [PubMed] [Google Scholar]
- 32.Wilson MS, Taylor MD, Balic A, Finney CAM, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med 2005;202:1199–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kullberg MC, Hay V, Cheever AW, Mamura M, Sher A, Letterio JJ, Shevach EM, Piccirillo CA. TGF-beta1 production by CD4+CD25+ regulatory T cells is not essential for suppression of intestinal inflammation. Eur J Immunol 2005;35:2886–2895. [DOI] [PubMed] [Google Scholar]
- 34.Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, Shevach EM. CD4+CD25+ regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta–1 production and responsiveness. J Exp Med 2002;196:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol 2000;164:183–190. [DOI] [PubMed] [Google Scholar]
- 36.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DAA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007;450:566–569. [DOI] [PubMed] [Google Scholar]
- 37.Shalev I, Liu H, Koscik C, Bartczak A, Javadi M, Wong KM, Maknojia A, He W, Liu MF, Diao J, et al. Targeted deletion of fgl2 leads to impaired regulatory T cell activity and development of autoimmune glomerulonephritis. J Immunol 2008;180:249–260. [DOI] [PubMed] [Google Scholar]
- 38.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 2004;21:589–601. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen KD, Vanichsarn C, Nadeau KC. Increased cytotoxicity of CD4+ invariant NKT cells against CD4+CD25hiCD127lo/− regulatory T cells in allergic asthma. Eur J Immunol 2008;38:2034–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gondek DC, Lu L-F, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B–dependent, perforin-independent mechanism. J Immunol 2005;174:1783–1786. [DOI] [PubMed] [Google Scholar]
- 41.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen J-F, Enjyoji K, Linden J, Oukka M, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007;204:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, Nelson DR. An immunomodulatory role for CD4+CD25+ regulatory T lymphocytes in hepatitis C virus infection. Hepatology 2004;40:1062–1071. [DOI] [PubMed] [Google Scholar]
- 43.Matriano JA, Socarras S, Streilein JW. Cellular mechanisms that maintain neonatally-induced tolerance of class II alloantigens: evidence that factor-mediated suppression silences cytotoxic T cell activity. J Immunol 1994;153:1505–1514. [PubMed] [Google Scholar]
- 44.Garin MI, Chu C-C, Golshayan D, Cernuda-Morollon E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood 2007;109:2058–2065. [DOI] [PubMed] [Google Scholar]
- 45.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre M-L, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol 2003;4:1206–1212. [DOI] [PubMed] [Google Scholar]
- 46.Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, Sharpe AH, Powrie F. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol 2006;177:4376–4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008;322:271–275. [DOI] [PubMed] [Google Scholar]
- 48.Liang B, Workman C, Lee J, Chew C, Dale BM, Colonna L, Flores M, Li N, Schweighoffer E, Greenberg S, et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene–3 engagement of MHC class II. J Immunol 2008;180:5916–5926. [DOI] [PubMed] [Google Scholar]
- 49.Glinka Y, Chang Y, Prud'homme GJ. Protective regulatory T cell generation in autoimmune diabetes by DNA covaccination with islet antigens and a selective CTLA-4 ligand. Mol Ther 2006;14:578–587. [DOI] [PubMed] [Google Scholar]
- 50.Milpied P, Renand A, Bruneau J, Mendes-Da-Cruz D, Jacquelin S, Asnafi V, Rubio M-T, MacIntyre E, Lepelletier Y, Hermine O. Neuropilin-1 is not a marker of human Foxp3+ Treg. Eur J Immunol 2009;39:1466–1471. [DOI] [PubMed] [Google Scholar]
- 51.Collison LW, Pillai MR, Chaturvedi V, Vignali DAA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35– and IL-10–dependent manner. J Immunol 2009;182:6121–6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de la Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol 2004;34:2480–2488. [DOI] [PubMed] [Google Scholar]
- 53.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol 2007;8:1353–1362. [DOI] [PubMed] [Google Scholar]
- 54.Szymczak-Workman AL, Workman CJ, Vignali DAA. Cutting edge: regulatory T cells do not require stimulation through their TCR to suppress. J Immunol 2009;182:5188–5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han Y, Guo Q, Zhang M, Chen Z, Cao X. CD69+CD4+CD25− T Cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta1. J Immunol 2009;182:111–120. [DOI] [PubMed] [Google Scholar]
- 56.Zhao D, Zhang C, Yi T, Lin C-L, Todorov I, Kandeel F, Forman S, Zeng D. In vivo–activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood 2008;112:2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Minigo G, Woodberry T, Piera K, Salwati E, Tjitra E, Kenangalem E, Price R, Engwerda C, Anstey N, Plebanski M. Parasite-dependent expansion of TNF receptor II–positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog 2009;5:e1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernandez I, Zeiser R, Karsunky H, Kambham N, Beilhack A, Soderstrom K, Negrin RS, Engleman E. CD101 surface expression discriminates potency among murine FoxP3+ regulatory T cells. J Immunol 2007;179:2808–2814. [DOI] [PubMed] [Google Scholar]
- 59.Gregori S, Mangia P, Bacchetta R, Tresoldi E, Kolbinger F, Traversari C, Carballido JM, de Vries JE, Korthauer U, Roncarolo M-G. An anti-CD45RO/RB monoclonal antibody modulates T cell responses via induction of apoptosis and generation of regulatory T cells. J Exp Med 2005;201:1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Olffen RW, Koning N, van Gisbergen KPJM, Wensveen FM, Hoek RM, Boon L, Hamann J, van Lier RAW, Nolte MA. GITR triggering induces expansion of both effector and regulatory CD4+ T cells in vivo. J Immunol 2009;182:7490–7500. [DOI] [PubMed] [Google Scholar]
- 61.Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam K-P, Coyle AJ, Kroczek RA, Hutloff A. ICOS controls the pool size of effector-memory and regulatory T cells. J Immunol 2008;180:774–782. [DOI] [PubMed] [Google Scholar]
- 62.Semitekolou M, Alissafi T, Aggelakopoulou M, Kourepini E, Kariyawasam HH, Kay AB, Robinson DS, Lloyd CM, Panoutsakopoulou V, Xanthou G. Activin-A induces regulatory T cells that suppress T helper cell immune responses and protect from allergic airway disease. J Exp Med 2009;206:1769–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon VR, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick J, Renauld J-C, et al. IL-9 induces differentiation of Th17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci USA 2009;106:12885–12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.George JF, Braun A, Brusko TM, Joseph R, Bolisetty S, Wasserfall CH, Atkinson MA, Agarwal A, Kapturczak MH. Suppression by CD4+CD25+ regulatory T cells is dependent on expression of heme oxygenase–1 in antigen-presenting cells. Am J Pathol 2008;173:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hansen W, Loser K, Westendorf AM, Bruder D, Pfoertner S, Siewert C, Huehn J, Beissert S, Buer J. G protein–coupled receptor 83 overexpression in naive CD4+CD25− T cells leads to the induction of Foxp3+ regulatory T cells in vivo. J Immunol 2006;177:209–215. [DOI] [PubMed] [Google Scholar]
- 66.Pino-Lagos K, Benson MJ, Noelle RJ. Retinoic acid in the immune system. Ann N Y Acad Sci 2008;1143:170–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ji J, Cloyd MW. HIV-1 binding to CD4 on CD4+CD25+ regulatory T cells enhances their suppressive function and induces them to home to, and accumulate in, peripheral and mucosal lymphoid tissues: an additional mechanism of immunosuppression. Int Immunol 2009;21:283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wohler J, Bullard D, Schoeb T, Barnum S. LFA-1 is critical for regulatory T cell homeostasis and function. Mol Immunol 2009;46:2424–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kitamura N, Murata S, Ueki T, Mekata E, Reilly RT, Jaffee EM, Tani T. OX40 costimulation can abrogate Foxp3+ regulatory T cell–mediated suppression of antitumor immunity. Int J Cancer 2009;125:630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Franceschini D, Paroli M, Francavilla V, Videtta M, Morrone S, Labbadia G, Cerino A, Mondelli MU, Barnaba V. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J Clin Invest 2009;119:551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hartigan-O'Connor DJ, Poon C, Sinclair E, McCune JM. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods 2007;319:41–52. [DOI] [PubMed] [Google Scholar]
- 72.Laderach D, Movassagh M, Johnson A, Mittler RS, Galy A. 4-1BB co-stimulation enhances human CD8+ T cell priming by augmenting the proliferation and survival of effector CD8+ T cells. Int Immunol 2002;14:1155–1167. [DOI] [PubMed] [Google Scholar]
- 73.Gereke M, Jung S, Buer J, Bruder D. Alveolar type II epithelial cells present antigen to CD4+ T cells and induce Foxp3+ regulatory T cells. Am J Respir Crit Care Med 2009;179:344–355. [DOI] [PubMed] [Google Scholar]
- 74.Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol 2008;8:142–152. [DOI] [PubMed] [Google Scholar]
- 75.Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T–cell response? Immunology 2007;123:326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Azuma T, Takahashi T, Kunisato A, Kitamura T, Hirai H. Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res 2003;63:4516–4520. [PubMed] [Google Scholar]
- 77.Jiang S, Game DS, Davies D, Lombardi G, Lechler R. Activated CD1d-restricted natural killer T cells secrete IL-2: innate help for CD4+CD25+ regulatory T cells? Eur J Immunol 2005;35:1193–1200. [DOI] [PubMed] [Google Scholar]
- 78.Zhao D-M, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood 2006;107:3925–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+CD25+ regulatory T cells. J Immunol 2005;175:4180–4183. [DOI] [PubMed] [Google Scholar]
- 80.Yanaba K, Bouaziz J-D, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell–dependent inflammatory responses. Immunity 2008;28:639–650. [DOI] [PubMed] [Google Scholar]
- 81.Meiler F, Klunker S, Zimmermann M, Akdis CA, Akdis M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and Toll-like receptors. Allergy 2008;63:1455–1463. [DOI] [PubMed] [Google Scholar]
- 82.Suto A, Nakajima H, Ikeda K, Kubo S, Nakayama T, Taniguchi M, Saito Y, Iwamoto I. CD4+CD25+ T-cell development is regulated by at least 2 distinct mechanisms. Blood 2002;99:555–560. [DOI] [PubMed] [Google Scholar]
- 83.Lewkowicz P, Lewkowicz N, Sasiak A, Tchorzewski H. Lipopolysaccharide-activated CD4+CD25+ T regulatory cells inhibit neutrophil function and promote their apoptosis and death. J Immunol 2006;177:7155–7163. [DOI] [PubMed] [Google Scholar]
- 84.Mahajan D, Wang Y, Qin X, Wang Y, Zheng G, Wang YM, Alexander SI, Harris DCH. CD4+CD25+ regulatory T cells protect against injury in an innate murine model of chronic kidney disease. J Am Soc Nephrol 2006;17:2731–2741. [DOI] [PubMed] [Google Scholar]
- 85.Feleszko W, Jaworska J, Rha RD, Steinhausen S, Avagyan A, Jaudszus A, Ahrens B, Groneberg DA, Wahn U, Hamelmann E. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory–dependent mechanisms in a murine model of asthma. Clin Exp Allergy 2007;37:498–505. [DOI] [PubMed] [Google Scholar]
- 86.Bashir MEH, Andersen P, Fuss IJ, Shi HN, Nagler-Anderson C. An enteric helminth infection protects against an allergic response to dietary antigen. J Immunol 2002;169:3284–3292. [DOI] [PubMed] [Google Scholar]
- 87.Kitagaki K, Businga TR, Racila D, Elliott DE, Weinstock JV, Kline JN. Intestinal helminths protect in a murine model of asthma. J Immunol 2006;177:1628–1635. [DOI] [PubMed] [Google Scholar]
- 88.Karimi K, Inman MD, Bienenstock J, Forsythe P. Lactobacillus reuteri–induced regulatory T cells protect against an allergic airway response in mice. Am J Respir Crit Care Med 2009;179:186–193. [DOI] [PubMed] [Google Scholar]
- 89.Dittrich AM, Erbacher A, Specht S, Diesner F, Krokowski M, Avagyan A, Stock P, Ahrens B, Hoffmann WH, Hoerauf A, et al. Helminth infection with Litomosoides sigmodontis induces regulatory T cells and inhibits allergic sensitization, airway inflammation, and hyperreactivity in a murine asthma model. J Immunol 2008;180:1792–1799. [DOI] [PubMed] [Google Scholar]
- 90.Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, Kemeny DM, Bowen G, Rook G, Walker C. Suppression of airway eosinophilia by killed Mycobacterium vaccae–induced allergen-specific regulatory T-cells. Nat Med 2002;8:625–629. [DOI] [PubMed] [Google Scholar]
- 91.Trujillo-Vargas CM, Werner-Klein M, Wohlleben G, Polte T, Hansen G, Ehlers S, Erb KJ. Helminth-derived products inhibit the development of allergic responses in mice. Am J Respir Crit Care Med 2007;175:336–344. [DOI] [PubMed] [Google Scholar]
- 92.Wohlleben G, Trujillo C, Muller J, Ritze Y, Grunewald S, Tatsch U, Erb KJ. Helminth infection modulates the development of allergen-induced airway inflammation. Int Immunol 2004;16:585–596. [DOI] [PubMed] [Google Scholar]
- 93.Mo HM, Lei JH, Jiang ZW, Wang CZ, Cheng YL, Li YL, Liu WQ. Schistosoma japonicum infection modulates the development of allergen-induced airway inflammation in mice. Parasitol Res 2008;103:1183–1189. [DOI] [PubMed] [Google Scholar]
- 94.Baumgart M, Tompkins F, Leng J, Hesse M. Naturally occurring CD4+Foxp3+ regulatory T cells are an essential, IL-10–independent part of the immunoregulatory network in Schistosoma mansoni egg–induced inflammation. J Immunol 2006;176:5374–5387. [DOI] [PubMed] [Google Scholar]
- 95.Preston JA, Essilfie A-T, Horvat JC, Wade MA, Beagley KW, Gibson PG, Foster PS, Hansbro PM. Inhibition of allergic airways disease by immunomodulatory therapy with whole killed Streptococcus pneumoniae. Vaccine 2007;25:8154–8162. [DOI] [PubMed] [Google Scholar]
- 96.Fenoy I, Giovannoni M, Batalla E, Martin V, Frank F, Piazzon I, Goldman A. Toxoplasma gondii infection blocks the development of allergic airway inflammation in BALB/c mice. Clin Exp Allergy 2009;155:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol 2008;8:218–230. [DOI] [PubMed] [Google Scholar]
- 98.Stephens R, Randolph DA, Huang G, Holtzman MJ, Chaplin DD. Antigen-nonspecific recruitment of Th2 cells to the lung as a mechanism for viral infection–induced allergic asthma. J Immunol 2002;169:5458–5467. [DOI] [PubMed] [Google Scholar]
- 99.Mehlhop P, van de Rijn M, Goldberg A, Brewer J, Kurup V, Martin T, Oettgen H. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proc Natl Acad Sci USA 1997;94:1344–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arkwright PD, David TJ. Intradermal administration of a killed Mycobacterium vaccae suspension (SRL 172) is associated with improvement in atopic dermatitis in children with moderate-to-severe disease. J Allergy Clin Immunol 2001;107:531–534. [DOI] [PubMed] [Google Scholar]
- 101.Vargas MH., Bernal-Alcántara DA, Vaca MA, Franco-Marina F, Lascurain R. Effect of BCG vaccination in asthmatic schoolchildren. Pediatr Allergy Immunol 2004;15:415–420. [DOI] [PubMed] [Google Scholar]
- 102.Tang M. Probiotics and prebiotics: immunological and clinical effects in allergic disease. Nestle Nutr Workshop Ser Pediatr Program 2009;64:219–235. [DOI] [PubMed] [Google Scholar]
- 103.Camporota L, Corkhill A, Long H, Lordan J, Stanciu L, Tuckwell N, Cross A, Stanford JL, Rook GAW, Holgate ST, et al. The effects of Mycobacterium vaccae on allergen-induced airway responses in atopic asthma. Eur Respir J 2003;21:287–293. [DOI] [PubMed] [Google Scholar]
- 104.Berth-Jones J, Arkwright PD, Marasovic D, Savani N, Aldridge CR, Leech SN, Morgan C, Clark SM, Ogilvie S, Chopra S, et al. Killed Mycobacterium vaccae suspension in children with moderate-to-severe atopic dermatitis: a randomized, double-blind, placebo-controlled trial. Clin Exp Allergy 2006;36:1115–1121. [DOI] [PubMed] [Google Scholar]
- 105.Brothers S, Asher MI, Jaksic M, Stewart AW. Effect of a Mycobacterium vaccae derivative on paediatric atopic dermatitis: a randomized, controlled trial. Clin Exp Dermatol 2009;34:770–775. [DOI] [PubMed] [Google Scholar]
- 106.Lloyd CM, Hawrylowicz CM, Regulatory T. Cells in Asthma. Immunity 2009;31:438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol 2007;119:780–789. [DOI] [PubMed] [Google Scholar]
- 108.O'Hehir RE, Gardner LM, de Leon MP, Hales BJ, Biondo M, Douglass JA, Rolland JM, Sandrini A. House dust mite sublingual immunotherapy: the role for transforming growth factor–β and functional regulatory T cells. Am J Respir Crit Care Med 2009;180:936–947. [DOI] [PubMed] [Google Scholar]
- 109.Rolland JM, Gardner LM, O'Hehir RE. Allergen-related approaches to immunotherapy. Pharmacol Ther 2009;121:273–284. [DOI] [PubMed] [Google Scholar]
- 110.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O'Garra A. In vitro generation of interleukin 10–producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)– and Th2-inducing cytokines. J Exp Med 2002;195:603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, Adikibi T, Pridgeon C, Dallman M, Loke T-K, et al. Reversing the defective induction of IL-10 secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest 2006;116:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]