Abstract
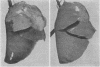
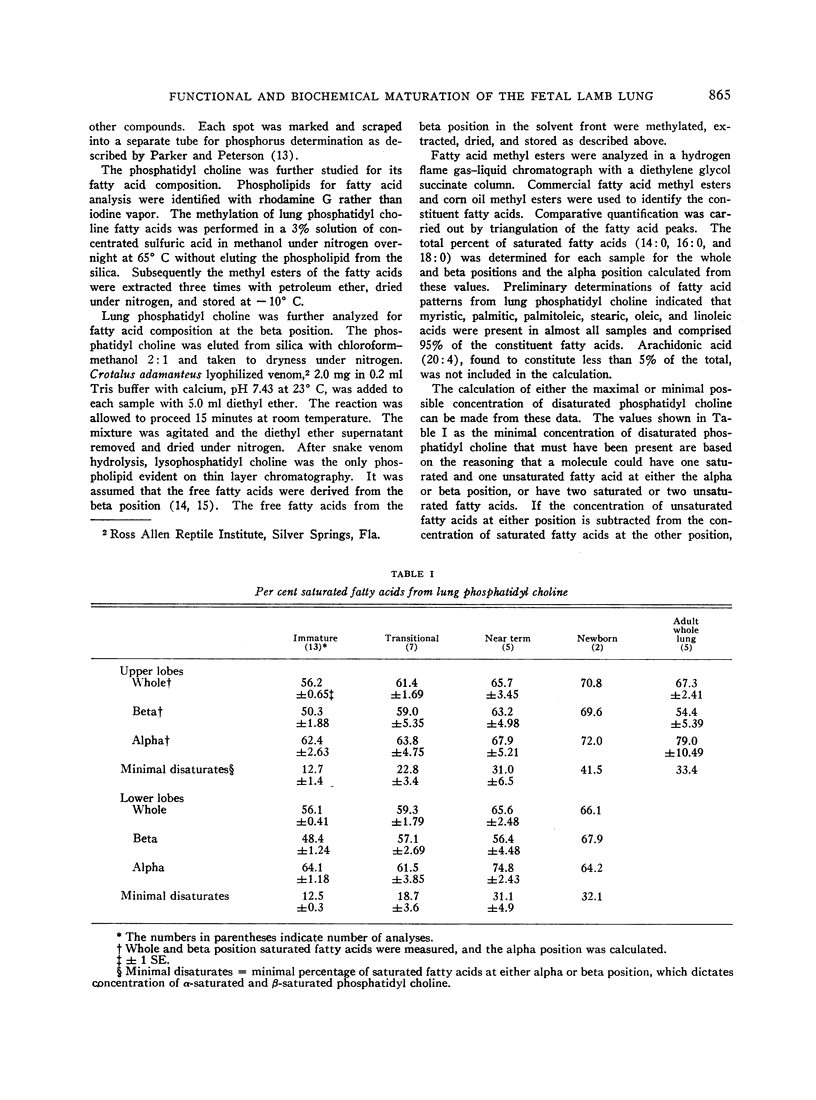
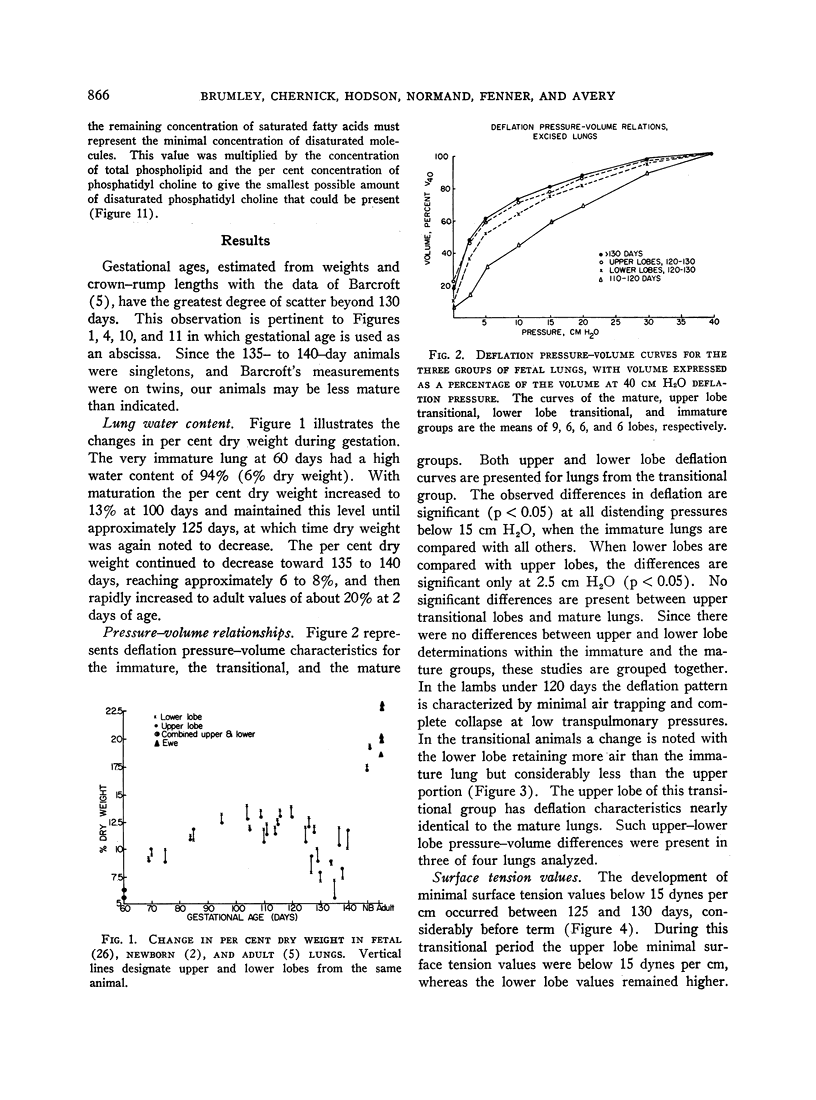
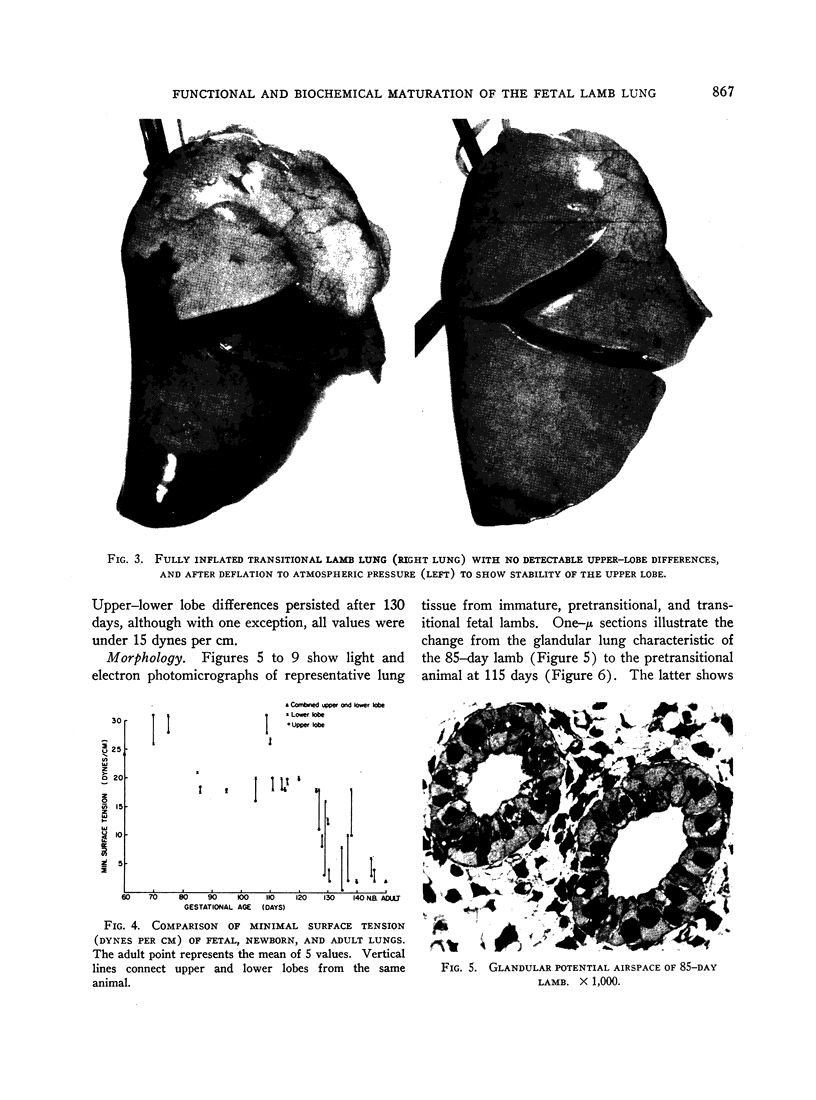
Pressure-volume characteristics and surface tension measurements of the lamb of 120 to 130 days gestational age were typical of the mature lung in the upper lobes and the immature lung in the lower lobes. By term both upper and lower lobes had findings characteristic of the mature animal.
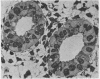
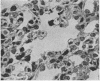
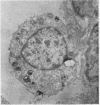
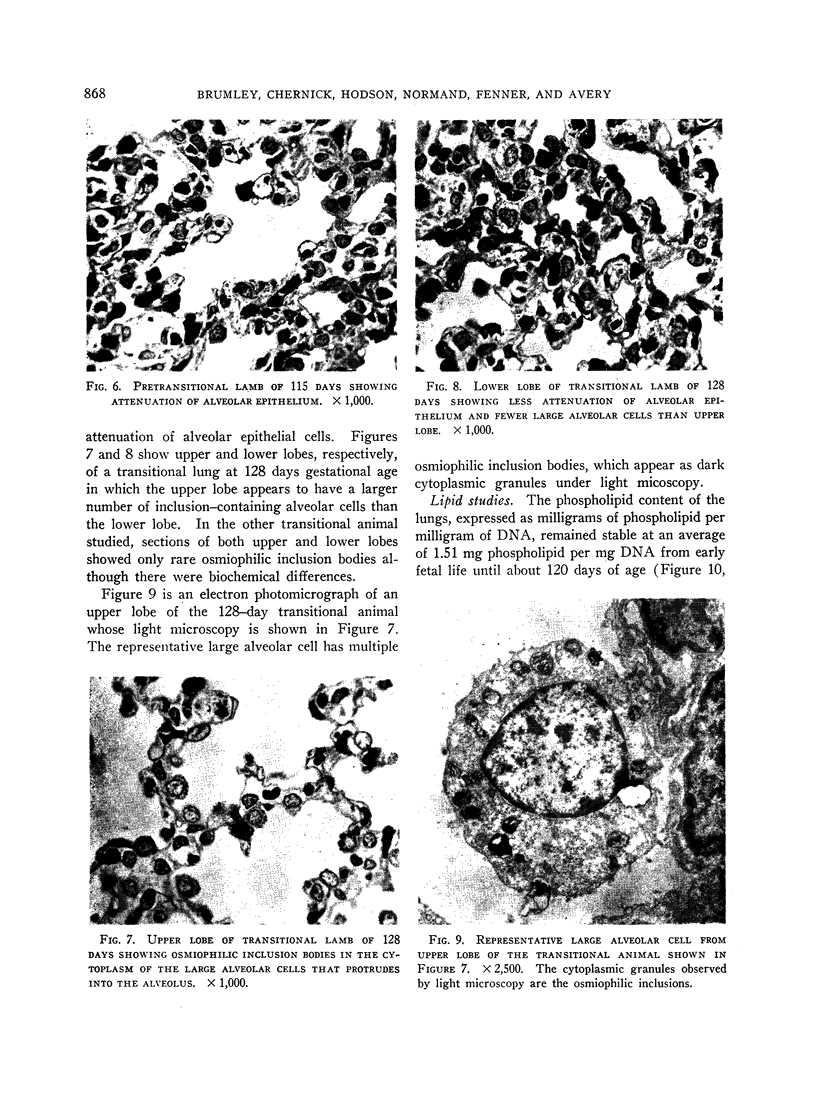
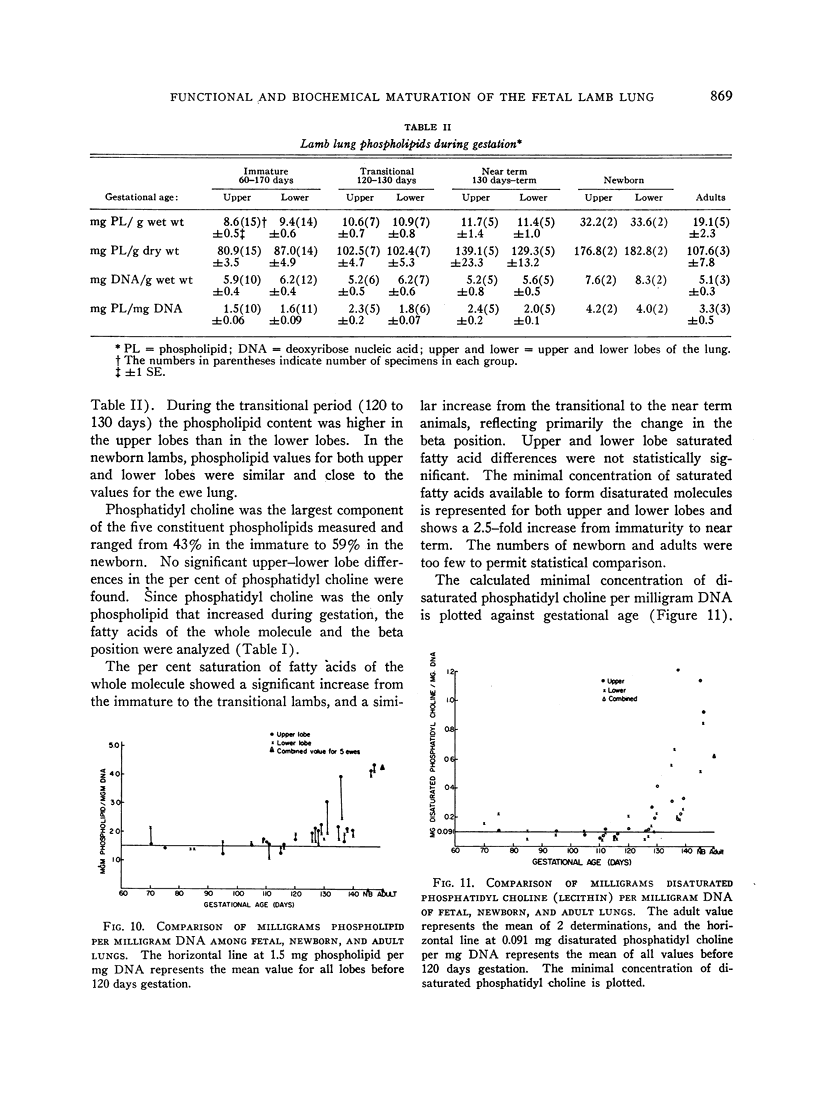
Phospholipid concentration per milligram DNA and per cent saturated fatty acids on pulmonary phosphatidyl choline were relatively constant from 60 to 120 days gestational age; thereafter there was a significant increase in both measurements. These changes usually coincided with an increase in osmiophilic inclusion bodies in the large alveolar cell.
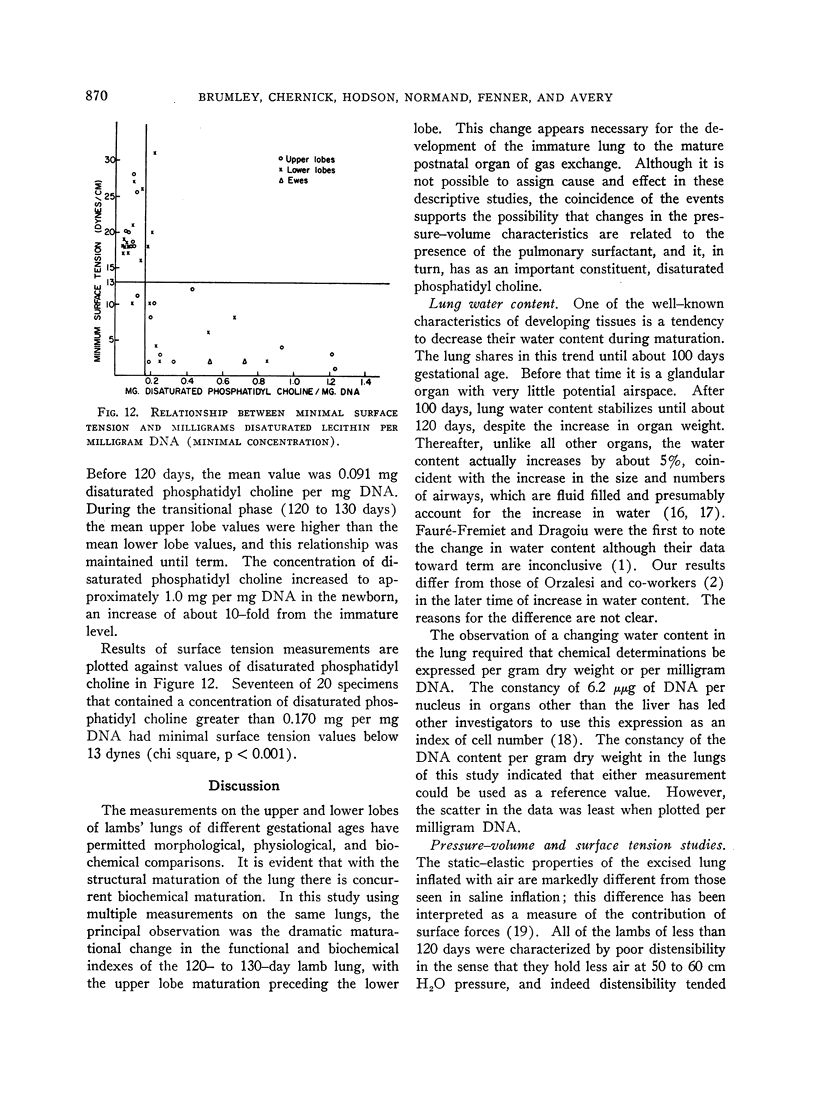
A concentration of disaturated phosphatidyl choline per milligram DNA in excess of 0.170 mg per mg was associated with a minimal surface tension below 13 dynes per cm (p < 0.001). Newborn animal lungs contained over 3 times this critical concentration, whereas adult lungs contained 1.5 times this value. The excess disaturated phosphatidyl choline per milligram DNA may represent a reservoir of pulmonary surfactant.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS F. H., FUJIWARA T., ROWSHAN G. THE NATURE AND ORIGIN OF THE FLUID IN THE FETAL LAMB LUNG. J Pediatr. 1963 Nov;63:881–888. doi: 10.1016/s0022-3476(63)80218-8. [DOI] [PubMed] [Google Scholar]
- AVERY M. E., COOK C. D. Volume-pressure relationships of lungs and thorax in fetal, newborn, and adult goats. J Appl Physiol. 1961 Nov;16:1034–1038. doi: 10.1152/jappl.1961.16.6.1034. [DOI] [PubMed] [Google Scholar]
- BALIS J. U., CONEN P. E. THE ROLE OF ALVEOLAR INCLUSION BODIES IN THE DEVELOPING LUNG. Lab Invest. 1964 Oct;13:1215–1229. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BENSCH K., SCHAEFER K., AVERY M. E. GRANULAR PNEUMOCYTES: ELECTRON MICROSCOPIC EVIDENCE OF THEIR EXOCRINIC FUNCTION. Science. 1964 Sep 18;145(3638):1318–1319. doi: 10.1126/science.145.3638.1318-a. [DOI] [PubMed] [Google Scholar]
- BORN G. V., DAWES G. S., MOTT J. C. The viability of premature lambs. J Physiol. 1955 Oct 28;130(1):191–212. doi: 10.1113/jphysiol.1955.sp005403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN E. S. ISOLATION AND ASSAY OF DIPALMITYL LECITHIN IN LUNG EXTRACTS. Am J Physiol. 1964 Aug;207:402–406. doi: 10.1152/ajplegacy.1964.207.2.402. [DOI] [PubMed] [Google Scholar]
- BUCKINGHAM S., AVERY M. E. Time of appearance of lung surfactant in the foetal mouse. Nature. 1962 Feb 17;193:688–689. doi: 10.1038/193688a0. [DOI] [PubMed] [Google Scholar]
- BUCKINGHAM S., MCNARY W. F., Jr, SOMMERS S. C. PULMONARY ALVEOLAR CELL INCLUSIONS: THEIR DEVELOPMENT IN THE RAT. Science. 1964 Sep 11;145(3637):1192–1193. doi: 10.1126/science.145.3637.1192. [DOI] [PubMed] [Google Scholar]
- Blank M. L., Nutter L. J., Privett O. S. Determination of the structure of lecithins. Lipids. 1966 Mar;1(2):132–135. doi: 10.1007/BF02533005. [DOI] [PubMed] [Google Scholar]
- CLEMENTS J. A. Surface phenomena in relation to pulmonary function. Physiologist. 1962 Feb;5:11–28. [PubMed] [Google Scholar]
- FELTS J. M. BIOCHEMISTRY OF THE LUNG. Health Phys. 1964 Dec;10:973–979. doi: 10.1097/00004032-196412000-00016. [DOI] [PubMed] [Google Scholar]
- FOLCH J., ASCOLI I., LEES M., MEATH J. A., LeBARON N. Preparation of lipide extracts from brain tissue. J Biol Chem. 1951 Aug;191(2):833–841. [PubMed] [Google Scholar]
- Howatt W. F., Avery M. E., Humphreys P. W., Normand I. C., Reid L., Strang L. B. Factors affecting pulmonary surface properties in the foetal lamb. Clin Sci. 1965 Oct;29(2):239–248. [PubMed] [Google Scholar]
- JOHNSON J. W., PERMUTT S., SIPPLE J. H., SALEM E. S. EFFECT OF INTRA-ALVEOLAR FLUID ON PULMONARY SURFACE TENSION PROPERTIES. J Appl Physiol. 1964 Jul;19:769–777. doi: 10.1152/jappl.1964.19.4.769. [DOI] [PubMed] [Google Scholar]
- KLAUS M., REISS O. K., TO OLEY W. H., PIEL C., CLEMENTS J. A. Alveolar epithelial cell mitochondria as source of the surface-active lung lining. Science. 1962 Sep 7;137(3532):750–751. doi: 10.1126/science.137.3532.750. [DOI] [PubMed] [Google Scholar]
- Kikkawa Y., Motoyama E. K., Cook C. D. The ultrastructure of the lungs of lambs. The relation of osmiophilic inclusions and alveolar lining layer to fetal maturation and experimentally produced respiratory distress. Am J Pathol. 1965 Nov;47(5):877–903. [PMC free article] [PubMed] [Google Scholar]
- ORZALESI M. M., MOTOYAMA E. K., JACOBSON H. N., KIKKAWA Y., REYNOLDS E. O., COOK C. D. THE DEVELOPMENT OF THE LUNGS OF LAMBS. Pediatrics. 1965 Mar;35:373–381. [PubMed] [Google Scholar]
- ROBERTSON A. F., LANDS W. E. Positional specificites in phospholipid hydrolyses. Biochemistry. 1962 Sep;1:804–810. doi: 10.1021/bi00911a012. [DOI] [PubMed] [Google Scholar]
- SKIPSKI V. P., PETERSON R. F., SANDERS J., BARCLAY M. THIN-LAYER CHROMATOGRAPHY OF PHOSPHOLIPIDS USING SILICA GEL WITHOUT CALCIUM SULFATE BINDER. J Lipid Res. 1963 Apr;4:227–228. [PubMed] [Google Scholar]
- Weinhold P. A., Villee C. A. Phospholipid metabolism in the liver and lung of rats during development. Biochim Biophys Acta. 1965 Dec 2;106(3):540–550. doi: 10.1016/0005-2760(65)90070-6. [DOI] [PubMed] [Google Scholar]