Abstract
How arsenic (As) is transported in phloem remains unknown. To help answer this question, we quantified the chemical species of As in phloem and xylem exudates of castor bean (Ricinus communis) exposed to arsenate [As(V)], arsenite [As(III)], monomethylarsonic acid [MMA(V)], or dimethylarsinic acid. In the As(V)- and As(III)-exposed plants, As(V) was the main species in xylem exudate (55%–83%) whereas As(III) predominated in phloem exudate (70%–94%). The ratio of As concentrations in phloem to xylem exudate varied from 0.7 to 3.9. Analyses of phloem exudate using high-resolution inductively coupled plasma-mass spectrometry and accurate mass electrospray mass spectrometry coupled to high-performance liquid chromatography identified high concentrations of reduced and oxidized glutathione and some oxidized phytochelatin, but no As(III)-thiol complexes. It is thought that As(III)-thiol complexes would not be stable in the alkaline conditions of phloem sap. Small concentrations of oxidized glutathione and oxidized phytochelatin were found in xylem exudate, where there was also no evidence of As(III)-thiol complexes. MMA(V) was partially reduced to MMA(III) in roots, but only MMA(V) was found in xylem and phloem exudate. Despite the smallest uptake among the four As species supplied to plants, dimethylarsinic acid was most efficiently transported in both xylem and phloem, and its phloem concentration was 3.2 times that in xylem. Our results show that free inorganic As, mainly As(III), was transported in the phloem of castor bean exposed to either As(V) or As(III), and that methylated As species were more mobile than inorganic As in the phloem.
Arsenic (As) is an environmental and food chain contaminant that has attracted much attention in recent years. Soil contamination with As may lead to phytotoxicity and reduced crop yield (Panaullah et al., 2009). Food crops are also an important source of inorganic As, a class-one carcinogen, in human dietary intake, and there is a need to decrease the exposure to this toxin (European Food Safety Authority, 2009). Paddy rice (Oryza sativa) is particularly efficient in As accumulation, which poses a potential risk to the population based on a rice diet (Meharg et al., 2009; Zhao et al., 2010a). Other terrestrial food crops generally do not accumulate as much As as paddy rice; however, where soils are contaminated, relatively high concentrations of As in wheat (Triticum aestivum) grain have been reported (Williams et al., 2007; Zhao et al., 2010b). On the other hand, some fern species in the Pteridaceae family are able to tolerate and hyperaccumulate As in the aboveground part to >1,000 mg kg−1 dry weight (e.g. Ma et al., 2001; Zhao et al., 2002); these plants offer the possibility for remediation of As-contaminated soil or water (Salido et al., 2003; Huang et al., 2004). A better understanding of As uptake and long-distance transport, metabolism, and detoxification is needed for developing strategies for mitigating As contamination, through either decreased As accumulation in food crops or enhanced As accumulation for phytoremediation.
The pathways of As uptake by plant roots differ between different As species; arsenate [As(V)] enters plant cells via phosphate transporters, whereas arsenite [As(III)] is taken up via some aquaporins (for review, see Zhao et al., 2009). In rice, a silicic acid efflux protein also mediates As(III) efflux toward stele for xylem loading (Ma et al., 2008). Methylated As species, such as monomethylarsonic acid [MMA(V)] and dimethylarsinic acid [DMA(V)], which may be present in the environment as products of microbial or algal methylation of inorganic As or from past uses of methylated As pesticides, are taken up by rice roots partly through the aquaporin NIP2;1 (for nodulin 26-like intrinsic protein; also named Lsi1; Li et al., 2009). Once inside plant cells, As(V) is reduced to As(III), possibly catalyzed by As(V) reductase(s) such as the plant homologs of the yeast (Saccharomyces cerevisiae) ACR2 (Bleeker et al., 2006; Dhankher et al., 2006; Ellis et al., 2006; Duan et al., 2007). As(III) has a high affinity to thiol (-SH) groups and is detoxified by complexation with thiol-rich phytochelatins (PCs; Pickering et al., 2000; Schmöger et al., 2000; Raab et al., 2005; Bluemlein et al., 2009; Liu et al., 2010). As(III)-PC complexation in roots was found to result in reduced mobility for efflux and for long-distance transport, possibly because the complexes are stored in the vacuoles (Liu et al., 2010). Excess As(III) causes cellular toxicity by binding to the vicinal thiol groups of enzymes, such as the plastidial lipoamide dehydrogenase, which has been shown to be a sensitive target of As toxicity (Chen et al., 2010). The As hyperaccumulating Pteris species differ from nonhyperaccumulating plants because of enhanced As(V) uptake (Wang et al., 2002; Poynton et al., 2004), little As(III)-thiol complexation (Zhao et al., 2003; Raab et al., 2004), and efficient xylem loading of As(III) (Su et al., 2008). Recently, an As(III) efflux transporter, PvACR3, has been found to play an important role in As(III) detoxification by transporting As(III) into vacuoles in Pteris vittata (Indriolo et al., 2010).
With the exception of As hyperaccumulators, most plant species have a limited root-to-shoot translocation of As (Zhao et al., 2009). The chemical species of As in xylem exudate have been determined in a number of plant species. As(III) was found to be the predominant species (80%–100%) in the xylem sap of rice, tomato (Solanum lycopersicum), cucumber (Cucumis sativus), and P. vittata even when these plants were fed As(V) (Mihucz et al., 2005; Xu et al., 2007; Ma et al., 2008; Su et al., 2010), suggesting that As(V) is reduced in roots before being loaded into the xylem. In other plant species, such as Brassica juncea (Pickering et al., 2000), wheat, and barley (Hordeum vulgare; Su et al., 2010), As(V) accounted for larger proportions (40%–50%) of the total As in the xylem sap. Studies using HPLC-inductively coupled plasma (ICP)-mass spectrometry (MS) coupled with electrospray (ES)-MS showed no evidence of As(III)-thiol complexation in the xylem sap of sunflower (Helianthus annuus; Raab et al., 2005). When rice plants were exposed to MMA(V) or DMA(V), both As species were found in the xylem sap (Li et al., 2009). Generally, methylated As species are taken up by roots at slower rates than inorganic As, but they are more mobile during the xylem transport from roots to shoots (Marin et al., 1992; Raab et al., 2007; Li et al., 2009).
It has been shown that phloem transport contributes substantially to As accumulation in rice grain (Carey et al., 2010). However, little is known about how As is transported in phloem (Zhao et al., 2009). There are no reports on the chemical species of As in phloem exudate. The speciation of As in phloem is important because it dictates how As is loaded in the source tissues and unloaded in the sink tissues, such as grain. Questions with regard to the oxidation state, methylation, and complexation of As in phloem sap remain to be answered. Unlike xylem sap, phloem sap is much more difficult to obtain in sufficient quantities for analysis. In this study, we investigated As speciation in phloem and xylem exudates of castor bean (Ricinus communis), which is widely used as a model plant to investigate phloem transport of solutes (e.g. Hall et al., 1971; Hall and Baker, 1972; Allen and Smith, 1986; Bromilow et al., 1987).
RESULTS
Sugar Composition in Phloem and Xylem Exudates of Castor Bean
The purity of phloem and xylem exudates was checked by analyzing the sugar composition. Phloem exudate contained high concentrations of Suc with the values similar to those reported before (Hall and Baker, 1972; Smith and Milburn, 1980), while reducing sugars were absent (Fru) or present at only trace levels (Glc; Table I). Xylem exudate contained very small concentrations of Suc, Fru, and Glc. These compositions are characteristic of phloem and xylem saps, suggesting little cross contamination.
Table I. The composition of sugars in phloem and xylem exudates of castor bean (mean ± se, n = 4).
| Exudate | Suc | Glc | Fru |
| mm | |||
| Phloem | 238 ± 15.8 | 0.12 ± 0.12 | 0 ± 0 |
| Xylem | 1.20 ± 0.32 | 0.29 ± 0.08 | 0.36 ± 0.11 |
As Speciation in Castor Bean Exposed to As(V) or As(III)
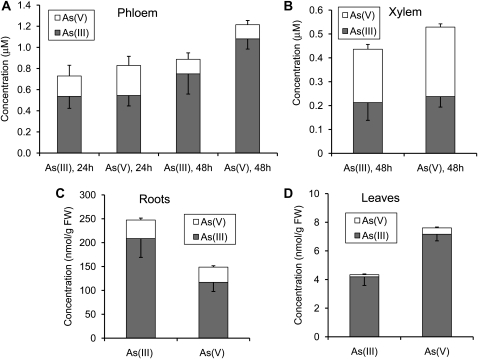
In the first experiment, 18-d-old plants were exposed to 10 μm As(III) or As(V) for 24 to 48 h. Total As concentration in phloem exudate increased slightly from 24 to 48 h after As exposure, from approximately 0.8 to 0.9 to 1.2 μm (Fig. 1A). The concentrations were not significantly different between the As(V) and As(III) treatments at either sampling times. The majority of As in phloem exudates was As(III), which accounted for 67% to 79% and 86% to 90% of the total As at 24 and 48 h, respectively, and the remainder was As(V). Again, there was no significant difference in this percentage between the As(V) and As(III) treatments. Xylem exudate was collected after 48 h exposure. Total As concentration in the xylem exudate was about half of that in the phloem exudate (Fig. 1B), with As(III) accounting for 44% in both treatments and the rest being As(V).
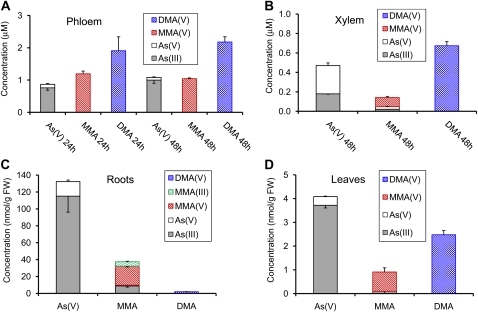
Figure 1.
As speciation in phloem exudate (A), xylem exudate (B), roots (C), and leaves (D) of castor bean exposed to 10 μm As(V) or As(III) for 24 or 48 h. Error bars are 1 se (n = 3–4).
Analysis of the plant samples collected after 48 h exposure showed much higher As concentrations in roots than in leaves (Fig. 1, C and D). Seventy-nine percent of the As in roots was As(III), compared with 96% in leaves, and there was no significant difference in this percentage between the As(III) and As(V) treatments. The sum of all As species detected in roots was smaller in the As(V) than in the As(III) treatment (P = 0.056), but the opposite was observed for the concentrations in leaves (P < 0.01). Total As concentrations were also determined by acid digestion followed by ICP-MS determination. The sum of As species determined by extraction with a phosphate buffer solution (PBS) and HPLC-ICP-MS analysis represented 81% and 43% of the total As in roots and shoots, respectively.
A considerable proportion (43% at 24 h) of As(III) in the nutrient solution was oxidized to As(V), whereas 9% of the As in the As(V) treatment was reduced to As(III) (data not shown). It has been shown that plant roots extrude As(III) to the external medium following As(V) uptake and reduction (Xu et al., 2007). Conversely, As(III) in the medium may be oxidized to As(V) because the nutrient solution was continuously aerated. The partial interconversion of As species in the medium may partly explain the lack of significant differences between the As(V) and As(III) treatments.
As Speciation in Castor Bean Exposed to Increasing Concentration of As(V)
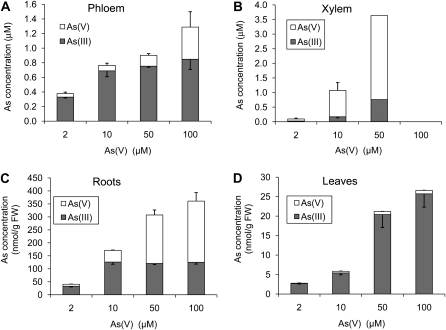
Total As concentration in phloem exudate collected 24 h after As(V) exposure increased with the increasing As(V) concentration in the nutrient solution (P < 0.001; Fig. 2A). The proportion of As(III) varied little (84%–90%) in the 2 to 50 μm As(V) treatment, but decreased to 71% in the 100 μm As(V) treatment. Xylem exudate could only be collected from the low As(V) treatments (2–10 μm) and from one of the four replicates of the 50 μm As(V)-treated plants; As toxicity at high As(V) concentrations might have affected water uptake and/or xylem exudation. As(V) was the main As species in the xylem exudate, accounting for 67% and 79% of the total As in the 2 and 10 μm treatments, respectively, with the rest being As(III) (Fig. 2B). The ratio of phloem to xylem As concentrations was 3.9 and 0.7 in the 2 and 10 μm treatments, respectively. No As species were detected in either xylem or phloem exudate from the control plants in the 0 μm As(V) treatment.
Figure 2.
As speciation in phloem exudate (A), xylem exudate (B), roots (C), and leaves (D) of castor bean exposed to 2 to 100 μm As(V) for 24 h. Error bars are 1 se (n = 3–4, except the 50 μm treatment in which only one replicate produced xylem exudate; the 100 μm treatment produced no xylem exudate).
As concentrations in roots and leaves increased with the external As(V) concentrations (Fig. 2, C and D). Most of the As in leaves (96%) was As(III) and the proportion was not significantly affected by the concentration of As(V) in the medium. In contrast, the proportion of As(III) in roots decreased progressively with the concentration of As(V) exposure, from 81% at 2 μm to 36% at 100 μm As(V). In fact, while the concentration of As(V) in roots increased with the As(V) exposure concentration, that of As(III) did not increase beyond the 10 μm As(V) treatment. In this experiment, the extraction and As speciation method recovered on average 84% and 59% of the total As in roots and leaves, respectively.
Phosphate Supply Affects As Concentration But Not Speciation in Phloem and Xylem Exudates of Castor Bean
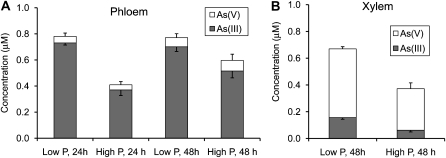
In this experiment, castor bean plants were exposed to 10 μm As(V) with either 10 or 200 μm phosphate. Phloem exudate was dominated by As(III), which accounted for 89% to 94% of the total As at 24 and 48 h, and the level of phosphate supply had no significant effect on this percentage (P > 0.80; Fig. 3A). Increasing phosphate from 10 to 200 μm did significantly decrease the concentration of As(III) in phloem exudate, by 50% (P < 0.001) and 26% (P < 0.05) at 24 and 48 h, respectively. In contrast, xylem exudate was dominated by As(V), with As(III) accounting for only 17% to 23% of the total As (Fig. 3B). The concentrations of As(V) and As(III) in xylem exudate were decreased by 40% (P < 0.05) and 60% (P < 0.01), respectively, by increasing phosphate concentration in the medium. The percentage of As(III) was not significantly affected. At 48 h, total As concentrations in phloem exudate were 15% to 60% higher than those in xylem exudate.
Figure 3.
As speciation in phloem exudate (A) and xylem exudate (B) of castor bean exposed to 10 μm As(V) with either 10 μm (low P) or 200 μm phosphate (high P) for 24 or 48 h. Error bars are 1 se (n = 3–4).
No Evidence of As(III)-Thiol Complexation in the Phloem and Xylem Exudates of Castor Bean
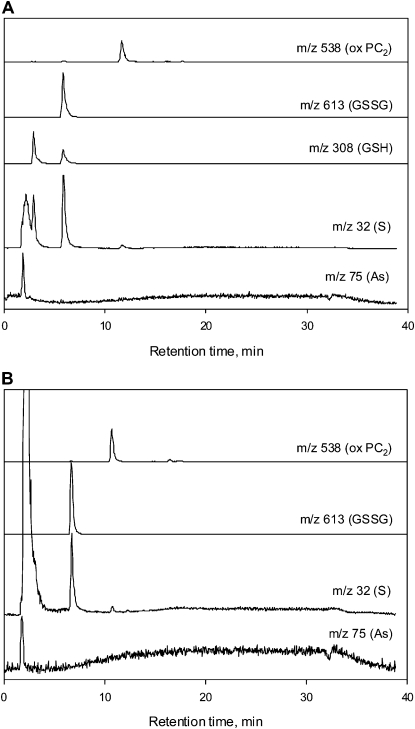
Phloem and xylem exudates collected after 48 h exposure to 10 μm As(V) were analyzed for As-thiol complexes and free thiol compounds using reverse-phase HPLC high-resolution ICP-MS/high-resolution ES-MS. The use of high-resolution ICP-MS allows quantification of sulfur (S; mass-to-charge ratio [m/z] 32), which is not possible with normal ICP-MS due to the polyatomic interference of O2 (m/z 32), in addition to simultaneous quantification of As (m/z 75). In addition, the use of high-resolution ES-MS allows molecular identification of the compounds. In both phloem and xylem exudate samples, As was eluted in the void volume corresponding to inorganic As; also eluted was sulfate. No As-thiol complexes were detected in either exudate (Fig. 4, A and B). High concentrations of reduced and oxidized glutathione (GSH and GSSG) were found in the phloem exudate, as well as some oxidized PC (PC2; Table II). GSSG and oxidized PC2 were detected in the xylem exudate, but at much lower concentrations than those in the phloem exudate. No GSH was present in the xylem exudate. Inorganic S (sulfate) was abundant in both exudates.
Figure 4.
Overlaid reverse-phase HPLC chromatograms for the phloem (A) and xylem (B) exudates detected online simultaneously by using ICP-MS (m/z 32 and 75) and ES-MS (m/z 308, 538, and 613 [M + H]+). The S trace (m/z 32) shows the relative quantity of the inorganic S compounds eluting near the void at 2 min compared to GSSG and oxidized PC2.
Table II. The concentrations of sulfate, GSH, and GSSG and oxidized PC2 in phloem and xylem exudates of castor bean exposed to 10 μm As(V) for 48 h (mean ± se, n = 2).
| Exudate | S Species | |||
| Sulfate | GSH | GSSG | Oxidized PC2 | |
| nmol g−1 | ||||
| Phloem | 141 ± 56 | 64 ± 33 | 22 ± 1.4 | 1.2 ± 0.7 |
| Xylem | 131 ± 8 | 0 ± 0 | 5.8 ± 0.8 | 0.2 ± 0.01 |
Methylated As Has a Greater Mobility in Phloem and Xylem Than Inorganic As
In this experiment, As speciation and the relative mobility were compared among plants exposed to 10 μm As(V), MMA(V), or DMA(V). Only inorganic As species were found in the phloem exudate from the As(V)-treated plants, with As(III) being the predominant species (88% and 92% at 24 and 48 h, respectively; Fig. 5A). In the MMA(V)-treated plants, MMA(V) was the only species detected in the phloem exudate, while only DMA(V) was found in the DMA(V)-treated plants. As concentrations in phloem exudate were similar in both 24 and 48 h samples. Among the three treatments, As concentration in phloem exudate was the highest in the DMA(V) treatment (P < 0.001; Fig. 5A).
Figure 5.
As speciation in phloem exudate (A), xylem exudate (B), roots (C), and leaves (D) of castor bean exposed to 10 μm As(V), MMA(V), or DMA(V) for 24 or 48 h. Error bars are 1 se (n = 3–4). FW, Fresh weight. [See online article for color version of this figure.]
In xylem exudate, total As concentration followed the order of treatment of MMA(V) < As(V) < DMA(V) (Fig. 5B). The xylem exudate from the As(V)-treated plants contained 38% and 62% As(III) and As(V), respectively. The xylem exudate from the MMA(V)-treated plants contained 67% MMA(V), 15% As(III), and 18% As(V). However, the MMA(V) stock solution used in the experiment contained a small amount (approximately 3%) of As(V) as an impurity, which likely explains the occurrence of As(V) and As(III) in the xylem exudate from the MMA(V)-treated plants. Only DMA(V) was found in the xylem exudate of the DMA(V)-treated plants. The ratios of As concentration in phloem exudate to that in xylem exudate were 2.3, 7.3, and 3.2 in the As(V), MMA(V), and DMA(V) treatments, respectively.
The concentrations and species of As in roots and leaves differed between the three treatments (Fig. 5, C and D). Similar to the experiments described above, As(III) was the dominant species in roots (87%) and leaves (91%) of the As(V)-treated plants, with As(V) being the minor As species. As speciation in the roots of the MMA(V)-treated plants was more complex, showing the presence of MMA(V), MMA(III), As(III), and As(V) with percentages of 60%, 14%, 23%, and 3%, respectively (Fig. 5C; Supplemental Fig. S1). In the leaves of this treatment, As species comprised 89% and 11% MMA(V) and As(III), respectively. In the plants exposed to DMA(V), only DMA(V) was found in both roots and leaves. The MMA(V)-treated plants had smaller (by about 75%) concentrations of total As (sum of all As species) in roots and leaves than the As(V)-treated plants. In the DMA(V)-treated plants, total As concentrations in roots and leaves were 2% and 61%, respectively, of those in the As(V)-treated plants.
In this experiment, the sum of As species accounted for 40% to 60% of the total As concentration by acid digestion, with the exception of a complete extraction (105%) in the DMA(V)-treated leaves. Based on the total As concentrations by acid digestion, uptake of As during the 48 h exposure were 358, 80, and 8.5 nmol g−1 root fresh weight in the As(V), MMA(V), and DMA(V) treatments, respectively.
DISCUSSION
This study was motivated by the gap of knowledge of As speciation in phloem exudate, which has important implications for how As is redistributed around the plant, especially toward the seeds (Zhao et al., 2009). Castor bean plants were exposed to four different As species, and As speciation in phloem exudate was compared to that in xylem exudate and roots and leaves.
In the plants exposed to either As(V) or As(III), As(III) was found to be the predominant As species in phloem exudate and As(V) the minor species. The proportion of As(III) varied somewhat among different experiments and was also influenced by the concentrations of As(V) in the external solution, but generally fell within the range of 80% to 90% (Figs. 1, 2, 3, and 5). The proportion dropped to around 70% when plants were exposed to a high concentration (100 μm) of As(V). Furthermore, inorganic As in the phloem exudate was not complexed with thiol compounds, even though the concentrations of GSH, GSSG, and PC2 far exceeded that of inorganic As (Fig. 4; Table II). The lack of thiolated As is not an analytical artifact, because the extraction and the chromatographic separation has been validated by using x-ray absorption spectrometry of plant tissues directly (Bluemlein et al., 2008). This lack of thiol complexation may be explained by the high pH of phloem sap in castor bean (7.4–8.2; Hall and Baker, 1972; Allen and Smith, 1986). It is known that As(III) forms stable complexes with thiol compounds under acidic conditions but not in neutral-alkaline environments (Schmöger et al., 2000). A number of As(III)-thiol complexes have been identified in roots and shoots of different plant species (Raab et al., 2004, 2005; Liu et al., 2010), while x-ray absorption studies indicate that most of As(III) in nonhyperaccumulating plant species is bonded with S (Pickering et al., 2000; Dhankher et al., 2002). As(III)-thiol complexes are likely to be stored in vacuoles where the acidic pH favors the stability of the complexes. Increasing As(III)-thiol complexation was found to decrease xylem transport of As(III) (Liu et al., 2010), and may also affect As(III) transport in phloem in the same way. Given that uncomplexed As(III) or at least only weakly complexed As(III), which is not detectable by chromatographic methods (Feldmann et al., 2009), and, to a smaller extent, As(V), are the only species of As in phloem exudate, transporters for these species are likely to be involved in the loading and unloading during phloem transport, e.g. some of the aquaporins, Lsi2-like or ACR3-like proteins for As(III), and phosphate transporters for As(V).
Similar to our study, thiol compounds (mainly glutathione and PC2) were found to be abundant in the phloem sap from Brassica napus exposed to cadmium (Cd; Mendoza-Cózatl et al., 2008). Experiments with grafting and shoot-specific expression of the genes involved in glutathione synthesis have shown that glutathione and PCs are transported via phloem from shoots to roots of Arabidopsis (Arabidopsis thaliana; Chen et al., 2006; Li et al., 2006). Unlike As, it is possible that Cd-PC complexes are transported in phloem (Mendoza-Cózatl et al., 2008), because these complexes are stable in the slightly alkaline conditions of phloem sap, but not in acidic environments (Johanning and Strasdeit, 1998).
Different from phloem exudate, more As(V) than As(III) was found in xylem exudate of castor bean, with the former typically accounting for 60% to 80% of the total As (Figs. 1, 2, 3, and 5). There was also no evidence of As(III)-thiol complexes (Fig. 4), even though xylem sap pH (approximately 5.0) in castor bean (Allen and Smith, 1986) is favorable to the stability of As(III)-thiol complexes. Small concentrations of GSSG and oxidized PC2 were found in xylem sap of castor bean (Table II), which was consistent with a previous study on sunflower (Raab et al., 2005). The study of Raab et al. (2005) also found no evidence of As(III)-thiol complexation in the xylem sap of sunflower. The presence of only low concentrations of nonreactive (oxidized) thiols in xylem sap probably explains the lack of As(III)-thiol complexation. Similarly, Mendoza-Cózatl et al. (2008) found small concentrations of glutathione and traces of PC2 in the xylem sap of B. napus exposed to Cd, although the analytical method used did not separate oxidized from reduced thiols.
Previous studies have shown that As(III) dominates in the xylem sap collected from a number of plant species exposed to As(V), including tomato, rice, cucumber, and the As hyperaccumulator P. vittata (Mihucz et al., 2005; Xu et al., 2007; Su et al., 2008, 2010). In contrast, As(V) was found to be an important As species in the xylem sap collected from B. juncea, Holcus lanatus, and wheat, accounting for between 30% to 60% of the total As (Pickering et al., 2000; Su et al., 2010). The relative percentages of As(III) and As(V) in xylem sap may depend on a number of factors, such as the capacity of As(V) reduction in roots, the abundance and the relative affinities of As(III) or As(V) transporters involved in xylem loading, the degree of As(III)-thiol complexation in roots, and the presence of competing ions. There is evidence that more As(V) was loaded into xylem when the As(V) reduction capacity in roots was exceeded in the treatments with high As(V) concentrations (Fig. 2); this also had the effect of increasing As(V) percentage in the phloem exudate. Complexation of As(III) with thiol compounds in roots may decrease As(III) loading into the xylem (Liu et al., 2010), resulting in a larger As(V)% in the xylem exudate than that in the root tissues. This explanation may also apply to the higher As(V)% in phloem sap than in leaves. Phosphate is known to inhibit As(V) uptake (Ullrich-Eberius et al., 1989; Abedin et al., 2002; Wang et al., 2002), and may also inhibit As(V) loading into xylem or phloem. However, this latter effect was not observed in our experiment (Fig. 3). Increasing phosphate supply decreased As concentrations in both xylem and phloem without significantly affecting the percentages of the two As species, suggesting that phosphate inhibits the influx of As(V) into roots rather than its loading into xylem. Comparing xylem and phloem exudates, the concentrations of As in the latter were, in most cases, higher than those in the former. The fact that both leaves and phloem exudate contained much higher proportions of As(III) than xylem exudate indicates that As(V) is reduced to As(III) in leaves as well as in roots.
Previous studies have shown that methylated As species are generally taken up more slowly than inorganic As, but translocated more efficiently from roots to shoots (Marin et al., 1992; Abedin et al., 2002; Raab et al., 2007; Li et al., 2009). Similar results were obtained with castor bean, with As uptake following the order of As(V) > MMA(V) > DMA(V). In castor bean roots, MMA(V) was partly reduced to MMA(III), although only MMA(V) was loaded into the xylem and phloem (Fig. 5; Supplemental Fig. S1). According to the Challenger pathway of As methylation established in microorganisms (Bentley and Chasteen, 2002), reduction of MMA(V) to MMA(III) is a prerequisite step before further addition of the methyl group. However, no DMA(V) was produced in the MMA(V)-treated plants, indicating no further methylation of MMA(III) in castor bean within the 48 h exposure period. Despite a very limited uptake of DMA(V), its concentration was the highest in both xylem and phloem exudates among the three As treatments, with the phloem concentration being about 3 times the xylem concentration (Fig. 5). MMA(V) concentrations in phloem exudate were also comparable to or larger than inorganic As despite smaller uptake of the former species. These results indicate that methylated As, particularly the dimethyl species, are more mobile than inorganic As in the phloem. Consistent with this finding, Carey et al. (2010) showed that DMA(V) was transported to rice grain much more efficiently than inorganic As. Uptake of undissociated MMA(V) and DMA(V) by rice roots is partly mediated by the aquaporin NIP2,1 (Li et al., 2009), but membrane transporters responsible for loading and unloading of methylated As species in phloem remain unknown. It has been suggested that a lack of thiol complexation of MMA(V) and DMA(V) may explain their high mobility in xylem transport (Raab et al., 2007); this explanation may also apply to the high mobility in phloem observed in this study. Increased lipophilicity with methylation may be another reason, although this is apparently associated with decreased uptake by roots.
The identification of the forms of As in phloem exudate should help future research to elucidate the mechanisms of As loading and unloading in the phloem. Future work should also investigate if the forms of As transported in phloem are similar in food crops such as rice.
MATERIALS AND METHODS
Plant Culture
Seeds of Castor bean (Ricinus communis) var Gibsonii were surface sterilized by immersing them in 0.5% NaOCl for 15 min. After rinsing, seeds were soaked in deionized water overnight and then germinated in moist vermiculite. After germination, seedlings were grown in hydroponic culture for 18 d with a modified one-half-strength Hoagland nutrient solution (one seedling per 1-L pot). The composition of the nutrient solution were 3 mm KNO3, 2 mm Ca(NO3)2, 0.5 mm NH4H2PO4, 1 mm MgSO4, 2 mm MES (pH adjusted to 6.0 with KOH), 100 μm Fe(III)-EDTA, 0.55 μm MnCl2, 46 μm H3BO3, 9 μm MnCl2, 0.75 μm ZnSO4, and 0.35 μm CuSO4. The nutrient solution was aerated continuously and renewed once every 3 d. The growth conditions were 16 h photoperiod with a light intensity of 350 μmol m−2 s−1, day:night temperatures 28°C:25°C, and 70% relative humidity. After 18-d growth, the fresh weight of shoots and roots were on average 30 and 18 g per plant.
As Exposure and Collection of Phloem and Xylem Exudates
A series of experiments were conducted with 18-d-old castor bean plants, varying in the species and concentrations of As added to the nutrient solution. The first experiment compared As exposure in the forms of As(V) (Na2HAsO4) or As(III) (NaAsO2) at 10 μm. The second experiment investigated the dose response to increasing As(V) concentrations (0, 2, 10, 50, and 100 μm). In the third experiment, plants were exposed to 10 μm As(V) with either 10 or 200 μm phosphate. In the fourth experiment, xylem and phloem exudates were collected from plants exposed to 10 μm As(V) for the analysis of thiol compounds and As(III)-thiol complexes. The fifth experiment compared the As mobility and speciation in xylem and phloem exudates after castor bean plants were exposed to 10 μm As(V), MMA(V), or DMA(V). Each treatment was replicated in three to four pots. During As exposure, the phosphate concentration in the nutrient solution was reduced to 10 μm to allow more uptake of As(V) (except the high phosphorus treatment in the third experiment). Phloem exudate was collected at 24 and 48 h after As exposure, and xylem exudate at 48 h exposure, except in the second experiment when both exudates were collected at 24 h. To collect phloem exudate, a shallow, V-shaped incision was made into the bark of the stem at about 2 cm above the stem/root junction with a razor blade (Hall et al., 1971). Phloem exudate was collected in a glass capillary tube (50 or 100 μL) for 1 h. After collection of phloem exudate, the bark (containing the phloem tissue) was removed from a 30 to 40 mm section of stem near the base by ring girdling (Allen and Smith, 1986). One hour later, the stem was cut at the top of the ring-girdled section and xylem exudate collected by pipette for 1 h after decapitation. The collected sap samples were stored on ice, and diluted with a PBS containing 2 mm NaH2PO4 and 0.2 mm Na2-EDTA (pH 6.0) before As speciation analysis. For analysis of thiols and As(III)-thiol complexes, phloem and xylem saps were diluted with 1% formic acid in 1:5 ratio. After collection of phloem and xylem exudates, plant roots were rinsed briefly in an ice-cold desorption solution containing 1 mm K2HPO4, 0.5 mm Ca(NO3)2, and 5 mm MES (pH 6.0), and immersed in 1 L of the same solution for 10 min to remove apoplastic As (Xu et al., 2007). Shoots were rinsed in deionized water. Root and leaf samples were blotted dry, frozen in liquid N2, and were ground to fine powder with a mortar and pestle. Aliquots of nutrient solutions were taken at 24 and 48 h to monitor the changes in As species.
Analysis of Sugar Composition in Xylem and Phloem Exudates
The predominant soluble sugars in both xylem and phloem exudates were identified and quantified by high-pH anion-exchange HPLC using a Dionex CarboPac PA1 guard (4 × 50 mm) and analytical (4 × 250 mm) column connected to a Dionex DX500, with refrigerated autosampler. Each separation was achieved within 20 min by isocratic elution using 40 mm sodium hydroxide at a flow rate of 1 mL min−1. Peak detection was by pulsed amperometry using a gold working electrode and an Ag/AgCl reference electrode together with the manufacturer’s electrode potential settings, optimized for carbohydrate detection. The calibration standards were linearly related to detector response over the range employed (0, 1.25, 2.5, and 5 nmols of Glc, Fru, and Suc) and the exudates were diluted as necessary to give component signals that fell within the standard range (xylem 20-fold; phloem 2,000-fold). An injection volume of 25 μL was used throughout.
Analysis of As Species Using Anion-Exchange HPLC-ICP-MS
Aliquots (0.2–0.5 g) of the ground materials were extracted with 20 mL PBS for 1 h under sonication. The extracts were filtered through 0.45 μm filters. As speciation in phloem and xylem saps, nutrient solutions, and plant extracts were determined using HPLC-ICP-MS (Agilent LC1100 series and Agilent ICP-MS 7500ce, Agilent Technologies). As species [As(III), As(V), DMA(V), MMA(V), and MMA(III)] were separated by an anion-exchange column (Hamilton PRP X-100) as described previously (Li et al., 2009). The mobile phase was either 6.6 mm NH4H2PO4 and 6.6 mm NH4NO3 (pH 6.3) with a flow rate of 0.6 mL min−1 (method 1), or 2 mm NH4H2PO4 and 2 mm NH4NO3 (pH 6.3) with a flow rate of 0.8 mL min−1 (method 2). Method 1 was used to separate As(III), As(V), MMA(V), and DMA(V), and method 2 As(III), MMA(V), MMA(III), and DMA(V) (Li et al., 2009). The outlet of the separation column was connected to a concentric nebulizer and a water-jacketed cyclonic spray chamber of the ICP-MS. Germanium (Ge) was used as the internal standard that was mixed continuously with the post-column solution through a peristaltic pump. Signals at m/z 75 (As), 72 (Ge), and 35 (chlorine [Cl]) were collected with a dwell time of 0.3 s for As and Ge and 0.1 s for Cl. Possible polyatomic interference of 40Ar35Cl on m/z 75 was removed by the Agilent octopole reaction system using helium as the reaction gas. The As signal was normalized by the Ge signal to correct any signal drift during the analysis. As peaks were identified by comparisons with the retention times of standard compounds. Analysis of As species was carried out immediately following sample collection or extraction. For each batch of samples, the analysis was completed within 12 h; repeated analyses showed no changes in As speciation during this period of time. To test the stability of As species in xylem exudate during collection and subsequent analysis, As(III) or As(V) was spiked to the exudate collected from plants untreated with As. No evidence of As(III) oxidation or As(V) reduction was found.
Analysis of Thiols and As(III)-Thiol Complexes Using Reverse-Phase HPLC High-Resolution ICP-MS—High-Resolution ES-MS
Analysis of thiols and As(III)-thiol complexes in phloem and xylem saps was carried out using HPLC coupled with high-resolution ICP-MS (element 2, Thermo Fisher Scientific) and high-resolution ES-MS (LTQ Orbitrap Discovery, Thermo Fisher Scientific). Separation was performed on a C18 reverse-phase column, using a water-methanol gradient described previously (Bluemlein et al., 2008, 2009; Liu et al., 2010). For the ICP-MS analysis, As and S were measured on m/z 75 and 32, respectively. Rhodium was added post column as internal standard and its signal was measured on m/z 103. The ICP-MS was used in medium resolution. The molecular masses of the eluted compounds were determined with high resolution on the ES-MS during a chromatographic run, where glutathione from a xylem sap sample was used as an internal standard for mass determination and correction. A retention time-dependent element-specific response using DMA(V) and Met was used for quantification of any As and S containing molecules as described in Bluemlein et al. (2008).
Analysis of Total As in Plant Tissues
Ground plant samples were digested in 5 mL high-purity HNO3/HClO4 (87/13, v/v). Total As concentrations in the samples were determined by ICP-MS (Agilent 7500ce) operating in the helium gas mode to remove possible interference of 40Ar35Cl on m/z 75.
Data Analysis
Significance of treatment effects was tested by ANOVA. Where necessary, data were logarithmically transformed prior to ANOVA. Treatment means were compared using the least significant difference.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. HPLC-ICP-MS chromatograms of As species in roots, leaves, xylem, and phloem exudates of castor bean exposed to 10 μm As(V), DMA, or MMA.
Supplementary Material
Acknowledgments
We thank Dr. Richard Bromilow for advice on phloem sap collection. Rothamsted Research is an institute of the Biotechnology and Biological Sciences Research Council of the United Kingdom.
References
- Abedin MJ, Feldmann J, Meharg AA. (2002) Uptake kinetics of arsenic species in rice plants. Plant Physiol 128: 1120–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S, Smith JAC. (1986) Ammonium nutrition in Ricinus communis—its effect on plant growth and the chemical composition of the whole plant, xylem and phloem saps. J Exp Bot 37: 1599–1610 [Google Scholar]
- Bentley R, Chasteen TG. (2002) Microbial methylation of metalloids: arsenic, antimony, and bismuth. Microbiol Mol Biol Rev 66: 250–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker PM, Hakvoort HWJ, Bliek M, Souer E, Schat H. (2006) Enhanced arsenate reduction by a CDC25-like tyrosine phosphatase explains increased phytochelatin accumulation in arsenate-tolerant Holcus lanatus. Plant J 45: 917–929 [DOI] [PubMed] [Google Scholar]
- Bluemlein K, Raab A, Feldmann J. (2009) Stability of arsenic peptides in plant extracts: off-line versus on-line parallel elemental and molecular mass spectrometric detection for liquid chromatographic separation. Anal Bioanal Chem 393: 357–366 [DOI] [PubMed] [Google Scholar]
- Bluemlein K, Raab A, Meharg AA, Charnock JM, Feldmann J. (2008) Can we trust mass spectrometry for determination of arsenic peptides in plants: comparison of LC-ICP-MS and LC-ES-MS/ICP-MS with XANES/EXAFS in analysis of Thunbergia alata. Anal Bioanal Chem 390: 1739–1751 [DOI] [PubMed] [Google Scholar]
- Bromilow RH, Rigitano RLO, Briggs GG, Chamberlain K. (1987) Phloem translocation of non-ionised chemicals in Ricinus communis. Pestic Sci 19: 85–99 [Google Scholar]
- Carey AM, Scheckel KG, Lombi E, Newville M, Choi Y, Norton GJ, Charnock JM, Feldmann J, Price AH, Meharg AA. (2010) Grain unloading of arsenic species in rice. Plant Physiol 152: 309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Komives EA, Schroeder JI. (2006) An improved grafting technique for mature Arabidopsis plants demonstrates long-distance shoot-to-root transport of phytochelatins in Arabidopsis. Plant Physiol 141: 108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Chi Y, Taylor NL, Lambers H, Finnegan PM. (2010) Disruption of ptLPD1 or ptLPD2, genes that encode isoforms of the plastidial lipoamide dehydrogenase, confers arsenate hypersensitivity in Arabidopsis. Plant Physiol 153: 1385–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhankher OP, Li YJ, Rosen BP, Shi J, Salt D, Senecoff JF, Sashti NA, Meagher RB. (2002) Engineering tolerance and hyperaccumulation of arsenic in plants by combining arsenate reductase and gamma-glutamylcysteine synthetase expression. Nat Biotechnol 20: 1140–1145 [DOI] [PubMed] [Google Scholar]
- Dhankher OP, Rosen BP, McKinney EC, Meagher RB. (2006) Hyperaccumulation of arsenic in the shoots of Arabidopsis silenced for arsenate reductase (ACR2). Proc Natl Acad Sci USA 103: 5413–5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan GL, Zhou Y, Tong YP, Mukhopadhyay R, Rosen BP, Zhu YG. (2007) A CDC25 homologue from rice functions as an arsenate reductase. New Phytol 174: 311–321 [DOI] [PubMed] [Google Scholar]
- Ellis DR, Gumaelius L, Indriolo E, Pickering IJ, Banks JA, Salt DE. (2006) A novel arsenate reductase from the arsenic hyperaccumulating fern Pteris vittata. Plant Physiol 141: 1544–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority (2009) Scientific opinion on arsenic in food. EFSA Journal 7: 1351 [Google Scholar]
- Feldmann J, Salaun P, Lombi E. (2009) Elemental speciation analysis methods in environmental chemistry—moving towards methodological integration. Environ Chem 6: 275–289 [Google Scholar]
- Hall SM, Baker DA. (1972) Chemical composition of Ricinus phloem exudate. Planta 106: 131–140 [DOI] [PubMed] [Google Scholar]
- Hall SM, Baker DA, Milburn JA. (1971) Phloem transport of 14C-labelled assimilates in Ricinus. Planta 100: 200–207 [DOI] [PubMed] [Google Scholar]
- Huang JWW, Poynton CY, Kochian LV, Elless MP. (2004) Phytofiltration of arsenic from drinking water using arsenic-hyperaccumulating ferns. Environ Sci Technol 38: 3412–3417 [DOI] [PubMed] [Google Scholar]
- Indriolo E, Na GN, Ellis D, Salt DE, Banks JA. (2010) A vacuolar arsenite transporter necessary for arsenic tolerance in the arsenic hyperaccumulating fern Pteris vittata is missing in flowering plants. Plant Cell 22: 2045–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanning J, Strasdeit H. (1998) A coordination-chemical basis for the biological function of the phytochelatins. Angew Chem Int Ed 37: 2464–2466 [DOI] [PubMed] [Google Scholar]
- Li RY, Ago Y, Liu WJ, Mitani N, Feldmann J, McGrath SP, Ma JF, Zhao FJ. (2009) The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol 150: 2071–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, Dankher OP, Carreira L, Smith AP, Meagher RB. (2006) The shoot-specific expression of gamma-glutamylcysteine synthetase directs the long-distance transport of thiol-peptides to roots conferring tolerance to mercury and arsenic. Plant Physiol 141: 288–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WJ, Wood BA, Raab A, McGrath SP, Zhao FJ, Feldmann J. (2010) Complexation of arsenite with phytochelatins reduces arsenite efflux and translocation from roots to shoots in Arabidopsis. Plant Physiol 152: 2211–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ. (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA 105: 9931–9935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LQ, Komar KM, Tu C, Zhang WH, Cai Y, Kennelley ED. (2001) A fern that hyperaccumulates arsenic. Nature 409: 579. [DOI] [PubMed] [Google Scholar]
- Marin AR, Masscheleyn PH, Patrick WH. (1992) The influence of chemical form and concentration of arsenic on rice growth and tissue arsenic concentration. Plant Soil 139: 175–183 [Google Scholar]
- Meharg AA, Williams PN, Adomako E, Lawgali YY, Deacon C, Villada A, Cambell RCJ, Sun G, Zhu YG, Feldmann J, et al. (2009) Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ Sci Technol 43: 1612–1617 [DOI] [PubMed] [Google Scholar]
- Mendoza-Cózatl DG, Butko E, Springer F, Torpey JW, Komives EA, Kehr J, Schroeder JI. (2008) Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus: a role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. Plant J 54: 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihucz VG, Tatár E, Virág I, Cseh E, Fodor F, Záray G. (2005) Arsenic speciation in xylem sap of cucumber (Cucumis sativus L.). Anal Bioanal Chem 383: 461–466 [DOI] [PubMed] [Google Scholar]
- Panaullah GM, Alam T, Hossain MB, Loeppert RH, Lauren JG, Meisner CA, Ahmed ZU, Duxbury JM. (2009) Arsenic toxicity to rice (Oryza sativa L.) in Bangladesh. Plant Soil 317: 31–39 [Google Scholar]
- Pickering IJ, Prince RC, George MJ, Smith RD, George GN, Salt DE. (2000) Reduction and coordination of arsenic in Indian mustard. Plant Physiol 122: 1171–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynton CY, Huang JWW, Blaylock MJ, Kochian LV, Elless MP. (2004) Mechanisms of arsenic hyperaccumulation in Pteris species: root As influx and translocation. Planta 219: 1080–1088 [DOI] [PubMed] [Google Scholar]
- Raab A, Feldmann J, Meharg AA. (2004) The nature of arsenic-phytochelatin complexes in Holcus lanatus and Pteris cretica. Plant Physiol 134: 1113–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab A, Schat H, Meharg AA, Feldmann J. (2005) Uptake, translocation and transformation of arsenate and arsenite in sunflower (Helianthus annuus): formation of arsenic-phytochelatin complexes during exposure to high arsenic concentrations. New Phytol 168: 551–558 [DOI] [PubMed] [Google Scholar]
- Raab A, Williams PN, Meharg A, Feldmann J. (2007) Uptake and translocation of inorganic and methylated arsenic species by plants. Environ Chem 4: 197–203 [Google Scholar]
- Salido AL, Hasty KL, Lim JM, Butcher DJ. (2003) Phytoremediation of arsenic and lead in contaminated soil using Chinese brake ferns (Pteris vittata) and Indian mustard (Brassica juncea). Int J Phytoremediation 5: 89–103 [DOI] [PubMed] [Google Scholar]
- Schmöger MEV, Oven M, Grill E. (2000) Detoxification of arsenic by phytochelatins in plants. Plant Physiol 122: 793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JAC, Milburn JA. (1980) Osmoregulation and the control of phloem sap composition in Ricinus communis L. Planta 148: 28–34 [DOI] [PubMed] [Google Scholar]
- Su YH, McGrath SP, Zhao FJ. (2010) Rice is more efficient in arsenite uptake and translocation than wheat and barley. Plant Soil 328: 27–34 [Google Scholar]
- Su YH, McGrath SP, Zhu YG, Zhao FJ. (2008) Highly efficient xylem transport of arsenite in the arsenic hyperaccumulator Pteris vittata. New Phytol 180: 434–441 [DOI] [PubMed] [Google Scholar]
- Ullrich-Eberius CI, Sanz A, Novacky AJ. (1989) Evaluation of arsenate- and vanadate-associated changes of electrical membrane potential and phosphate transport in Lemna gibba-G1. J Exp Bot 40: 119–128 [Google Scholar]
- Wang JR, Zhao FJ, Meharg AA, Raab A, Feldmann J, McGrath SP. (2002) Mechanisms of arsenic hyperaccumulation in Pteris vittata: uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol 130: 1552–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ, Feldmann J, Meharg AA. (2007) Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ Sci Technol 41: 6854–6859 [DOI] [PubMed] [Google Scholar]
- Xu XY, McGrath SP, Zhao FJ. (2007) Rapid reduction of arsenate in the medium mediated by plant roots. New Phytol 176: 590–599 [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Dunham SJ, McGrath SP. (2002) Arsenic hyperaccumulation by different fern species. New Phytol 156: 27–31 [Google Scholar]
- Zhao FJ, Ma JF, Meharg AA, McGrath SP. (2009) Arsenic uptake and metabolism in plants. New Phytol 181: 777–794 [DOI] [PubMed] [Google Scholar]
- Zhao FJ, McGrath SP, Meharg AA. (2010a) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61: 535–559 [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Stroud JL, Eagling T, Dunham SJ, McGrath SP, Shewry PR. (2010b) Accumulation, distribution, and speciation of arsenic in wheat grain. Environ Sci Technol 44: 5464–5468 [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Wang JR, Barker JHA, Schat H, Bleeker PM, McGrath SP. (2003) The role of phytochelatins in arsenic tolerance in the hyperaccumulator Pteris vittata. New Phytol 159: 403–410 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.