Abstract
The mechanism by which adrenocortical steroids induce granulocytosis in man has been investigated using granulocytes labeled with radioactive diisopropylfluorophosphate.
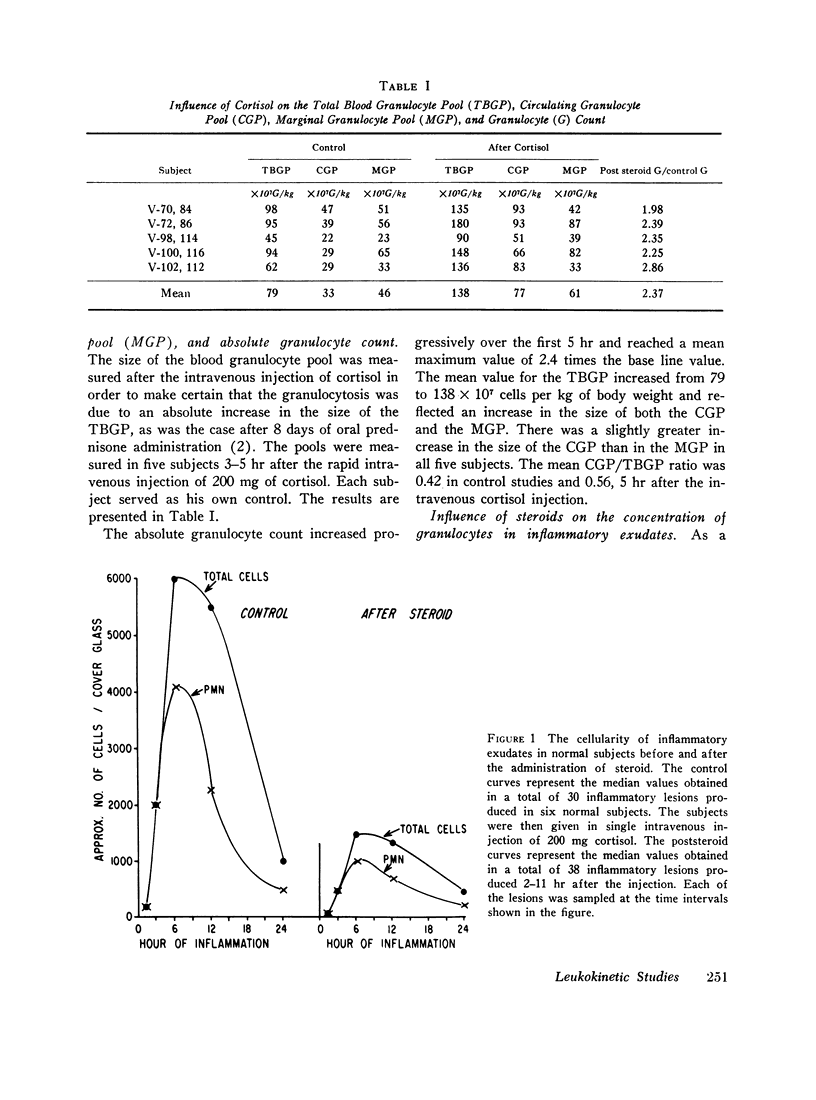
After an intravenous injection of 200 mg of cortisol was given to five normal subjects, the mean value for the total blood granulocyte pool increased from 79 to 138 × 107 cells per kg of body weight and reflected an increase in the size of both the circulating granulocyte pool and the marginal granulocyte pool.
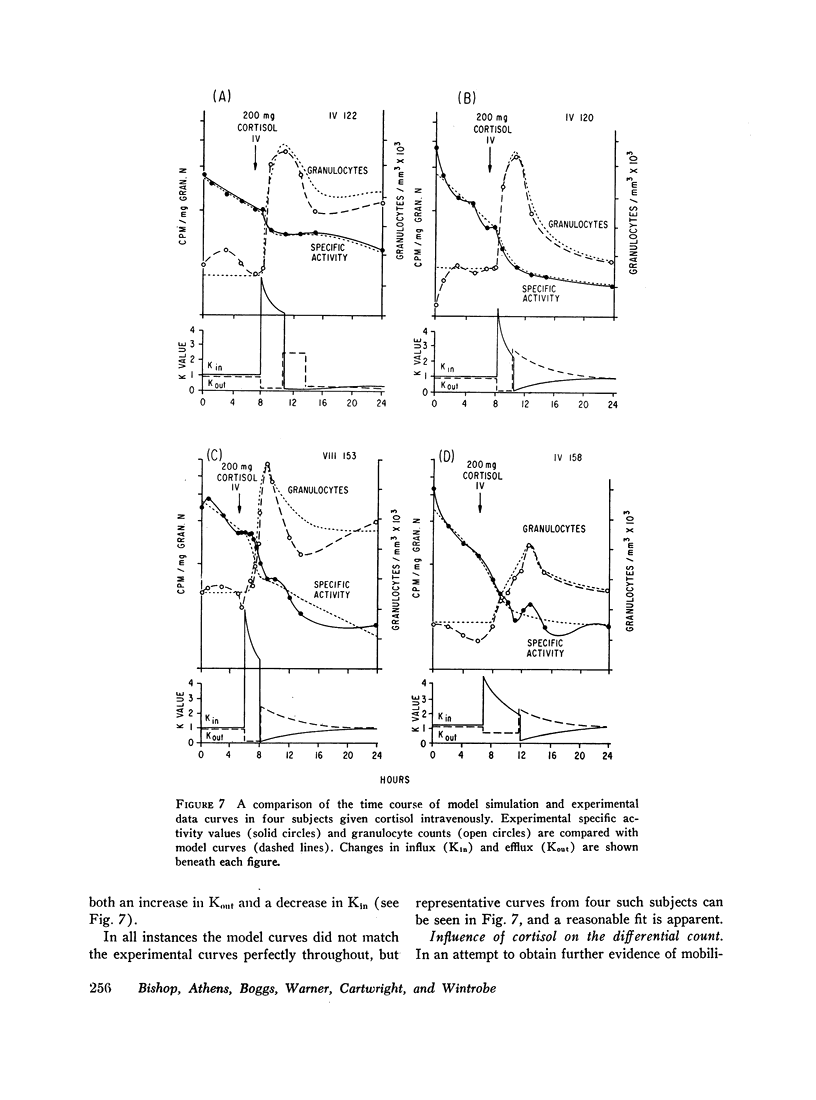
When granulocytes in the circulation were labeled with diisopropylfluorophosphate and granulocytosis was induced later by the intravenous administration of cortisol, the rate of decline of granulocyte specific activity was increased, indicating that the blood pool was being diluted at an accelerated rate by unlabeled cells entering from the bone marrow.
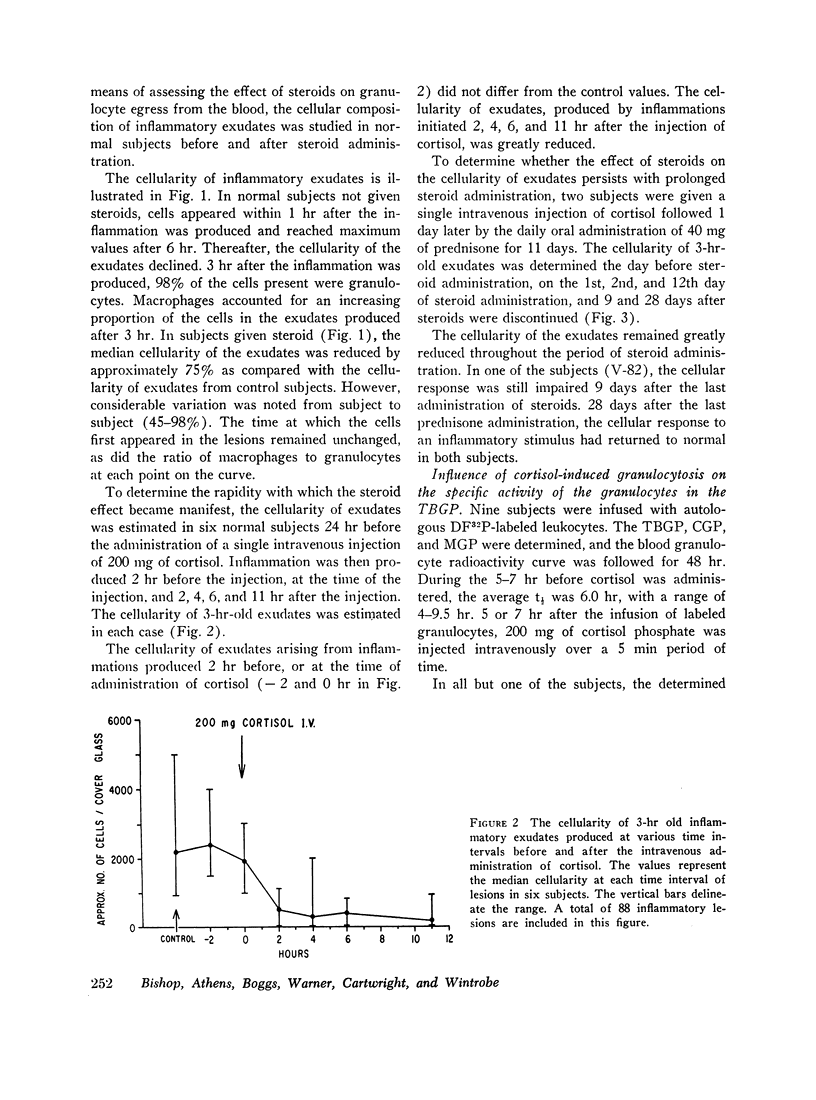
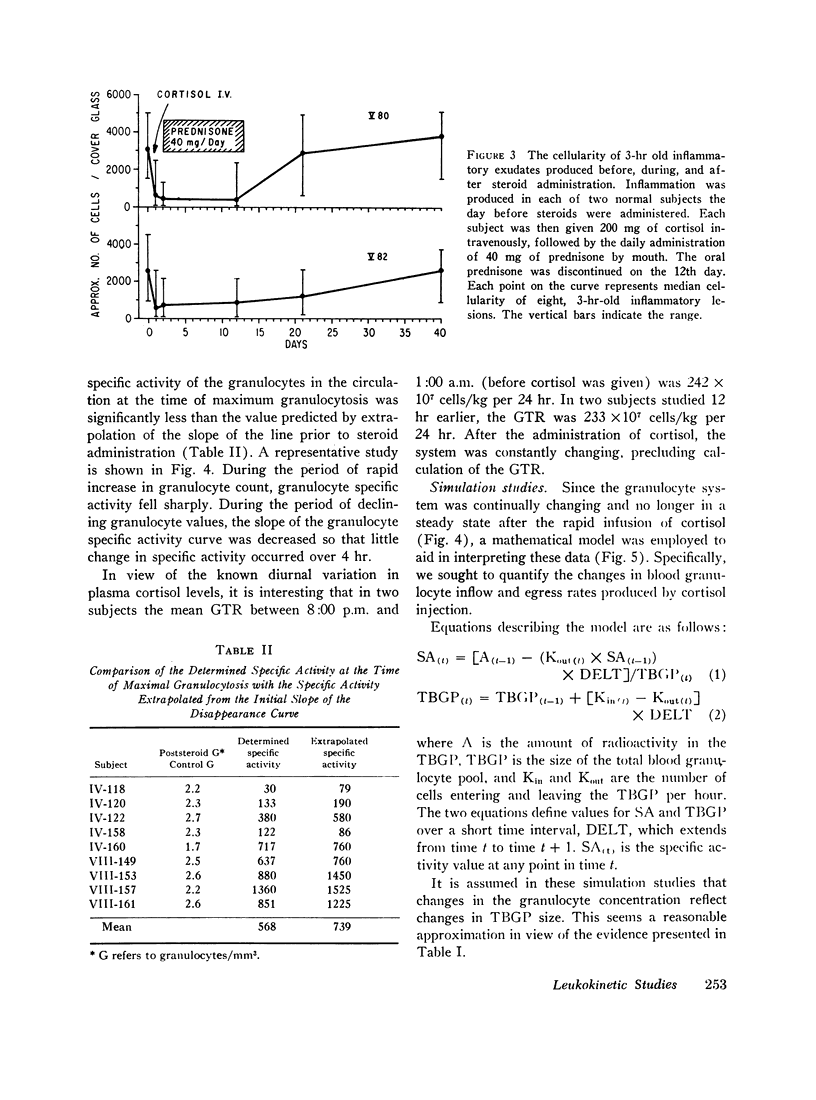
The rate of egress of granulocytes from the blood pool to an inflammatory exudate was studied by the “skin window” technique. After the administration of cortisol, there was a mean reduction in the cellularity of induced inflammatory exudates of 75%. However, this reduction in cellularity varied considerably from subject to subject (45-98%).
From these studies we can infer that steroids induce an absolute granulocytosis by decreasing the rate of egress of cells from the total blood granulocyte pool as well as by increasing the influx of cells from the bone marrow.
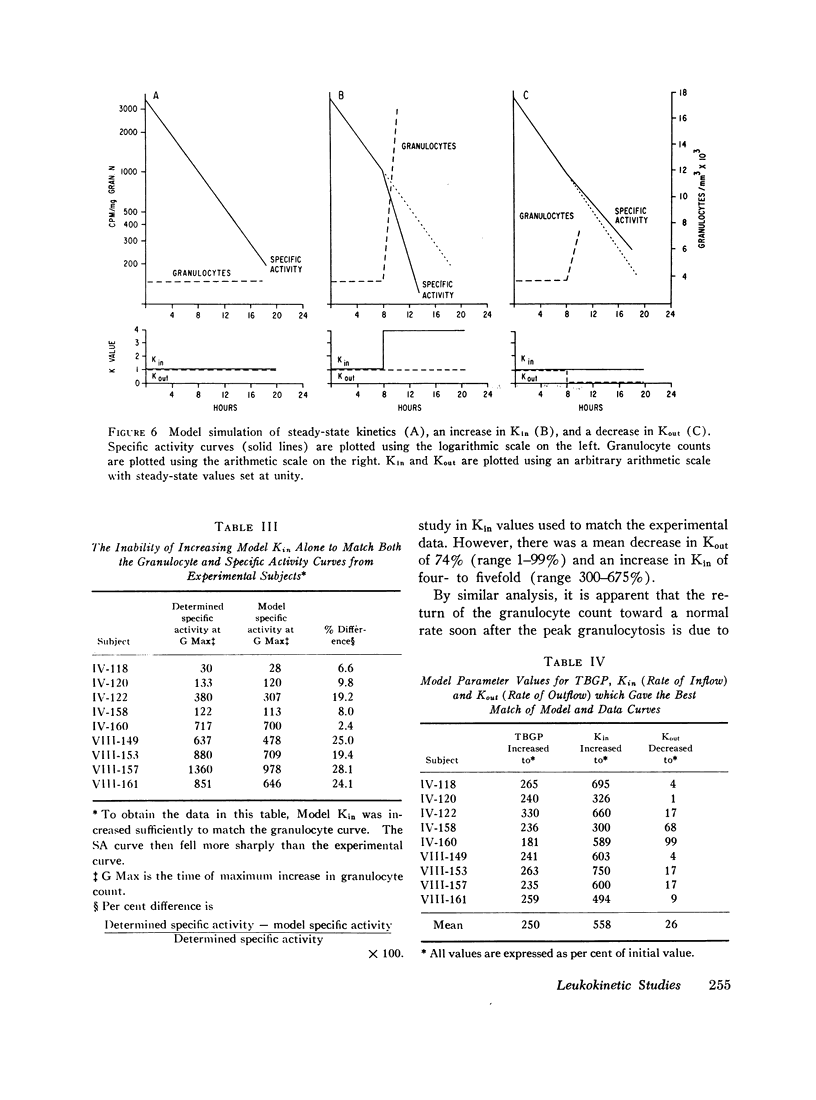
By model simulation studies of the non-steady state induced by cortisol injection, it has been possible to quantitate these rate changes. In the present studies cortisol injection resulted in a mean decrease in blood granulocyte egress of 74% (1-99%) and a mean increase in cell inflow of 450% (300-750%).
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON F., Jr, SMITH M. R., WOOD W. B., Jr Studies on the pathogenesis of acute inflammation. II. The action of cortisone on the inflammatory response to thermal injury. J Exp Med. 1955 Dec 1;102(6):669–676. doi: 10.1084/jem.102.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATHENS J. W., HAAB O. P., RAAB S. O., BOGGS D. R., ASHENBRUCKER H., CARTWRIGHT G. E., WINTROBE M. M. LEUKOKINETIC STUDIES. XI. BLOOD GRANULOCYTE KINETICS IN POLYCYTHEMIA VERA, INFECTION, AND MYELOFIBROSIS. J Clin Invest. 1965 May;44:778–788. doi: 10.1172/JCI105190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATHENS J. W., HAAB O. P., RAAB S. O., MAUER A. M., ASHENBRUCKER H., CARTWRIGHT G. E., WINTROBE M. M. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Invest. 1961 Jun;40:989–995. doi: 10.1172/JCI104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATHENS J. W., RAAB S. O., HAAB O. P., BOGGS D. R., ASHENBRUCKER H., CARTWRIGHT G. E., WINTROBE M. M. LEUKOKINETIC STUDIES. X. BLOOD GRANULOCYTE KINETICS IN CHRONIC MYELOCYTIC LEUKEMIA. J Clin Invest. 1965 May;44:765–777. doi: 10.1172/JCI105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATHENS J. W., RAAB S. O., HAAB O. P., MAUER A. M., ASHENBRUCKER H., CARTWRIGHT G. E., WINTROBE M. M. Leukokinetic studies. III. The distribution of granulocytes in the blood of normal subjects. J Clin Invest. 1961 Jan;40:159–164. doi: 10.1172/JCI104230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER H., KUDO Y., ARGENTON H., FISCHER H. [Cytological research on local inflammation]. Folia Haematol (Frankf) 1961 Apr;5:91–138. [PubMed] [Google Scholar]
- BIERMAN H. R., MARSHALL G. J., MAEKAWA T., KELLY K. H. Granulocytic activity of human plasma: I. Acta Haematol. 1962;27:217–228. doi: 10.1159/000206783. [DOI] [PubMed] [Google Scholar]
- BOGGS D. R., ATHENS J. W., CARTWRIGHT G. E., WINTROBE M. M. THE EFFECT OF ADRENAL GLUCOCORTICOSTEROIDS UPON THE CELLULAR COMPOSITION OF INFLAMMATORY EXUDATES. Am J Pathol. 1964 May;44:763–773. [PMC free article] [PubMed] [Google Scholar]
- BOGGS D. R., ATHENS J. W., HAAB O. P., RAAB S. O., CARTWRIGHT G. E., WINTROBE M. M. LEUKOKINETIC STUDIES. VIII. A SEARCH FOR AN EXTRAMEDULLARY TISSUE POOL OF NEUTROPHILIC GRANULOCYTES. Proc Soc Exp Biol Med. 1964 Mar;115:792–796. doi: 10.3181/00379727-115-29040. [DOI] [PubMed] [Google Scholar]
- Boggs D. R., Cartwright G. E., Wintrobe M. M. Neutrophilia-inducing activity in plasma of dogs recovering from drug-induced myelotoxicity. Am J Physiol. 1966 Jul;211(1):51–60. doi: 10.1152/ajplegacy.1966.211.1.51. [DOI] [PubMed] [Google Scholar]
- CARTWRIGHT G. E., ATHENS J. W., WINTROBE M. M. THE KINETICS OF GRANULOPOIESIS IN NORMAL MAN. Blood. 1964 Dec;24:780–803. [PubMed] [Google Scholar]
- Cline M. J., Melmon K. L. Plasma kinins and cortisol: a possible explanation of the anti-inflammatory action of cortisol. Science. 1966 Sep 2;153(3740):1135–1138. doi: 10.1126/science.153.3740.1135. [DOI] [PubMed] [Google Scholar]
- DELMONTE L., LIEBELT R. A. GRANULOCYTOSIS-PROMOTING EXTRACT OF MOUSE TUMOR TISSUE: PARTIAL PURIFICATION. Science. 1965 Apr 23;148(3669):521–523. doi: 10.1126/science.148.3669.521. [DOI] [PubMed] [Google Scholar]
- DOUGHERTY T. F., SCHNEEBELI G. L. The use of steroids as anti-inflammatory agents. Ann N Y Acad Sci. 1955 May 27;61(2):328–348. doi: 10.1111/j.1749-6632.1955.tb42483.x. [DOI] [PubMed] [Google Scholar]
- EBERT R. H., BARCLAY W. R. [Changes in connective tissue reaction induced by cortisone]. Ann Intern Med. 1952 Sep;37(3):506–518. doi: 10.7326/0003-4819-37-3-506. [DOI] [PubMed] [Google Scholar]
- GERMUTH F. G., Jr The role of adrenocortical steroids in infection, immunity and hypersensitivity. Pharmacol Rev. 1956 Mar;8(1):1–24. [PubMed] [Google Scholar]
- GORDON A. S., HANDLER E. S., SIEGEL C. D., DORNFEST B. S., LOBUE J. PLASMA FACTORS INFLUENCING LEUKOCYTE RELEASE IN RATS. Ann N Y Acad Sci. 1964 Feb 28;113:766–789. doi: 10.1111/j.1749-6632.1964.tb40703.x. [DOI] [PubMed] [Google Scholar]
- KASS E. H., FINLAND M. Adrenocortical hormones in infection and immunity. Annu Rev Microbiol. 1953;7:361–388. doi: 10.1146/annurev.mi.07.100153.002045. [DOI] [PubMed] [Google Scholar]
- KETCHEL M. M. Physiological and radioistopic evidence for the binding of hydrocortisone by human leucocytes in vitro. Endocrinology. 1961 Jul;69:60–66. doi: 10.1210/endo-69-1-60. [DOI] [PubMed] [Google Scholar]
- MICHAEL M., Jr, WHORTON C. M. Delay of the early inflammatory response by cortisone. Proc Soc Exp Biol Med. 1951 Apr;76(4):754–756. doi: 10.3181/00379727-76-18620. [DOI] [PubMed] [Google Scholar]
- NELSON D. H., SANDBERG A. A., PALMER J. G., TYLER F. H. Blood levels of 17-hydroxycorticosteroids following the administration of adrenal steroids and their relation to levels of circulating leukocytes. J Clin Invest. 1952 May;31(9):843–849. doi: 10.1172/JCI102671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAUCH H. C., LOOMIS M. E., JOHNSON M. E., FAVOUR C. B. In vitro suppression of polymorphonuclear leukocyte and lymphocyte glycolysis by cortisol. Endocrinology. 1961 Mar;68:375–385. doi: 10.1210/endo-68-3-375. [DOI] [PubMed] [Google Scholar]
- REBUCK J. W., SMITH R. W., MARGULIS R. R. The modification of leukocytic function in human windows by ACTH. Gastroenterology. 1951 Dec;19(4):644–657. [PubMed] [Google Scholar]
- TRUBOWITZ S., MOSCHIDES E., FELDMAN D. Alkaline phosphatase activity of the polymorphonuclear leukocyte in rapidly induced leukopenia and leukocytosis. J Lab Clin Med. 1961 May;57:747–754. [PubMed] [Google Scholar]


