Abstract
Gender and sex hormones can influence a variety of mental health states, including mood, cognitive development and function, and vulnerability to neurodegenerative diseases and brain damage. Functions of neuronal cells may be altered by estrogens depending upon the availability of different physiological estrogenic ligands; these ligands and their effects vary with life stages, the genetic or postgenetic regulation of receptor levels in specific tissues, or the intercession of competing nonphysiological ligands (either intentional or unintentional, beneficial to health or not). Here we review evidence for how different estrogens (physiological and environmental/dietary), acting via different estrogen receptor subtypes residing in alternative subcellular locations, influence brain functions and behavior. We also discuss the families of receptors and transporters for monoamine neurotransmitters and how they may interact with the estrogenic signaling pathways.
Keywords: estrogen receptor α, estrogen receptor β, GPR30, GPER, xenoestrogens, phytoestrogens, transporters, brain function, neurotransmitter receptors
Estrogens, or the immediate downstream products that they induce, have long been known to alter reproductive behaviors. Prime examples are sexual receptivity and maternal behavior.1,2 However, estrogens can also modify nonreproductive behaviors and cellular responses including mood, affect, anxiety, fear, locomotor activity,3–5 tumor susceptibility,6 and vulnerability to addictive drugs.7 In some cases these estrogenic influences on behavior have been localized to specific brain areas. For example, estrogens alter locomotor activity via actions in the medial preoptic area,8 while anxiety and conditioned fear appear to be controlled by the amygdala,9 and developmental and tumor growth effects have been documented in the cerebellum.10 Each of these brain regions expresses both α and β subtypes of estrogen receptors (ERs),11 although their balance varies between locations. Other, more novel ER candidates found in multiple brain areas12–14 are also beginning to be examined.
Life stage-specific, fluctuating levels of several physiological estrogens, and their relationship to diseases and vulnerabilities in women
There are major sex-based differences in diseases in which neurotransmitters, and their transporters and receptors, play a role. For example, depression is more prevalent in women,15 especially during periods of fluctuating estrogen levels.16,17 Diseases involving the dopamine transporter (DAT) such as Parkinson’s, Alzheimer’s, Tourette’s, and attention-deficit hyperactivity disorder (ADHD), worsen in women after menopause,18 or are different in premenopausal versus postmenopausal females,19–25 suggesting a protective effect of estrogens, or altered vulnerabilities. Receptors and transporters for other catecholamines [notably the serotonin transporter (SERT) and the norepinephrine transporter (NET)] may also be involved in these sex-biased diseases.26–28
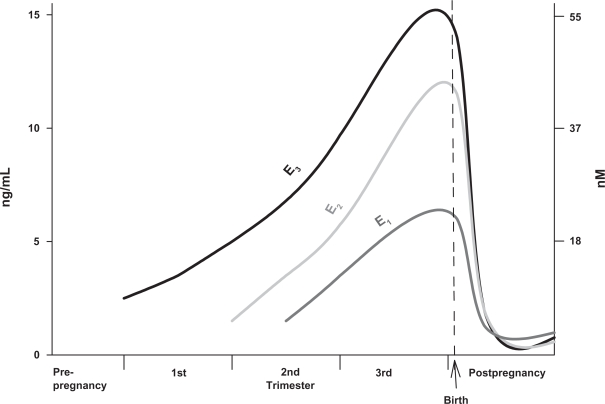
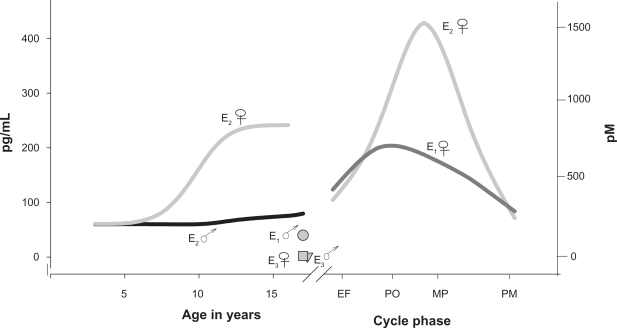
Because estrogen actions can alter the function of these machineries for neurotransmission, it is important to review the fluctuations in hormone levels that affect women. Levels of the most prominent physiological estrogens rise dramatically during pregnancy (see Figure 1), and return to prepregnancy levels very rapidly after parturition; this abrupt change can be correlated with the onset of postpartum depression.29 Levels of these hormones also vary widely between the sexes, and between women’s cycle stages and life stages (Figure 2). These changes are a likely basis for age- or pregnancy status-specific disease biases in women.30–32 Ovarian hormones fluctuate in perimenopause, followed eventually by chronically lower levels33 that can be correlated with the onset of mood disorders and reward circuit-based or other behavioral disturbances. Likewise, pubertal and menstrual cycle-based fluctuations can also lead to phase-dependent mood disorders.34–40 Females are more vulnerable to cocaine use disorders than males,4,7,41,42 and depressive states associated with drug addiction vulnerability or lack of recovery success can coincide with the rise and decline of estrogens.43 Crises in schizophrenia/bipolar disorders can sometimes be directly correlated to menstrual cycle-related hormonal fluctuations.17,44 Estradiol (E2) can rapidly reverse the effects of selective serotonin reuptake inhibitors (SSRIs) used to treat depression.45 Estrogens may also be involved in cognitive function and attention.46,47 These observations suggest that dramatic fluctuations in estrogens or their downstream effectors are key to our understanding of these life stage-specific disease biases in women.
Figure 1.
Hormone level changes in predominant physiological estrogens in the nonpregnant state versus the trimesters of pregnancy.
Note: The levels of the estrogens estrone, estradiol, and estriol (E1, E2, and E3, respectively) drop rapidly to nonpregnant levels at parturition. Graphed from published data tables.226
Figure 2.
Hormone level changes in predominant physiological estrogens with increasing age in females compared to males, and during menstrual cycle phases.
Note: These levels are depicted on scales three orders of magnitude lower than those used in Figure 1. The levels of the estrogens estrone, estradiol, and estriol (E 1, E2, and E3, respectively) are shown for females (♀) and males (♂). The cycle phases depicted are early follicular (EF), pre-ovulatory (PO), midcyle peak (MP), luteal (L), and postmenopausal (PMlevels). Graphed from published data tables.226
Is there evidence that treatment with estrogens can alleviate some of these conditions and diseases caused by deficits or dramatic decreases in estrogens? Although it has been proposed that a more rapid decline in E2 is associated with postpartum depression, some recent evidence does not fully support this notion.48 However, treatment with estrogens can relieve some cases of postpartum depression,31,49–51 and some experimental designs that simulate pre- and postpartum estrogen levels also support this conclusion.52 Yet E2 therapy in humans can be ineffective in reversing mood depression or other purportedly estrogen-influenced diseases.50,52–56 One explanation for these discrepancies could be the involvement of other prominent estrogen metabolites [eg, estrone (E1) and estriol (E3); see Figures 1 and 2] that have not been studied nearly as extensively for these activities. They can have potent nongenomic actions,57,58 in contrast to their previously determined minor role in genomic responses as “weak” estrogens. A few studies have looked at the effects of E1 or E3 on behavior,59–61 but most have focused on treatments with E2, with substrates for several estrogens (DHEA), or mixtures of estrogens such as Premarin®, making it difficult to interpret effects of individual estrogens in those preparations.
The primary physiological estrogens (E1, E2, and E3) are predominantly synthesized in the ovaries, though they can also be synthesized in placenta (especially E3),62 brain,63,64 and fat cells.65 The levels of these hormones are therefore affected by the quantity and state of such non ovarian tissues. In addition, reports that only large doses of estrogens can improve mood disorders66 may suggest the involvement of metabolites of the administered compound (usually E2); these would be present in smaller amounts and could only accumulate to active levels after a large dose of the precursor estrogen is given.
Effects mediated by peptide hormones downstream of estrogens
Besides direct actions of estrogens on behavior, there are also indirect effects that cause synthesis of other receptors,67 or synthesis and secretion of peptide hormones which act downstream. A classic example of such indirect action is production and secretion of the hormone prolactin (PRL). In the pituitary, estrogens facilitate both synthesis and regulated secretion of PRL.68 PRL and its receptors are widely distributed throughout the body. Most actions elicited by this hormone are directly or indirectly related to reproductive processes (such as lactation). However, behavioral changes that facilitate reproductive success also result. Behavioral hallmarks associated with high PRL levels are diverse, and can be elicited in both pregnancy and pseudopregnancy (when PRL levels rise without a pregnancy). These include maternal behavior (including aggressiveness associated with protectiveness and territoriality) and sexual dysfunction (which may prevent a subsequent pregnancy during a critical infant developmental period). PRL overstimulation can also be correlated with depression, changed affect, and abnormal responses to stress.69 As dopamine of hypothalamic origin provides D2 dopamine receptor-mediated inhibitory control over PRL secretion,70 and PRL and/or estrogens may also affect dopamine71 and serotonin signaling,72 there is clear interplay among these factors. Low dopamine levels (associated with depression) also relieve dopamine’s suppressive effect on PRL secretion in the pituitary, thus perhaps compounding adverse effects on mood. Estrogen-induced cell proliferation is also part of the normal response of the pituitary and many other reproduction-related tissues.73,74 Estrogen exposures at the wrong levels or of inappropriate types can cause disregulated proliferation, and even produce tumors of those tissues,75–77 including the pituitary;78 behavioral issues are compounded if these tumors are prolactinomas.
Models for cellular mechanisms of estrogen action
The vast majority of studies on the mechanisms of estrogen (and other steroid) actions over the past 40–50 years focused on nuclear transcription (genomic) effects.79–81 However, more recent evidence (including our own)82–89 also supports nongenomic steroid actions initiated at the level of the cell membrane.90–93 While we are beginning to understand the various ways in which E2 acts via membrane receptor-initiated pathways, we still know very little about nongenomic responses to other prominent physiological estrogens (such as E1 and E3) or xenoestrogens (see below), and still less about other metabolites of these compounds. Membrane-initiated signaling pathways include complex webs of interacting signals that can converge to ramp a particular function up or down, and can have either immediate mechanistic consequences due to rapid signaling, or later downstream consequences after the accumulation of signaling cascade intermediates, or phosphorylation of transcription factors.94 Multiple individual pathways must thus be tested to comprehensively understand functional control via such regulatory mechanisms, and their effects on women’s health.
Which receptors mediate these responses?
Many areas of the brain express both ERα and ERβ,95 although the receptors and their functions can vary during different stages of development. Various approaches have been used to detect selective actions of these subtypes96 the most recent and convincing of which are ERα versus ERβ-selective ligands (PPT versus DPN, respectively) or knockdowns/knockouts of the ERs. DPN selectively regulates AMPA receptor subunits GluR2/3 in the hipopocampus97 and also opposes ERα induction of progesterone receptors in the ventromedial nucleus.98 ERβ can modulate DATs and D2 receptors in rats.99 ERα is thought to participate in striatal dopamine neuroprotection.100 However, the neuroprotective effects of estrogens are usually seen at much higher than physiological concentrations, and therefore may also act via nonreceptor-mediated mechanisms, such as changing fluidity of membranes surrounding the receptors, in which steroids dissolve readily at these high concentrations. Few studies have as yet been aimed at examining α- versus β-selective behavior; though some have been inconclusive,101 others have shown ERβ-specific effects on object recognition and placement tasks.102
In our own studies we examined nongenomic effects of estrogens on the stimulation of dopamine efflux in PC12 cells;103 we showed that plasma membrane versions of ERs (mERα and mERβ) and the newly renamed GPER (formerly called GPR30) are all involved in nongenomic estrogenic effects.85–89,104,105 GPER is a membrane ER of a different receptor family106–108 that works by activating matrix metalloproteinase that in turn cleaves active epidermal growth factor (EGF) from a tethered heparin-bound EGF membrane protein precursor, triggering subsequent action via the EGF receptor. A family of GPER-related receptors was identified in a wide variety of tissues and species, including humans; multiple reports indicate that GPER is present in the brain,12–14,109 though knowledge of its behavioral effects is still pending. We determined that GPER RNA and protein are expressed in PC12 cells,58,103,110 where a recently developed GPER-selective ligand111 appears to have inhibitory effects on ERα-stimulated dopamine efflux via the DAT, similar to GPER’s inhibitory effects in other tissues.109,112
Signaling from both the cell surface and from the nucleus – fitting estrogenic actions into the big picture
Ligands first encountered at a cell’s surface generally initiate cellular responses to a changing environment. Other classes of plasma membrane receptors have long been associated with membrane-initiated rapid signaling cascades; ERs that employ these signaling mechanisms are relatively new considerations. Such events can set into motion coordinated actions eventually leading to one of three main cell fates: proliferation, differentiation, or death. To direct the cell toward one of these decisions, multiple signaling pathways must funnel into a final common pathway signal, such as those involving mitogen-activated protein kinases (MAPKs). These enzymatic “signal receiving stations” sum many inputs from multiple signaling cascades to result in a tally of active MAPKs (with ERKs, JNKs, and p38 subtypes). Thus many stimuli can reconcile to a final decision for a major cellular response. Acting via their membrane receptors, steroids are only one class of input signals to the MAPK “signal integrator”. Estrogenic signals combine with those from other pathways, originating either from the cell surface or from intracellular locations.
The integration of these signal inputs is complex. Not all estrogens elicit identical responses (in level or timing) along these pathways.82 Also, as each tissue may contain a different repertoire of signaling machineries, the complex mixture of patterns leading to pivotal cellular fate decisions will likely also be tissue-specific. Fluctuating endogenous metabolites, along with introduced pharmaceutical estrogens or other nonphysiological estrogen mimetics (see below) can all contribute to a different final tally with distinct kinetics, and so lead to alternative final cellular responses. Therefore, discovering the spectrum of responses within the complex signaling web particular to each part of the brain will be an important goal for understanding the impact of estrogens on women’s behavioral health.
The cell biology and biochemistry of transporter function, and their regulation by estrogens
Many drugs currently used to treat behavioral disorders target the DAT and/or the SERT.113,114 Transporters of this family are recognized as the predominant mechanism for maintaining adequate synaptic levels of the corresponding neurotransmitters. For instance, in DAT or SERT knockout mice the synthetic machinery for producing new neurotransmitters cannot compensate for the loss of neurotransmitter reuptake via these transporters.115 Transporters in this family (DAT, SERT, and NET) all have 12 transmembrane regions, with both the N- and C-termini located within the cytoplasm, and a proposed structure-based mechanism for opening and closing extracellular versus cytoplasmic substrate (neurotransmitter) gates.116–118 Various therapeutic drugs and the addictive drugs cocaine, methamphetamine, and amphetamine bind to the DAT and inhibit or reverse its activity119–121 via mechanisms now beginning to be understood at the cellular and molecular levels. Some evidence also suggests that agents that cause DAT and SERT phosphorylation may regulate their removal from the plasma membrane and sequestration to an intracellular compartment.122–126 Protein kinases PKC and PKG and the p38 MAPK127 probably128 mediate these effects by modifying a C-terminal pentapeptide sequence that is homologous across the DAT, SERT, and NET proteins.
It is also possible that many different kinases controlled by estrogens regulate neurotransmitter transporters. We recently determined that E2 can rapidly alter several signaling pathways in PC12 cells to cause efflux of dopamine via the DAT;58 PKC and MEK (the enzyme upstream of the MAPK-ERKs) are activated by E2. E2 also increases intracellular calcium levels via release from stores. In addition, from our work in the pituitary field, and the work of others, we know that multiple estrogens induce activation of MAPKs.129,130 The estrogenic activation of other kinases likely to act on DAT’s N-terminal tail have yet to be investigated;93,131 these include PKA, PKG, the subtypes of PKC (α, βI, and II, γ), calmodulin kinase II (CamKII)132,133 and Cdk5.134 Such modifiers of phosphorylation and activity states could affect DAT in a variety of ways, including reversing the direction of transport,120,121,135,136 and/or degradation or removal of the transporter from the membrane.115,123,125,137 Specific phosphatases are also now being investigated for their role in maintaining a balance of phosphorylation at specific serines, threonines, and tyrosines at the cytosolic accessible regulatory tails of transporters;133 the part played by estrogens in these processes is largely unknown.
Both neurotransmitter transporters and receptors can be found in the same specialized membrane compartment as ERs – the cholesterol-rich microdomains or caveolae.138–140 Many kinases and phosphatases also reside here.132,138,140,141 However, nonraft or caveolar plasma membrane populations of these groups of proteins also exist, and the regulated movement between compartments is not yet understood. ER-induced kinase and phosphatase effects on neurotransporters and neurotransmitter receptors could be either direct or indirect (via intervening enzymes in signaling cascades), so mERs may or may not need to interact directly with these parts of the neurotransmission machinery in the same membrane compartment.
There are also sex differences in the expression levels and localization of DAT; females express higher DAT levels in the striatum than men,142 although men experience higher amplification of amphetamine-stimulated striatal dopamine release,143 perhaps because of their lower baseline levels due to lower endogenous estrogen levels. Sex steroid levels in females also correlate with different behavioral/neurochemical responses to drugs.144 The euphoric effects of psychoactive drugs are greatest during the follicular phase of the menstrual cycle, when the highest E2 levels occur (see Figure 2).145
New parallels between the actions of estrogens and drugs of abuse on the DAT have recently been identified. Both amphetamines118,146,147 and estrogens58,103,148 can induce reversal of the DAT to cause dopamine efflux. Other coincident actions include DAT trafficking caused by amphetamines and some estrogens (though sometimes in different directions),149,150 and the dependence of efflux caused by both compounds on PKC actions and release of intracellular calcium stores. However, outcomes can depend upon whether transporter expression is under the control of endogenous or transfection-driven expression.128 Interactions between CamKIIα and DAT’s cytoplasmic C-terminus are thought to bring about phosphorylation of nearby N-terminal tail serines to cause amphetamine-induced efflux.146 It will be interesting to see if CamKIIα is similarly involved in estrogen-induced dopamine efflux.
Currently, we only know that DAT function is differentially regulated by different physiological and nonphysiological estrogens.58,148 Functional and structural homologies of the transporters suggest that similar estrogenic mechanisms could affect all transporters in this family (DAT, SERT, and NET). Estrogens are already implicated in control of SERT and NET function and related disease etiologies.47,104 So while it is now well recognized that these transporters can be regulated by acute and selective responses via kinases and phosphatases, and that estrogens can activate kinases and phosphatases,140,151 it is unknown if estrogens will be one of the initial triggers of phosphoregulation of cellular neurotransmitter machineries, as has been shown for other targets.152,153
Xenoestrogens (nonphysiological estrogens) and their role in women’s mental health
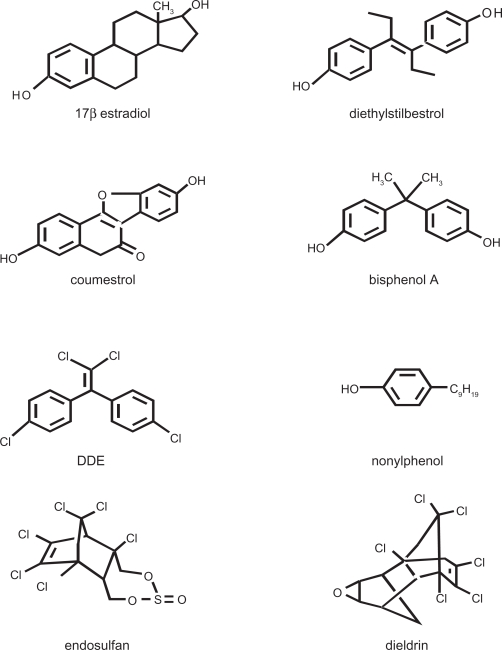
Estrogenic toxins or environmental estrogens (see examples in Figure 3) are capable of mimicking the effects of endogenous estrogens, but usually not perfectly. Thus they can initiate more, less, different, and/or mistimed estrogenic actions that can lead to disruptions of estrogenic signaling, as shown in several recent studies.151,154–159 Common human exposure levels have been associated with a variety of reproductive, neurological, and other impairments.160–163 Bisphenol A (BPA), a monomer of polycarbonate plastics, is found in beverage bottles, canned food liners, and epoxy dental sealants.164–166 Nonylphenol (NP) and structurally related alkylphenols are surfactant manufacturing byproducts and also found in detergents, cleaning materials, and pesticides.167 Diethylstilbestrol (DES) is a potent pharmaceutical estrogen that was prescribed to prevent miscarriages in the 1950s to 1970s; unfortunately, although not really preventive for miscarriage, DES frequently caused multiple reproductive tract abnormalities in offspring, and cancers in some.168 DDE, endosulfan, and dieldrin are estrogenic pesticides that have been associated with neurological impairments.169–172 Besides manufacturing exposures, these compounds break down slowly, so persistent deposits are found in the soil and water, where plants and animals, and thus food supplies become exposed, subsequently passing these exposures on to humans and their infants.173,174 Because many of these xenoestrogenic compounds bioaccumulate in fat tissues, resulting in prolonged and escalating human exposures, the exposure levels causing deleterious health effects are actively debated. Other discrepancies between reports arise from the insensitivity of some animal models to the effects of xenoestrogens.175 However, toxicities to cellular signaling functions can occur at much lower concentrations than the maximum currently allowed by law.155,157,176–179 We also know that some pharmaceutical estrogens become environmental contaminants because of pervasive human use (eg, ethinylestradiol in birth control pills). The known behavioral effects of these compounds at environmentally relevant concentrations are still relatively few, due to limited data. However, BPA is now known to adversely affect some sociosexual behaviors,180–182 locomotion,183 spatial learning/memory,184 and fear/anxiety185,186 at relatively low doses.
Figure 3.
Structurally diverse xenoestrogens compared to the predominant physiological estrogen, E2.
Note: Diethylstilbestrol is a pharmaceutical estrogen. Coumestrol is a plant estrogen. Bisphenol A is a monomer from which polycarbonate plastics are polymerized. Nonylphenol is an industrial surfactant. DDE (a metabolite of DDT), endosulfan, and dieldrin are pesticides. Though some are structurally less similar to estradiol, the most important receptor contact points for ERs α and β are maintained in these chemicals.227
Like E2, xenoestrogens can increase dopamine efflux by changing the amount or function of DAT in the cell membrane.187 Xenoestrogens could further exacerbate the effects of physiological estrogens on transporters via these mechanisms, perhaps with behavioral consequences. In rodent models, prenatal and neonatal exposure to BPA leads to enhanced sensitivity to the rewarding effects of methamphetamine188 and morphine.189 It remains to be seen if there are associations between human xenoestrogen exposure during specific developmental stages and an increased vulnerability to drug addictions later in life, with possible gender differences. Developmental effects of xenoestrogen exposure have recently been shown in rodents in diseases of the immune system such as asthma179 and in cerebellar neurons.178
Phytoestrogens (derived from plant sources) are another type of nonphysiological or xenoestrogen. Many are important constituents of Asian diets, which contain approximately 10-fold higher concentrations of many phytoestrogens than Western diets.190,191 Phytoestrogen-rich diets are thought to be one reason why women in cultures who eat them have less dramatic symptoms of menopause (such as hot flashes, osteoporosis, rise in heart disease), presumably due to the ability of phytoestrogens to replace some of the beneficial effects of estrogens.192 These cultures also have lower incidences of estrogen exposure-related cancers,193 suggesting that some phytoestrogens may oppose the carcinogenic effects of physiological estrogens and some xenoestrogens. Finally, phytoestrogens may protect against brain damage and aging,194,195 although studies are still few and conflicting.196
Unlike E2, which binds to both ER subtypes with relatively equal affinity, some phytoestrogens bind with higher affinity to ERβ (measured on nuclear version of the receptors), and therefore could affect behaviors quite selectively if the affinities for the membrane versions of ERβ are the same. Because membrane receptors are in a different chemical environment (lipid) and therefore expected to assume alternate protein conformations, it is not surprising that they have different potencies for estrogenic effects initiated there, compared to transcriptional effects initiated in the nucleus. Phytoestrogens and many other xenoestrogens show a much higher potency in nongenomic responses, therefore we expect their binding affinities could be higher for mERs. It is probably not correct to just “adopt” the literature on nuclear measurements of binding affinity to fit the membrane receptor. Though we would like to measure the binding affinities for membrane steroid receptors directly, these data are very difficult to interpret because binding of a lipophilic ligand to a receptor lodged in a lipid membrane is subject to very high levels of nonspecific binding. However, if binding to the nuclear receptor has any relevance for predicting binding affinities for the membrane forms of the receptors, there are several examples which might predict higher activities via ERβ. For example, the plant estrogens coumestrol and several isoflavonoids bind more tightly to ERβ.190,197–199
Phytoestrogens have been implicated in memory and learning,196,200 and can have anxiolytic effects.200–202 Some phytoestrogenic compounds can also antagonize the effects of E2; for example, while coumestrol by itself does not affect locomotor activity, it can antagonize the effects of E2.203 Besides its higher affinity for ERβ, coumestrol might act by triggering ERβ-mediated compensatory inhibition in the face of ERα activity in both genomic204,205 and nongenomic activation systems. The latter recent result demonstrated that estrogenic effects on the DAT (reversal of the transporter to cause efflux) are mainly mediated via ERα, but that an ERβ-selective synthetic ligand is inhibitory in the presence of ERα activity.103 Phytoestrogens can also act as agonists directly via ERβ in the brain206 and at the cellular level,103 in the absence of any ERα stimulation.
Estrogen replacement therapeutic strategies: pros and cons
It is very important to obtain low dose, wide dose, and temporal response information about compounds that mimic estrogens, to determine if and when they are safe for use as therapeutics. Many previous researchers have examined the actions of only very high concentrations of nonphysiological estrogens, under the mistaken assumption that dose-response relationships are always monotonic and entirely predictable, and that the effects of lower and noneffective doses could be extrapolated downwards. We now know that such extrapolations are incorrect,207 and that estrogenic actions via nongenomic responses are nonmonotoic.157,178 We have also learned that the temporal phasing of estrogenic and xenoestrogenic responses is different,177,208 suggesting that combinations of these compounds with one another might disrupt normal regulation by causing sustained responses, or cancelling each other out,148 rather than demonstrating the oscillating signals caused by endogenous estrogens. Thus the actions of multiple different estrogens and their pathways are complex.154,209 To understand the breadth of possible disease vulnerabilities influenced by variant endogenous and exogenous hormone levels we need to establish the principles of individual and combinatorial action of estrogenic compounds for each brain region, tissue type, and developmental stage.
To treat diseases associated with loss or imbalance of physiological estrogens (due to menopause, surgery, pregnancy, parturition, or cycle disturbances), or perhaps to counteract the effects of harmful nonphysiological estrogens, it is important to design estrogen replacement or augmentation strategies that deliver the most effective estrogens, over the lowest possible effective doses, with the most effective scheduling and fewest side effects. Currently, E2 and equine urine estrogen mixtures (Premarin®) are the most frequently used replacement therapies. While there are numerous suggestions in the clinical literature that replacing lost estrogens can be beneficial (to bones and skin, in specific cognitive and mood states, and perhaps for the cardiovascular system), there are also risks involved. Long term use of replacement estrogens can increase the risk of some cancers, notably those of the breast and uterus,210 complicate diagnostic procedures such as breast imaging,211 or exacerbate some cardiovascular problems.32 Though some studies have linked replacement estrogens to a decline in specific cognitive functions and increased heart disease,212–215 or have concluded that estrogens do not help prevent disease,216,217 these effects may also depend upon the dose, the use of the most appropriate estrogen metabolites, how long estrogen withdrawal occurred before replacement,218–220 or whether progestins are coadministered.221 Most of these parameters have yet to be systematically studied and agreed upon.
Protective effects of some estrogens against ischemic, glucocorticoid-induced, or other induced brain injury have been touted;222–224 however, such studies have been focused on very high doses of estrogens that, while acceptable for acute therapies to prevent death, are unacceptable for chronic therapeutic use because of the cancer risk. Therefore, we clearly do not yet understand how different estrogens and their metabolites at various doses and schedules may interact, especially given the nonmonotonic dose-response patterns that are becoming recognized as typical of nongenomic steroid actions.225 It is thus critical to know the lowest effective dose ranges of specific estrogens that regulate given functions such as neurotransmitter transporter and receptor activity. It remains to be proven conclusively if some phytoestrogens or E2 metabolites could act therapeutically to either restore estrogenic effects on transporters when endogenous estrogens are absent (such as to control hot flashes), or to act preventatively as inhibitory estrogens in scenarios where estrogenic overstimulation results in cancers.
Summary
There are important differences between males and females in a number of functional responses and vulnerabilities to behavioral disorders. Signaling mechanisms, both genomic and nongenomic, operating via several different ER proteins residing in different subcellular compartments, are beginning to be found responsible for diverse actions of estrogens involved in these functions. Complex signaling cascades and receptor systems can be influenced by multiple physiological estrogens, as well as some nonphysiological (dietary, pharmaceutical) and contaminant (environmental) estrogens. Such influences could have profound effects on the functioning of the brain and nervous system. Elucidating the underlying cellular mechanisms via which variant estrogens and their receptors act will provide explanations of how we might intervene medically to address severely imbalanced estrogens that cause disease, or enlighten our choices among commercial products or foods/dietary supplements that contain estrogens. These considerations should also inform future decisions about hormone replacements, analogs, and antagonists that could alleviate life stage-specific effects of estrogens or their withdrawal. An enhanced focus on the relatively new area of nongenomic estrogenic effects may allow entirely new understandings and approaches to treatment of these maladies, and perhaps change current treatment standards. One such change could be the preservation of ovaries in women undergoing hysterectomies, potentially justified because of the multiple beneficial estrogens that they provide.18 Hopefully, among these new understandings and opportunities will be ones that improve the diagnosis and treatment of mental state diseases for women.
Acknowledgments
We would like to thank Dr David Konkel for carefully editing our manuscript. CSW is funded by the NIEHS, NIDA, and American Institute for Cancer Research. KAC is funded by NIDA and the Klarman Family Foundation.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfaff D. Hormone-driven mechanisms in the central nervous system facilitate the analysis of mammalian behaviours. J Endocrinol. 2005;184:447–453. doi: 10.1677/joe.1.05897. [DOI] [PubMed] [Google Scholar]
- 3.Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 4.Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther. 2000;293:879–886. [PubMed] [Google Scholar]
- 5.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protocols. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belcher SM, Ma X, Le HH. Blockade of estrogen receptor signaling inhibits growth and migration of medulloblastoma. Endocr. 2009;150:1112–1121. doi: 10.1210/en.2008-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- 8.Fahrbach SE, Meisel RL, Pfaff DW. Preoptic implants of estradiol increase wheel running but not the open field activity of female rats. Physiol Behav. 1985;35:985–992. doi: 10.1016/0031-9384(85)90270-7. [DOI] [PubMed] [Google Scholar]
- 9.Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belcher SM. Rapid signaling mechanisms of estrogens in the developing cerebellum. Brain Res Rev. 2008;57:481–492. doi: 10.1016/j.brainresrev.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Qiu J, Ronnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;73:985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brailoiu E, Dun SL, Brailoiu GC, et al. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 14.Hazell GG, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, Lolait SJ. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol. 2009;202:223–236. doi: 10.1677/JOE-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staley JK, Sanacora G, Tamagnan G, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry. 2006;59:40–47. doi: 10.1016/j.biopsych.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Halbreich U, Kahn LS. Role of estrogen in the aetiology and treatment of mood disorders. CNS Drugs. 2001;15:797–817. doi: 10.2165/00023210-200115100-00005. [DOI] [PubMed] [Google Scholar]
- 17.Felthous AR, Robinson DB. Oral contraceptive medication in prevention of psychotic exacerbations associated with phases of the menstrual cycle. J Prev Psychiatry. 1981;1:5–14. [PubMed] [Google Scholar]
- 18.Parker WH, Shoupe D, Broder MS, Liu Z, Farquhar C, Berek JS. Elective oophorectomy in the gynecological patient: when is it desirable? Curr Opin Obstet Gynecol. 2007;19:350–354. doi: 10.1097/GCO.0b013e32821642d1. [DOI] [PubMed] [Google Scholar]
- 19.Dluzen DE, Mickley KR. Gender differences in modulatory effects of tamoxifen upon the nigrostriatal dopaminergic system. Pharmacol Biochem Behav. 2005;80:27–33. doi: 10.1016/j.pbb.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Foltynie T, Lewis SG, Goldberg TE, et al. The BDNF Val66Met polymorphism has a gender specific influence on planning ability in Parkinson’s disease. J Neurol. 2005;252:833–838. doi: 10.1007/s00415-005-0756-5. [DOI] [PubMed] [Google Scholar]
- 21.Benedetti MD, Maraganore DM, Bower JH, et al. Hysterectomy, menopause, and estrogen use preceding Parkinson’s disease: an exploratory case-control study. Mov Disord. 2001;16:830–837. doi: 10.1002/mds.1170. [DOI] [PubMed] [Google Scholar]
- 22.Quinn PO. Treating adolescent girls and women with ADHD: gender-specific issues. J Clin Psychol. 2005;61:579–587. doi: 10.1002/jclp.20121. [DOI] [PubMed] [Google Scholar]
- 23.Kurlan R. The pathogenesis of Tourette’s syndrome. A possible role for hormonal and excitatory neurotransmitter influences in brain development. Arch Neurol. 1992;49:874–876. doi: 10.1001/archneur.1992.00530320106020. [DOI] [PubMed] [Google Scholar]
- 24.Yoon DY, Rippel CA, Kobets AJ, et al. Dopaminergic polymorphisms in Tourette syndrome: Association with the DAT gene (SLC6A3) Am J Med Genet B Neuropsychiatr Genet. 2007;144:605–610. doi: 10.1002/ajmg.b.30466. [DOI] [PubMed] [Google Scholar]
- 25.Compton J, van AT, Murphy D. Mood, cognition and Alzheimer’s disease. Best Pract Res Clin Obstet Gynaecol. 2002;16:357–370. doi: 10.1053/beog.2002.0285. [DOI] [PubMed] [Google Scholar]
- 26.Markowitz JS, DeVane CL, Pestreich LK, Patrick KS, Muniz R. A comprehensive in vitro screening of d-, l-, and dl-threo-methylphenidate: an exploratory study. J Child Adolesc Psychopharmacol. 2006;16:687–698. doi: 10.1089/cap.2006.16.687. [DOI] [PubMed] [Google Scholar]
- 27.Osterlund MK, Overstreet DH, Hurd YL. The flinders sensitive line rats, a genetic model of depression, show abnormal serotonin receptor mRNA expression in the brain that is reversed by 17β-estradiol. Brain Res Mol Brain Res. 1999;74:158–166. doi: 10.1016/s0169-328x(99)00274-0. [DOI] [PubMed] [Google Scholar]
- 28.Nutt DJ. Relationship of neurotransmitters to the symptoms of major depressive disorder. J Clin Psychiatry. 2008;(69 Suppl E1):4–7. [PubMed] [Google Scholar]
- 29.Doornbos B, Fokkema DS, Molhoek M, Tanke MA, Postema F, Korf J. Abrupt rather than gradual hormonal changes induce postpartum blues-like behavior in rats. Life Sci. 2009;84:69–74. doi: 10.1016/j.lfs.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Williams CL. Estrogen effects on cognition across the lifespan. Horm Behav. 1998;34:80–84. doi: 10.1006/hbeh.1998.1480. [DOI] [PubMed] [Google Scholar]
- 31.Moses-Kolko EL, Berga SL, Kalro B, Sit DK, Wisner KL. Transdermal estradiol for postpartum depression: a promising treatment option. Clin Obstet Gynecol. 2009;52:516–529. doi: 10.1097/GRF.0b013e3181b5a395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loucks TL, Berga SL. Does postmenopausal estrogen use confer neuroprotection? Semin Reprod Med. 2009;27:260–274. doi: 10.1055/s-0029-1216279. [DOI] [PubMed] [Google Scholar]
- 33.Prior JC. Ovarian aging and the perimenopausal transition: the paradox of endogenous ovarian hyperstimulation. Endocrine. 2005;26:297–300. doi: 10.1385/ENDO:26:3:297. [DOI] [PubMed] [Google Scholar]
- 34.Yonkers KA, Brown C, Pearlstein TB, Foegh M, Sampson-Landers C, Rapkin A. Efficacy of a New Low-Dose Oral Contraceptive With Drospirenone in Premenstrual Dysphoric Disorder. Obstet Gynecol. 2005;106:492–501. doi: 10.1097/01.AOG.0000175834.77215.2e. [DOI] [PubMed] [Google Scholar]
- 35.Almeida OP, Barclay L. Sex hormones and their impact on dementia and depression: a clinical perspective. Expert Opin Pharmacother. 2001;2:527–535. doi: 10.1517/14656566.2.4.527. [DOI] [PubMed] [Google Scholar]
- 36.Stein D, Hanukoglu A, Blank S, Elizur A. Cyclic psychosis associated with the menstrual cycle. Br J Psychiatry. 1993;163:824–828. doi: 10.1192/bjp.163.6.824. [DOI] [PubMed] [Google Scholar]
- 37.Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. J Affect Disord. 2003;74:67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- 38.Taylor M. Psychological consequences of surgical menopause. J Reprod Med. 2001;46:317–324. [PubMed] [Google Scholar]
- 39.Oinonen KA, Mazmanian D. Does body fat protect against negative moods in women? Med Hypotheses. 2001;57:387–388. doi: 10.1054/mehy.2001.1365. [DOI] [PubMed] [Google Scholar]
- 40.Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sell SL, Thomas ML, Cunningham KA. Influence of estrous cycle and estradiol on behavioral sensitization to cocaine in female rats. Drug Alcohol Depend. 2002;67:281–290. doi: 10.1016/s0376-8716(02)00085-6. [DOI] [PubMed] [Google Scholar]
- 42.Elman I, Karlsgodt KH, Gastfriend DR. Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. Am J Drug Alcohol Abuse. 2001;27:193–202. doi: 10.1081/ada-100103705. [DOI] [PubMed] [Google Scholar]
- 43.Soares CN, Poitras JR, Prouty J. Effect of reproductive hormones and selective estrogen receptor modulators on mood during menopause. Drugs Aging. 2003;20:85–100. doi: 10.2165/00002512-200320020-00001. [DOI] [PubMed] [Google Scholar]
- 44.Coromina SM, Rodie JU, de Montagut LM, Sanchez AM. The use of oral contraceptives as a prevention of recurrent premenstrual psychosis. Psychiatry Res. 2009;170:290–291. doi: 10.1016/j.psychres.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 45.Benmansour S, Piotrowski JP, Altamirano AV, Frazer A. Impact of ovarian hormones on the modulation of the serotonin transporter by fluvoxamine. Neuropsychopharmacology. 2009;34:555–564. doi: 10.1038/npp.2008.23. [DOI] [PubMed] [Google Scholar]
- 46.Bender CM, Sereika SM, Berga SL, et al. Cognitive impairment associated with adjuvant therapy in breast cancer. Psychooncology. 2006;15:422–430. doi: 10.1002/pon.964. [DOI] [PubMed] [Google Scholar]
- 47.Shively CA, Bethea CL. Cognition, mood disorders, and sex hormones. ILAR J. 2004;45:189–199. doi: 10.1093/ilar.45.2.189. [DOI] [PubMed] [Google Scholar]
- 48.Klier CM, Muzik M, Dervic K, et al. The role of estrogen and progesterone in depression after birth. J Psychiatr Res. 2007;41:273–279. doi: 10.1016/j.jpsychires.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Genazzani AR, Spinetti A, Gallo R, Bernardi F. Menopause and the central nervous system: intervention options. Maturitas. 1999;31:103–110. doi: 10.1016/s0378-5122(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 50.Grigoriadis S, Kennedy SH. Role of estrogen in the treatment of depression. Am J Ther. 2002;9:503–509. doi: 10.1097/00045391-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Epperson CN, Wisner KL, Yamamoto B. Gonadal steroids in the treatment of mood disorders. Psychosom Med. 1999;61:676–697. doi: 10.1097/00006842-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 52.Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- 53.Morrison MF, Kallan MJ, Ten HT, Katz I, Tweedy K, Battistini M. Lack of efficacy of estradiol for depression in postmenopausal women: a randomized, controlled trial. Biol Psychiatry. 2004;55:406–412. doi: 10.1016/j.biopsych.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 54.Huttner RP, Shepherd JE. Gonadal steroids, selective serotonin reuptake inhibitors, and mood disorders in women. Med Clin North Am. 2003;87:1065–1076. doi: 10.1016/s0025-7125(03)00061-0. [DOI] [PubMed] [Google Scholar]
- 55.Miller KJ. The other side of estrogen replacement therapy: outcome study results of mood improvement in estrogen users and nonusers. Curr Psychiatry Rep. 2003;5:439–444. doi: 10.1007/s11920-003-0082-5. [DOI] [PubMed] [Google Scholar]
- 56.Klaiber EL, Broverman DM, Vogel W, Kobayashi Y. Estrogen therapy for severe persistent depressions in women. Arch Gen Psychiatry. 1979;36:550–554. doi: 10.1001/archpsyc.1979.01780050060006. [DOI] [PubMed] [Google Scholar]
- 57.Watson CS, Jeng YJ, Kochukov MY. Nongenomic actions of estradiol compared with estrone and estriol in pituitary tumor cell signaling and proliferation. FASEB J. 2008;22:3328–3336. doi: 10.1096/fj.08-107672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alyea RA, Watson CS. Nongenomic mechanisms of physiological estrogen-mediated dopamine efflux. BMC Neurosci. 2009;10:59. doi: 10.1186/1471-2202-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lebrun CE, van der Schouw YT, De Jong FH, Pols HA, Grobbee DE, Lamberts SW. Endogenous oestrogens are related to cognition in healthy elderly women. Clin Endocrinol (Oxf) 2005;63:50–55. doi: 10.1111/j.1365-2265.2005.02297.x. [DOI] [PubMed] [Google Scholar]
- 60.Almeida OP, Lautenschlager N, Vasikaram S, Leedman P, Flicker L. Association between physiological serum concentration of estrogen and the mental health of community-dwelling postmenopausal women age 70 years and over. Am J Geriatr Psychiatry. 2005;13:142–149. doi: 10.1176/appi.ajgp.13.2.142. [DOI] [PubMed] [Google Scholar]
- 61.Barha CK, Galea LA. Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochim Biophys Acta. 2010;(10):S0304–4165. 00011–00015. doi: 10.1016/j.bbagen.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 62.Newby D, Aitken DA, Howatson AG, Connor JM. Placental synthesis of oestriol in Down’s syndrome pregnancies. Placenta. 2000;21:263–267. doi: 10.1053/plac.1999.0469. [DOI] [PubMed] [Google Scholar]
- 63.Vanson A, Arnold AP, Schlinger BA. 3 β-hydroxysteroid dehydrogenase/isomerase and aromatase activity in primary cultures of developing zebra finch telencephalon: dehydroepiandrosterone as substrate for synthesis of androstenedione and estrogens. Gen Comp Endocrinol. 1996;102:342–350. doi: 10.1006/gcen.1996.0077. [DOI] [PubMed] [Google Scholar]
- 64.Reddy VV. Estriol synthesis in rat brain and pituitary. Brain Res. 1979;175:165–168. doi: 10.1016/0006-8993(79)90526-2. [DOI] [PubMed] [Google Scholar]
- 65.Deslypere JP, Verdonck L, Vermeulen A. Fat tissue: a steroid reservoir and site of steroid metabolism. J Clin Endocrinol Metab. 1985;61:564–570. doi: 10.1210/jcem-61-3-564. [DOI] [PubMed] [Google Scholar]
- 66.Richardson TA, Robinson RD. Menopause and depression: a review of psychologic function and sex steroid neurobiology during the menopause(1) Prim Care Update Ob Gyns. 2000;7:215–223. doi: 10.1016/s1068-607x(00)00049-4. [DOI] [PubMed] [Google Scholar]
- 67.Moffatt CA, Rissman EF, Shupnik MA, Blaustein JD. Induction of progestin receptors by estradiol in the forebrain of estrogen receptor-α gene-disrupted mice. J Neurosci. 1998;18:9556–9563. doi: 10.1523/JNEUROSCI.18-22-09556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dannies PS. Control of prolactin production by estrogen. In: Litwack G, editor. Biochemical Actions of Hormones. 12 ed. Orlando: Academic Press Inc.; 1985. pp. 289–310. [Google Scholar]
- 69.Sobrinho LG. Prolactin, psychological stress and environment in humans: adaptation and maladaptation. Pituitary. 2003;6:35–39. doi: 10.1023/a:1026229810876. [DOI] [PubMed] [Google Scholar]
- 70.Bression D, Brandi AM. In vitro and in vivo antagonistic regulation by estradiol and progesterone of the rat pituitary domperidone binding sites: correlation with ovarian steroid regulation of the dopaminergic inhibition of prolactin secretion in vitro. Endocr. 1985;116:1905. doi: 10.1210/endo-116-5-1905. [DOI] [PubMed] [Google Scholar]
- 71.Arbogast LA, Voogt JL. Hyperprolactinemia increases and hypoprolactinemia decreases tyrosine hydroxylase messenger ribonucleic acid levels in the arcuate nuclei, but not the substantia nigra or zona incerta. Endocr. 1991;128:997–1005. doi: 10.1210/endo-128-2-997. [DOI] [PubMed] [Google Scholar]
- 72.van Amelsvoort TA, Abel KM, Robertson DM, et al. Prolactin response to d-fenfluramine in postmenopausal women on and off ERT: comparison with young women. Psychoneuroendocrinology. 2001;26:493–502. doi: 10.1016/s0306-4530(01)00008-7. [DOI] [PubMed] [Google Scholar]
- 73.Adams AB. Human breast cancer: concerted role of diet, prolactin and adrenal C19-delta 5 steroids in tumorigenesis. Int J Cancer. 1992;50:854–858. doi: 10.1002/ijc.2910500603. [DOI] [PubMed] [Google Scholar]
- 74.Nevalainen MT, Valve EM, Ingleton PM, Nurmi M, Martikainen PM, Harkonen PL. Prolactin and prolactin receptors are expressed and functioning in human prostate. J Clin Invest. 1997;99:618–627. doi: 10.1172/JCI119204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24:1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gutzman JH, Miller KK, Schuler LA. Endogenous human prolactin and not exogenous human prolactin induces estrogen receptor α and prolactin receptor expression and increases estrogen responsiveness in breast cancer cells. J Steroid Biochem Mol Biol. 2004;88:69–77. doi: 10.1016/j.jsbmb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 77.Rose-Hellekant TA, Arendt LM, Schroeder MD, Gilchrist K, Sandgren EP, Schuler LA. Prolactin induces ERα-positive and ERα-negative mammary cancer in transgenic mice. Oncogene. 2003;22:4664–4674. doi: 10.1038/sj.onc.1206619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gorski J, Wendell D, Gregg D, Chun TY. Estrogens and the genetic control of tumor growth. [Review] [23 refs] Prog Clin Bio Res. 1997;396:233–243. [PubMed] [Google Scholar]
- 79.Lee KC, Lee KW. Nuclear receptors, coactivators and chromatin: new approaches, new insights. Trends Endocrinol Metab. 2001;12:191–197. doi: 10.1016/s1043-2760(01)00392-7. [DOI] [PubMed] [Google Scholar]
- 80.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. [Review] Ann Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 81.Evans RM. The steroid and thyroid hormone receptor superfamily [Review] Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bulayeva NN, Gametchu B, Watson CS. Quantitative measurement of estrogen-induced ERK 1 and 2 activation via multiple membrane-initiated signaling pathways. Steroids. 2004;69:181–192. doi: 10.1016/j.steroids.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Campbell CH, Bulayeva N, Brown DB, Gametchu B, Watson CS. Regulation of the membrane estrogen receptor-a: role of cell density, serum, cell passage number, and estradiol. FASEB J. 2002;16:1917–1927. doi: 10.1096/fj.02-0182com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clarke CH, Norfleet AM, Clarke MSF, Watson CS, Cunningham KA, Thomas ML. Peri-membrane localization of the estrogen receptor-a protein in neuronal processes of cultured hippocampal neurons. Neuroendocrinology. 2000;71:34–42. doi: 10.1159/000054518. [DOI] [PubMed] [Google Scholar]
- 85.Norfleet AM, Clarke C, Gametchu B, Watson CS. Antibodies to the estrogen receptor-a modulate prolactin release from rat pituitary tumor cells through plasma membrane estrogen receptors. FASEB J. 2000;14:157–165. doi: 10.1096/fasebj.14.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Norfleet AM, Thomas ML, Gametchu B, Watson CS. Estrogen receptor-a detected on the plasma membrane of aldehyde-fixed GH3/B6/F10 rat pituitary cells by enzyme-linked immunocytochemistry. Endocr. 1999;140:3805–3814. doi: 10.1210/endo.140.8.6936. [DOI] [PubMed] [Google Scholar]
- 87.Pappas TC, Gametchu B, Yannariello-Brown J, Collins TJ, Watson CS. Membrane estrogen receptors in GH3/B6 cells are associated with rapid estrogen-induced release of prolactin. Endocrine. 1994;2:813–822. [Google Scholar]
- 88.Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptor-enriched GH3/B6 cells have an enhanced non-genomic response to estrogen. Endocrine. 1995;3:743–749. doi: 10.1007/BF03000207. [DOI] [PubMed] [Google Scholar]
- 89.Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- 90.Szego CM. Cytostructural correlates of hormone action: new common ground in receptor-mediated signal propagation for steroid and peptide agonists. Endocrine. 1994;2:1079–1093. [Google Scholar]
- 91.Watson CS. The Identities of Membrane Steroid Receptors .... and Other Proteins Mediating Nongenomic Steroid Action. Boston: Kluwer Academic Publishers; 2003. [Google Scholar]
- 92.Watson CS, Gametchu B. Proteins of multiple classes participate in nongenomic steroid actions. Exp Biol Med. 2003;228:1272–1281. doi: 10.1177/153537020322801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Watson CS, Gametchu B. Membrane-initiated steroid actions and the proteins that mediate them. Proc Soc Exp Biol Med. 1999;220:9–19. doi: 10.1046/j.1525-1373.1999.d01-2.x. [DOI] [PubMed] [Google Scholar]
- 94.Watson CS. Signaling themes shared between peptide and steroid hormones at the plasma membrane [Review] STKE E1. Science’s Signal Transduction Knowledge Environment. 1999 doi: 10.1126/stke.1999.12.pe1. [serial online] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ikeda Y, Nagai A, Ikeda MA, Hayashi S. Sexually dimorphic and estrogen-dependent expression of estrogen receptor β in the ventromedial hypothalamus during rat postnatal development. Endocr. 2003;144:5098–5104. doi: 10.1210/en.2003-0267. [DOI] [PubMed] [Google Scholar]
- 96.Morissette M, Le SM, D’Astous M, et al. Contribution of estrogen receptors α and β to the effects of estradiol in the brain. J Steroid Biochem Mol Biol. 2008;108:327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 97.Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA. Estrogen receptor α and β specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res. 2009;1290:1–11. doi: 10.1016/j.brainres.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gonzales KL, Tetel MJ, Wagner CK. Estrogen receptor (ER) β modulates ERα responses to estrogens in the developing rat ventromedial nucleus of the hypothalamus. Endocr. 2008;149:4615–4621. doi: 10.1210/en.2008-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Le SM, Di PT. Influence of oestrogenic compounds on monoamine transporters in rat striatum. J Neuroendocrinol. 2006;18:25–32. doi: 10.1111/j.1365-2826.2005.01380.x. [DOI] [PubMed] [Google Scholar]
- 100.D’Astous M, Mendez P, Morissette M, Garcia-Segura LM, Di PT. Implication of the phosphatidylinositol-3 kinase/protein kinase B signaling pathway in the neuroprotective effect of estradiol in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mice. Mol Pharmacol. 2006;69:1492–1498. doi: 10.1124/mol.105.018671. [DOI] [PubMed] [Google Scholar]
- 101.Lacreuse A, Wilson ME, Herndon JG. No effect of different estrogen receptor ligands on cognition in adult female monkeys. Physiol Behav. 2009;96:448–456. doi: 10.1016/j.physbeh.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor β knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89:513–521. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alyea RA, Laurence SE, Kim SH, Katzenellenbogen BS, Katzenellenbogen JA, Watson CS. The roles of membrane estrogen receptor subtypes in modulating dopamine transporters in PC-12 cells. J Neurochem. 2008;106:1525–1533. doi: 10.1111/j.1471-4159.2008.05491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koldzic-Zivanovic N, Seitz PK, Watson CS, Cunningham KA, Thomas ML. Intracellular signaling involved in estrogen regulation of serotonin reuptake. Mol Cell Endocrinol. 2004;226:33–42. doi: 10.1016/j.mce.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 105.Watson CS, Pappas TC, Gametchu B. The other estrogen receptor in the plasma membrane: implications for the actions of environmental estrogens. Environ Health Perspect. 1995;103(Suppl 7):41–50. doi: 10.1289/ehp.95103s741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Filardo EJ, Quinn JA, Bland KI, Frackelton AR. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 107.Filardo EJ, Quinn JA, Frackelton AR, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: Stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 108.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 109.Xu H, Qin S, Carrasco GA, et al. Extra-nuclear estrogen receptor GPR30 regulates serotonin function in rat hypothalamus. Neuroscience. 2009;158:1599–1607. doi: 10.1016/j.neuroscience.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Watson CS, Alyea RA, Hawkins BE, Thomas ML, Cunningham KA, Jakubas AA. Estradiol effects on the dopamine transporter – protein levels, subcellular location, and function. J Mol Signal. 2006;1:5. doi: 10.1186/1750-2187-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bologa CG, Revankar CM, Young SM, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 112.Ariazi EA, Brailoiu E, Yerrum S, et al. The G protein-coupled receptor GPR30 inhibits proliferation of estrogen receptor-positive breast cancer cells. Cancer Res. 2010;70:1184–1194. doi: 10.1158/0008-5472.CAN-09-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thase ME, Haight BR, Richard N, et al. Remission rates following antidepressant therapy with bupropion or selective serotonin reuptake inhibitors: a meta-analysis of original data from 7 randomized controlled trials. J Clin Psychiatry. 2005;66:974–981. doi: 10.4088/jcp.v66n0803. [DOI] [PubMed] [Google Scholar]
- 114.Bannon MJ. The dopamine transporter: role in neurotoxicity and human disease. Toxicol Appl Pharmacol. 2005;204:355–360. doi: 10.1016/j.taap.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 115.Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 116.Giros B, Caron MG. Molecular characterization of the dopamine transporter. Trends Pharmacol Sci. 1993;14:43–49. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- 117.Blakely RD, Bauman AL. Biogenic amine transporters: regulation in flux. Curr Opin Neurobiol. 2000;10:328–336. doi: 10.1016/s0959-4388(00)00088-x. [DOI] [PubMed] [Google Scholar]
- 118.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl− dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 119.Pifl C, Drobny H, Reither H, Hornykiewicz O, Singer EA. Mechanism of the dopamine-releasing actions of amphetamine and cocaine: plasmalemmal dopamine transporter versus vesicular monoamine transporter. Mol Pharmacol. 1995;47:368–373. [PubMed] [Google Scholar]
- 120.Sandoval V, Riddle EL, Ugarte YV, Hanson GR, Fleckenstein AE. Methamphetamine-induced rapid and reversible changes in dopamine transporter function: an in vitro model. J Neurosci. 2001;21:1413–1419. doi: 10.1523/JNEUROSCI.21-04-01413.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kokoshka JM, Vaughan RA, Hanson GR, Fleckenstein AE. Nature of methamphetamine-induced rapid and reversible changes in dopamine transporters. Eur J Pharmacol. 1998;361:269–275. doi: 10.1016/s0014-2999(98)00741-9. [DOI] [PubMed] [Google Scholar]
- 122.Holton KL, Loder MK, Melikian HE. Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization – on line in press. Nat Neurosci. 2005;8:881–888. doi: 10.1038/nn1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jayanthi LD, Samuvel DJ, Blakely RD, Ramamoorthy S. Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis, and phosphorylation. Mol Pharmacol. 2005;67:2077–2087. doi: 10.1124/mol.104.009555. [DOI] [PubMed] [Google Scholar]
- 124.Ramamoorthy S, Blakely RD. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science. 1999;285:763–766. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- 125.Melikian HE. Neurotransmitter transporter trafficking: endocytosis, recycling, and regulation. Pharmacol Ther. 2004;104:17–27. doi: 10.1016/j.pharmthera.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 126.Gulley JM, Doolen S, Zahniser NR. Brief, repeated exposure to substrates down-regulates dopamine transporter function in Xenopus oocytes in vitro and rat dorsal striatum in vivo. J Neurochem. 2002;83:400–411. doi: 10.1046/j.1471-4159.2002.01133.x. [DOI] [PubMed] [Google Scholar]
- 127.Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD. Enhanced activity of human serotonin transporter variants associated with autism. Philos Trans R Soc Lond B Biol Sci. 2009;364:163–173. doi: 10.1098/rstb.2008.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Eriksen J, Rasmussen SG, Rasmussen TN, et al. Visualization of dopamine transporter trafficking in live neurons by use of fluorescent cocaine analogs. J Neurosci. 2009;29:6794–6808. doi: 10.1523/JNEUROSCI.4177-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jeng YJ, Kochukov MY, Watson CS. Membrane estrogen receptor-α-mediated nongenomic actions of phytoestrogens in GH3/B6/F10 pituitary tumor cells. J Mol Signal. 2009;4:2. doi: 10.1186/1750-2187-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jeng YJ, Watson CS. Proliferative and anti-proliferative effects of dietary levels of phytoestrogens in rat pituitary GH3/B6/F10 cells – the involvement of rapidly activated kinases and caspases. BMC Cancer. 2009;9:334. doi: 10.1186/1471-2407-9-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Watson CS. Signaling themes shared between peptide and steroid hormones at the plasma membrane. Sci STKE. 1999;1999:E1. doi: 10.1126/stke.1999.12.pe1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–1699. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gorentla BK, Moritz AE, Foster JD, Vaughan RA. Proline-directed phosphorylation of the dopamine transporter N-terminal domain. Biochem. 2009;48:1067–1076. doi: 10.1021/bi801696n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Price DA, Sorkin A, Zahniser NR. Cyclin-dependent kinase 5 inhibitors: inhibition of dopamine transporter activity. Mol Pharmacol. 2009;76:812–823. doi: 10.1124/mol.109.056978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eshleman AJ, Henningsen RA, Neve KA, Janowsky A. Release of dopamine via the human transporter. Mol Pharmacol. 1994;45:312–316. [PubMed] [Google Scholar]
- 136.Holton KL, Loder MK, Melikian HE. Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization. Nat Neurosci. 2005;8:881–888. doi: 10.1038/nn1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Torres GE, Carneiro A, Seamans K, et al. Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. J Biol Chem. 2003;278:2731–2739. doi: 10.1074/jbc.M201926200. [DOI] [PubMed] [Google Scholar]
- 138.Chambliss KL, Yuhanna IS, Mineo C, et al. Estrogen receptor α and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87:E44–E52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- 139.Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor α variant (ER46) in human endothelial cells. Proc Natl Acad Sci U S A. 2003;100:4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zivadinovic D, Watson CS. Membrane estrogen receptor-α levels predict estrogen-induced ERK1/2 activation in MCF-7 cells. Breast Cancer Res. 2005;7:R130–R144. doi: 10.1186/bcr959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Smart EJ, Ying YS, Anderson RG. Hormonal regulation of caveolae internalization. J Cell Biol. 1995;131:929–938. doi: 10.1083/jcb.131.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mozley LH, Gur RC, Mozley PD, Gur RE. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry. 2001;158:1492–1499. doi: 10.1176/appi.ajp.158.9.1492. [DOI] [PubMed] [Google Scholar]
- 143.Munro CA, McCaul ME, Wong DF, et al. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006;59:966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 144.Evans SM. The role of estradiol and progesterone in modulating the subjective effects of stimulants in humans 1. Exp Clin Psychopharmacol. 2007;15:418–426. doi: 10.1037/1064-1297.15.5.418. [DOI] [PubMed] [Google Scholar]
- 145.White TL, Justice AJ, de WH. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase 1. Pharmacol Biochem Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- 146.Fog JU, Khoshbouei H, Holy M, et al. Calmodulin Kinase II Interacts with the Dopamine Transporter C Terminus to Regulate Amphetamine-Induced Reverse Transport. Neuron. 2006;51:417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 147.Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annual Review of Pharmacology and Toxicology. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 148.Alyea RA, Watson CS. Differential regulation of dopamine transporter function and location by low concentrations of environmental estrogens and 17β-estradiol. Environ Health Perspect. 2009;117:778–783. doi: 10.1289/ehp.0800026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Furman CA, Chen R, Guptaroy B, Zhang M, Holz RW, Gnegy M. Dopamine and amphetamine rapidly increase dopamine transporter trafficking to the surface: live-cell imaging using total internal reflection fluorescence microscopy. J Neurosci. 2009;29:3328–3336. doi: 10.1523/JNEUROSCI.5386-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boudanova E, Navaroli DM, Melikian HE. Amphetamine-induced decreases in dopamine transporter surface expression are protein kinase C-independent. Neuropharmacology. 2008;54:605–612. doi: 10.1016/j.neuropharm.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Belcher SM, Le HH, Spurling L, Wong JK. Rapid estrogenic regulation of extracellular signal- regulated kinase 1/2 signaling in cerebellar granule cells involves a G protein- and protein kinase A-dependent mechanism and intracellular activation of protein phosphatase 2A. Endocr. 2005;146:5397–5406. doi: 10.1210/en.2005-0564. [DOI] [PubMed] [Google Scholar]
- 152.Foster JD, Cervinski MA, Gorentla BK, Vaughan RA. Regulation of the dopamine transporter by phosphorylation. Handb Exp Pharmacol. 2006:197–214. doi: 10.1007/3-540-29784-7_10. [DOI] [PubMed] [Google Scholar]
- 153.Zahniser NR, Sorkin A. Trafficking of dopamine transporters in psychostimulant actions. Semin Cell Dev Biol. 2009;20:411–417. doi: 10.1016/j.semcdb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bulayeva NN, Watson CS. Xenoestrogen-induced ERK 1 and 2 activation via multiple membrane-initiated signaling pathways. Environ Health Perspect. 2004;112:1481–1487. doi: 10.1289/ehp.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Narita SI, Goldblum RM, Watson CS, et al. Environmental estrogens induce mast cell degranulation and enhance IgE-mediated release of allergic mediators PMCID:17366818. Env Health Perspect. 2007;115:48–52. doi: 10.1289/ehp.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Watson CS, Alyea RA, Jeng YJ, Kochukov MY. Nongenomic actions of low concentration estrogens and xenoestrogens on multiple tissues PMCID:17601655. Mol Cell Endocrinol. 2007;274:1–7. doi: 10.1016/j.mce.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wozniak AL, Bulayeva NN, Watson CS. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-α-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ Health Perspect. 2005;113:431–439. doi: 10.1289/ehp.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic β-cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006;114:106–112. doi: 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Midoro-Horiuti T, Tiwari R, Watson CS, Goldblum RM. Maternal bisphenol a exposure promotes the development of experimental asthma in mouse pups. Environ Health Perspect. 2010;118:273–277. doi: 10.1289/ehp.0901259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McKinlay R, Plant JA, Bell JN, Voulvoulis N. Calculating human exposure to endocrine disrupting pesticides via agricultural and non-agricultural exposure routes. Sci Total Environ. 2008;398:1–12. doi: 10.1016/j.scitotenv.2008.02.056. [DOI] [PubMed] [Google Scholar]
- 161.Hotchkiss AK, Rider CV, Blystone CR, et al. Fifteen years after “Wing-spread” – environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicol Sci. 2008;105:235–259. doi: 10.1093/toxsci/kfn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Colborn T. Neurodevelopment and endocrine disruption. Environ Health Perspect. 2004;112:944–949. doi: 10.1289/ehp.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB. The pancreatic β-cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol. 2009;304:63–68. doi: 10.1016/j.mce.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 164.Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocr. 1993;132:2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- 165.Milligan SR, Balasubramanian AV, Kalita JC. Relative potency of xenobiotic estrogens in an acute in vivo mammalian assay. Environ Health Perspect. 1998;106:23–26. doi: 10.1289/ehp.9810623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.vom Saal FS, Cooke PS, Buchanan DL, et al. A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol Ind Health. 1998;14:239–260. doi: 10.1177/074823379801400115. [DOI] [PubMed] [Google Scholar]
- 167.Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol. 2003;37:4543–4553. doi: 10.1021/es0264596. [DOI] [PubMed] [Google Scholar]
- 168.Herbst AL. Behavior of estrogen-associated female genital tract cancer and its relation to neoplasia following intrauterine exposure to diethylstilbestrol (DES) Gynecol Oncol. 2000;76:147–156. doi: 10.1006/gyno.1999.5471. [DOI] [PubMed] [Google Scholar]
- 169.Lakshmana MK, Raju TR. Endosulfan induces small but significant changes in the levels of noradrenaline, dopamine and serotonin in the developing rat brain and deficits in the operant learning performance. Toxicology. 1994;91:139–150. doi: 10.1016/0300-483x(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 170.Bornman MS, Pretorius E, Marx J, Smit E, van der Merwe CF. Ultrastructural effects of DDT, DDD, and DDE on neural cells of the chicken embryo model. Environ Toxicol. 2007;22:328–336. doi: 10.1002/tox.20261. [DOI] [PubMed] [Google Scholar]
- 171.Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free Radic Biol Med. 2001;31:1473–1485. doi: 10.1016/s0891-5849(01)00726-2. [DOI] [PubMed] [Google Scholar]
- 172.Naqvi SM, Vaishnavi C. Bioaccumulative potential and toxicity of endosulfan insecticide to non-target animals. Comp Biochem Physiol C. 1993;105:347–361. doi: 10.1016/0742-8413(93)90071-r. [DOI] [PubMed] [Google Scholar]
- 173.Otaka H, Yasuhara A, Morita M. Determination of bisphenol A and 4-nonylphenol in human milk using alkaline digestion and cleanup by solid-phase extraction. Anal Sci. 2003;19:1663–1666. doi: 10.2116/analsci.19.1663. [DOI] [PubMed] [Google Scholar]
- 174.Sajiki J, Takahashi K, Yonekubo J. Sensitive method for the determination of bisphenol-A in serum using two systems of high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1999;736:255–261. doi: 10.1016/s0378-4347(99)00471-5. [DOI] [PubMed] [Google Scholar]
- 175.Myers JP, vom Saal FS, Akingbemi BT, et al. Why public health agencies cannot depend on good laboratory practices as a criterion for selecting data: the case of bisphenol A. Environ Health Perspect. 2009;117:309–315. doi: 10.1289/ehp.0800173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Alonso-Magdalena P, Laribi O, Ropero AB, et al. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic α-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environ Health Perspect. 2005;113:969–977. doi: 10.1289/ehp.8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bulayeva NN, Watson CS. Xenoestrogen-induced ERK-1 and ERK-2 activation via multiple membrane-initiated signaling pathways. Environ Health Perspect. 2004;112:1481–1487. doi: 10.1289/ehp.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zsarnovszky A, Le HH, Wang HS, Belcher SM. Ontogeny of rapid estrogen-mediated extracellular signal-regulated kinase signaling in the rat cerebellar cortex: potent nongenomic agonist and endocrine disrupting activity of the xenoestrogen bisphenol A. Endocr. 2005;146:5388–5396. doi: 10.1210/en.2005-0565. [DOI] [PubMed] [Google Scholar]
- 179.Midoro-Horiuti T, Tiwari R, Watson CS, Goldblum RM. Maternal bisphenol A exposure promotes the development of experimental asthma in mouse pups. Environ Health Perspect. 2010;118:273–277. doi: 10.1289/ehp.0901259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Della SD, Farabollini F, Dessi-Fulgheri F, Fusani L. Environmental-like exposure to low levels of estrogen affects sexual behavior and physiology of female rats. Endocrinology. 2008;149:5592–5598. doi: 10.1210/en.2008-0113. [DOI] [PubMed] [Google Scholar]
- 181.MacLusky NJ, Hajszan T, Leranth C. The environmental estrogen bisphenol a inhibits estradiol-induced hippocampal synaptogenesis. Environ Health Perspect. 2005;113:675–679. doi: 10.1289/ehp.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Palanza P, Gioiosa L, vom Saal FS, Parmigiani S. Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environ Res. 2008;108:150–157. doi: 10.1016/j.envres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 183.Zhou R, Zhang Z, Zhu Y, Chen L, Sokabe M, Chen L. Deficits in development of synaptic plasticity in rat dorsal striatum following prenatal and neonatal exposure to low-dose bisphenol A. Neuroscience. 2009;159:161–171. doi: 10.1016/j.neuroscience.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 184.Xu X, Liu Y, Sadamatsu M, et al. Perinatal bisphenol A affects the behavior and SRC-1 expression of male pups but does not influence on the thyroid hormone receptors and its responsive gene. Neurosci Res. 2007;58:149–155. doi: 10.1016/j.neures.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 185.Fujimoto T, Kubo K, Aou S. Prenatal exposure to bisphenol A impairs sexual differentiation of exploratory behavior and increases depression-like behavior in rats. Brain Res. 2006;1068:49–55. doi: 10.1016/j.brainres.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 186.Negishi T, Kawasaki K, Suzaki S, et al. Behavioral alterations in response to fear-provoking stimuli and tranylcypromine induced by perinatal exposure to bisphenol A and nonylphenol in male rats. Environ Health Perspect. 2004;112:1159–1164. doi: 10.1289/ehp.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Watson CS, Bulayeva NN, Wozniak AL, Alyea RA. Xenoestrogens are potent activators of nongenomic estrogenic responses. Steroids. 2007;72:124–134. doi: 10.1016/j.steroids.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Suzuki T, Mizuo K, Nakazawa H, et al. Prenatal and neonatal exposure to bisphenol-a enhances the central dopamine d1 receptor-mediated action in mice: enhancement of the methamphetamine-induced abuse state. Neuroscience. 2003;117:639–644. doi: 10.1016/s0306-4522(02)00935-1. [DOI] [PubMed] [Google Scholar]
- 189.Miyatake M, Miyagawa K, Mizuo K, Narita M, Suzuki T. Dynamic changes in dopaminergic neurotransmission induced by a low concentration of bisphenol-A in neurones and astrocytes. J Neuroendocrinol. 2006;18:434–444. doi: 10.1111/j.1365-2826.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- 190.Whitten PL, Patisaul HB. Cross-species and interassay comparisons of phytoestrogen action [Review] Environ Health Perspect. 2001;109:5–20. doi: 10.1289/ehp.01109s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Adlercreutz H, Yamada T, Wahala K, Watanabe S. Maternal and neonatal phytoestrogens in Japanese women during birth. Am J Obstet Gynecol. 1999;180:737–743. doi: 10.1016/s0002-9378(99)70281-4. [DOI] [PubMed] [Google Scholar]
- 192.Adlercreutz H, Gorbach SL, Goldin BR, Woods MN, Dwyer JT, Hamalainen E. Estrogen metabolism and excretion in oriental and caucasian women. J Natl Cancer Inst. 1994;86:1076–1082. doi: 10.1093/jnci/86.14.1076. [DOI] [PubMed] [Google Scholar]
- 193.Adlercreutz H. Phytoestrogens: epidemiology and a possible role in cancer protection. [Review] Environ Health Perspect. 1995;103(Suppl 7):103–112. doi: 10.1289/ehp.95103s7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kumar P, Padi SS, Naidu PS, Kumar A. Effect of resveratrol on 3-nitropropionic acid-induced biochemical and behavioural changes: possible neuroprotective mechanisms. Behav Pharmacol. 2006;17:485–492. doi: 10.1097/00008877-200609000-00014. [DOI] [PubMed] [Google Scholar]
- 195.Sonmez U, Sonmez A, Erbil G, Tekmen I, Baykara B. Neuroprotective effects of resveratrol against traumatic brain injury in immature rats. Neurosci Lett. 2007;420:133–137. doi: 10.1016/j.neulet.2007.04.070. [DOI] [PubMed] [Google Scholar]
- 196.Zhao L, Brinton RD. WHI and WHIMS follow-up and human studies of soy isoflavones on cognition. Expert Rev Neurother. 2007;7:1549–1564. doi: 10.1586/14737175.7.11.1549. [DOI] [PubMed] [Google Scholar]
- 197.Whitten PL, Patisaul HB, Young LJ. Neurobehavioral actions of coumestrol and related isoflavonoids in rodents. Neurotoxicol Teratol. 2002;24:47–54. doi: 10.1016/s0892-0362(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 198.Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocr. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 199.Harris DM, Besselink E, Henning SM, Go VLW, Heber D. Phytoestrogens Induce Differential Estrogen Receptor α- or β-Mediated Responses in Transfected Breast Cancer Cells. Experimental Biology and Medicine. 2005;230:558–568. doi: 10.1177/153537020523000807. [DOI] [PubMed] [Google Scholar]
- 200.Lephart ED, West TW, Weber KS, et al. Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicol Teratol. 2002;24:5–16. doi: 10.1016/s0892-0362(01)00197-0. [DOI] [PubMed] [Google Scholar]
- 201.Lund TD, Lephart ED. Dietary soy phytoestrogens produce anxiolytic effects in the elevated plus-maze. Brain Res. 2001;913:180–184. doi: 10.1016/s0006-8993(01)02793-7. [DOI] [PubMed] [Google Scholar]
- 202.Fernandez SP, Nguyen M, Yow TT, et al. The flavonoid glycosides, myricitrin, gossypin and naringin exert anxiolytic action in mice. Neurochem Res. 2009;34:1867–1875. doi: 10.1007/s11064-009-9969-9. [DOI] [PubMed] [Google Scholar]
- 203.Garey J, Morgan MA, Frohlich J, McEwen BS, Pfaff DW. Effects of the phytoestrogen coumestrol on locomotor and fear-related behaviors in female mice. Horm Behav. 2001;40:65–76. doi: 10.1006/hbeh.2001.1660. [DOI] [PubMed] [Google Scholar]
- 204.Paech K, Webb P, Kuiper GGJM, et al. Differential ligand activation of estrogen receptors ERa and ERb at AP1 sites. Science. 1997;227:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 205.Hall JM, McDonnell DP. The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocr. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 206.Lee YB, Lee HJ, Sohn HS. Soy isoflavones and cognitive function. J Nutr Biochem. 2005;16:641–649. doi: 10.1016/j.jnutbio.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 207.Sheehan DM. No-threshold dose-response curves for nongenotoxic chemicals: findings and applications for risk assessment. Environ Res. 2006;100:93–99. doi: 10.1016/j.envres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 208.Kochukov MY, Jeng YJ, Watson CS. Alkylphenol xenoestrogens with varying carbon chain lengths differentially and potently activate signaling and functional responses in GH3/B6/F10 somatomammotropes. Env Health Perspect. 2009;117:723–730. doi: 10.1289/ehp.0800182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sheffler DJ, Conn PJ. Allosteric potentiators of metabotropic glutamate receptor subtype 1a differentially modulate independent signaling pathways in baby hamster kidney cells. Neuropharmacology. 2008;55:419–427. doi: 10.1016/j.neuropharm.2008.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lipsett MB. Estrogen use and cancer risk. J Am Med Assoc. 1977;237:1112–1115. [PubMed] [Google Scholar]
- 211.Crandall CJ, Guan M, Laughlin GA, et al. Increases in Serum Estrone Sulfate Level Are Associated with Increased Mammographic Density during Menopausal Hormone Therapy. Cancer Epidemiol Biomarkers Prev. 2008;17:1674–1681. doi: 10.1158/1055-9965.EPI-07-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.File SE, Heard JE, Rymer J. Trough oestradiol levels associated with cognitive impairment in post-menopausal women after 10 years of oestradiol implants. Psychopharmacology (Berl) 2002;161:107–112. doi: 10.1007/s00213-002-1032-3. [DOI] [PubMed] [Google Scholar]
- 213.Maki PM. A systematic review of clinical trials of hormone therapy on cognitive function: effects of age at initiation and progestin use. Ann N Y Acad Sci. 2005;1052:182–197. doi: 10.1196/annals.1347.012. [DOI] [PubMed] [Google Scholar]
- 214.Prentice RL, Langer R, Stefanick ML, et al. Combined postmenopausal hormone therapy and cardiovascular disease: toward resolving the discrepancy between observational studies and the Women’s Health Initiative clinical trial. Am J Epidemiol. 2005;162:404–414. doi: 10.1093/aje/kwi223. [DOI] [PubMed] [Google Scholar]
- 215.Krieger N, Lowy I, Aronowitz R, et al. Hormone replacement therapy, cancer, controversies, and women’s health: historical, epidemiological, biological, clinical, and advocacy perspectives. J Epidemiol Community Health. 2005;59:740–748. doi: 10.1136/jech.2005.033316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Barrett-Connor E, Laughlin GA. Endogenous and exogenous estrogen, cognitive function, and dementia in postmenopausal women: evidence from epidemiologic studies and clinical trials. Semin Reprod Med. 2009;27:275–282. doi: 10.1055/s-0029-1216280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Laughlin GA, Kritz-Silverstein D, Barrett-Connor E. Endogenous oestrogens predict 4-year decline in verbal fluency in postmenopausal women: the Rancho Bernardo Study. Clin Endocrinol (Oxf) 2010;72:99–106. doi: 10.1111/j.1365-2265.2009.03599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Henderson VW. Aging, estrogens, and episodic memory in women. Cogn Behav Neurol. 2009;22:205–214. doi: 10.1097/WNN.0b013e3181a74ce7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sherwin BB. Estrogen and cognitive functioning in women. Endocr Rev. 2003;24:133–151. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- 220.Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 221.Knuuti J, Kalliokoski R, Janatuinen T, et al. Effect of estradiol-drospirenone hormone treatment on myocardial perfusion reserve in postmenopausal women with angina pectoris. Am J Cardiol. 2007;99:1648–1652. doi: 10.1016/j.amjcard.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 222.Simpkins JW, Green PS, Gridley KE, Singh M, de Fiebre NC, Rajakumar G. Role of estrogen replacement therapy in memory enhancement and the prevention of neuronal loss associated with Alzheimer’s disease. Am J Med. 1997;103:19S–25S. doi: 10.1016/s0002-9343(97)00260-x. [DOI] [PubMed] [Google Scholar]
- 223.Haynes LE, Lendon CL, Barber DJ, Mitchell IJ. 17 β-oestradiol attenuates dexamethasone-induced lethal and sublethal neuronal damage in the striatum and hippocampus. Neuroscience. 2003;120:799–806. doi: 10.1016/s0306-4522(03)00167-2. [DOI] [PubMed] [Google Scholar]
- 224.Arvin M, Fedorkova L, Disshon KA, Dluzen DE, Leipheimer RE. Estrogen modulates responses of striatal dopamine neurons to MPP(+): evaluations using in vitro and in vivo techniques. Brain Res. 2000;872:160–171. doi: 10.1016/s0006-8993(00)02511-7. [DOI] [PubMed] [Google Scholar]
- 225.vom Saal FS, Hughes C. An extensive new literature concenring low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Greenspan FS, Gardner DG. Appendix: Normal Hormone Reference Ranges. In: Greenspan FS, Gardner DG, editors. Basic and Clinical Endocrinology. 7th ed. New York: Lange Medical Books, McGraw Hill; 2004. pp. 925–926. [Google Scholar]
- 227.Katzenellenbogen BS, Bhardwaj B, Fang H, et al. Hormone binding and transcription activation by estrogen receptors: analyses using mammalian and yeast systems. J Steroid Biochem Mol Biol. 1993;47:39–48. doi: 10.1016/0960-0760(93)90055-2. [DOI] [PubMed] [Google Scholar]