Introduction
Mammography has widespread acceptance as a screening modality for breast cancer, with mortality reduction of 30–40% in screened populations (1). However, mammography has limitations. Its sensitivity for cancer detection when used for screening lies in the 80% range, ranging from 70.8% in the 40–44 age group to 84.5% for 75–89 year old patients (2). In addition, there is an accepted recall rate of 10% (with 90% of those recalled findings proving to be non actionable) and an expected negative biopsy rate in range of 60–75% for mammographically-identified suspicious lesions, both of which result in added cost, anxiety, and potential morbidity.
One inherent limitation of planar mammography is that a three-dimensional, volumetric structure (the breast) is imaged, but the resultant data are displayed in a two- dimensional manner. This leads to superimposition of tissue and limits effectiveness, especially in women with dense breasts. Carney et al showed a decrease in mammographic sensitivity as a function of increasing breast density (87.0% sensitivity in women with fatty breasts versus 62.9% in women with extremely dense breasts) (3). Berg and colleagues demonstrated an even lower sensitivity rate of 45% among women with extremely dense breasts (4). Yankaskas and colleagues showed that as breast density increases, so does recall rate from 2.4% in almost entirely fat breasts to 6.9% in extremely dense breasts (5). This makes intuitive sense, as most non-calcified cancers and normal fibroglandular tissue share similar X-ray attenuation values; as a result, the presence of a significant amount of this “background noise” (heterogeneously or extremely dense parenchymal pattern) can lead to misrepresentation of normal tissue as a potential abnormality requiring recall (false positive) or, more ominously, non-detection of a cancer obscured by adjacent or overlapping tissue (false negative). Obtaining two near-orthogonal mammographic views (rather than a single view) serves as an attempt at three-dimensionality, helping to sort out real from spurious findings, and aiding in localization of potential abnormalities. However, even when additional views (e.g. 90° lateral, step-obliques, rolled view s) are obtained, it remains the mammographer’s burden to construct a mental 3-D virtual image of the breast, and superimposition of tissue in women with any significant degree of density persists as a limiting constraint.
Full-field digital mammography (FFDM), with its improved dynamic range, tissue contrast, and ability to post-process digital images, was expected to improve cancer detection, particularly in dense-breasted women. This was confirmed in the Digital Mammographic Imaging Screening Trial (DMIST), which showed statistically significant improved sensitivity for cancer detection with FFDM compared to SFM, but only in women under 50, pre- or peri-menopausal, and with dense breasts, the latter factor appearing to be the key (6,7). However, the issue of tissue superimposition lingers, for while the digital images can be manipulated in many ways, they remain planar.
Imaging the breast with ultrasound (US) more closely approximates a 3-D examination, with 2 planes (depth and the chosen transducer orientation plane) visualized at any single moment with the third dimension added by the operator’s real time sweep of the transducer. In most cases, all tissue from skin to chest wall can be interrogated in a single frame, such that superimposition of tissue is not a factor. The effectiveness of US as an adjunctive breast cancer screening tool has been demonstrated recently, with American College of Radiology Imaging Network (ACRIN) protocol 6666 showing a supplemental detection yield (over and above cancers found by mammography alone) of 4.2 cancers per 1000 high risk women undergoing screening (8). However, the real time nature of breast US introduces operator-dependence to a far greater degree than is found in mammography and US is most effectively used when the imaging physician performs or is present during scanning. This can be time consuming, and therefore physician labor-intensive and cost-ineffective. Another limitation of breast US, as demonstrated by Berg and colleagues, is a relatively low positive predictive value for biopsy at 8.9% versus 22.6% for mammography, in the screening setting (8). Evolving technological enhancements, including elastography and 3D/4D breast US, may improve its performance in the near future.
An additional exciting bonus to the advent of FFDM is its ability to allow new, novel computer-based technologies. Digital breast tomosynthesis (DBT) is one example that partially addresses the 2-D limitations of planar mammography. DBT is performed using a mammographic X-ray tube but differs significantly from standard digital mammography in that it obtains multiple low-dose images at angled increments along an arc centered around the compressed breast. This information is computer-compiled and subsequently displayed on a workstation that allows the breast to be examined on a slice-by-slice basis, at a variety of slice thicknesses, chosen by the interpreter. Each individual slice has the appearance of a planar mammographic view (either craniocaudal or mediolateral oblique, depending on how the breast was positioned at acquisition), albeit displaying information from only one tomographic plane at a time. The third dimension is added as the examiner scrolls through the breast from skin to skin. Using this imaging technique reduces the element of tissue superimposition, theoretically improving lesion detection capability and mass margin characterization. Smith et al showed that DBT combined with FFDM resulted in improved clinical performance (as measured by area under the ROC and recall rate reduction) compared with the use of FFDM alone, independent of radiologist experience (9). However, despite its theoretical promise, DBT has not yet found its niche in routine clinical practice, in part perhaps due to lack of a validated optimal viewing protocol, well-defined indications, dose considerations and the large number of reconstructed images that must be reviewed.
Stereoscopic digital mammography (SDM) is another spin-off of FFDM whose purpose is to mitigate the limitations of superimposed breast tissue. The technique is patterned after the concept of binocular vision; having two eyes separated by several centimeters allows each eye to receive slightly discrepant views of the world that the brain is able to compile into a 3-D construct. In SDM, two “discrepant” images are obtained in both the CC and MLO projections, each angled +/− 5° relative to standard tube position. These slightly angled images are sent to a specially-designed workstation where each image is projected separately, then filtered through a polarizing screen. The observer, who is wearing cross-polarized viewing glass, can see only one of the two angled images with each eye. From this data set, the observer’s brain constructs a virtual 3-D image of the breast. While tissue is still superimposed (the way trees viewed in a forest would be), the viewer can perceive the relative depth and location of structures, helping to differentiate significant findings from spurious ones related to simple superimposition. Getty et al, demonstrated a PPV of true lesion detection of 32% for SDM and 26% for standard 2D digital mammography (10). However, these promising results came with a “cost”. Since each of the two stereo views was obtained at a standard dose, each stereo acquisition in the MLO and CC views was twice that of the standard dose. This is untenable in a population based screening situation and work is underway to duplicate the study with a dose similar to standard digital mammography.
While strides have been made toward advancing breast imaging beyond planar mammography, each modality described above has significant limitations. As a result, the search for other promising technologies continues. Dedicated breast CT (DBCT) represents a new technology which may allow true three dimensional imaging of the breast using the same fundamental x-ray contrast mechanism with which breast imagers are already familiar.
Technical Aspects of Dedicated Breast CT
Dedicated Breast CT Basic Geometry
Conventional whole body CT scanners use detector arrays which are arranged in an arc around the patient (Figure 1A). The detector arrays are composed of individual detector modules, which are small, modular, planar arrays, arranged on an arced support. The arc in conventional whole body scanners usually spans an angle of about 60°, and the collimated beam width in the z-axis direction typically span from 20 mm to 40 mm.
Figure 1.


Figure 1a Traditional multiple detector array CT makes use of a fan beam of radiation will very little divergence in the z-dimension. The cone angle of typical whole body CT scanners is on the order of 2°.
Figure 1b Breast CT systems generally use a flat panel detector which covers not only the fan beam of the scanner, but also the full extent in the z-dimension as well. The wider cone beam that results is capable of creating a complete CT data set of the breast in one rotation of the scanner around the breast.
Flat panel detectors for fluoroscopy are the enabling technology behind many cone beam CT systems, including those for DBCT (Figure 1B). Such detectors replace the entire fluoroscopic imaging detector, including the image intensifier, optical coupling, and optical camera. Presently, flat panel detectors are capable of real time readout (30 frames per second), in reduced resolution modes.
Prone patient positioning, with the breast imaged as it projects through a hole in the table top, is used for DBCT (Figure 2a). The x-ray tube and flat panel detector rotate around the breast in the horizontal plane and acquire the image data. Prototype breast CT systems (11, 12) make use of flat panel detectors, which give rise to the half cone beam geometry (Figure 2b) because the x-ray tube and detector need to rotate below the plane of the table This geometry is required to obtain x-ray projections far enough posterior into the breast to capture CT images of the chest wall.
Figure 2.

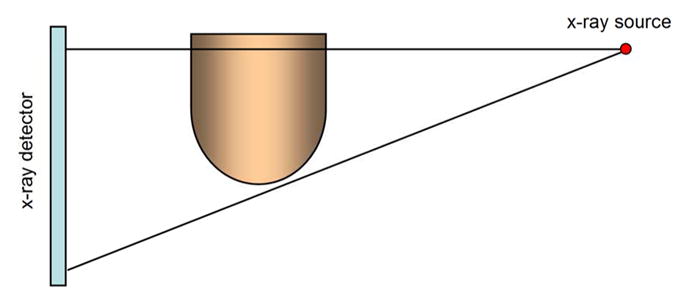
Figure 2a The breast to be imaged is positioned in a hole in the center of the breast CT table, as then women lies prone with her breast in the pendant position. This allows the CT scanner hardware to rotate in the horizontal plane and acquire the projection data necessary for cone beam CT reconstruction.
Figure 2b The cone angle in breast CT is quite large compared to whole body scanners, with cone angles up to about 15°. In whole body imaging, such a large cone angle would be prone to artifacts, but the breast has no highly attenuating anatomy (i.e. bones) and tapers in diameter anteriorly, and this reduces the propensity for cone beam artifacts.
System Hardware
All of the currently fabricated DBCT systems use the PAXSCAN flat panel detector (Varian Imaging Systems, Salt Lake City, UT) which has a 40 cm × 30 cm field of view at the detector plane, and represents a 2048 × 1536 array of 194 μm × 194 μm detector elements. In order to achieve 30 frame per second readout of the panel, the individual detector elements must be “binned” into 2 × 2 elements, creating an effective detector element which is 388 μm on a side. The readout array in this mode becomes 1024 × 768.
Scanning Protocols
The scanner developed at the University of Rochester (12) uses a pulsed x-ray source and a 10 second acquisition. A total of 300 views are acquired at 30 frames per second, using the PAXSCAN 4030CB detector. This scanner operates at a maximum of 49 kVp, and uses a digital mammography x-ray tube designed with a tungsten anode. There is a benefit of the pulsed x-ray tube design, where the short duration (~8 ms) of each x-ray pulse essentially freezes the rotational motion of the gantry, improving spatial resolution. The 10 second scan time should also be accepted well by women who undergo this breath-hold examination.
The University of California Davis scanners (11, 13–15) also use the PAXSCAN 4030CB detector system as well, but employ a continuous (non-pulsed) x-ray source operating typically at 80 kVp. At 30 frames per second, this results in a 33 ms acquisition time per frame with a total of 500 frames or projection images acquired over a 16.6 second acquisition sequence. This slightly longer acquisition time has also been well tolerated by patients with a single breath hold.
Data Processing
The reconstruction algorithm utilizes the entire data set of projection images acquired during the 360 degree rotation of the gantry around the breast. For the 500 projection images and the 786,000 pixels in each image, a total of 393 million data points are used to reconstruct the breast CT images. The reconstruction algorithm uses a cone beam reconstruction procedure to produce an isotropic three dimensional CT volume data set, consisting of a series of 5122 × 512 images.
Radiation Dose from Dedicated Breast CT
The radiation dose from CT in body imaging is substantially more than that required for digital radiography, and thus, for many years it was thought that CT would be dose prohibitive for breast imaging. However, the smaller dimensions of the breast of 140 mm average diameter, (range from 100 to 180 mm) in the pendant position combined with the lower tissue density of adipose (density ~ 0.93 g/cm3) and a higher x-ray beam energy (49–80 kVp) than mammography, suggest that breast CT can be performed at low radiation dose levels.
Monte Carlo techniques, which involve computer simulations of the x-ray beam passing through a mathematically-defined breast phantom, x-ray photon by x-ray photon, were employed for dose calculation in DBCT (16). Using these dose coefficients and the measured doses of two view mammography, the technique factors for DBCT are adjusted at UC Davis such that the average glandular dose is equal to that of two view mammography.
Clinical Experience with Dedicated Breast CT
Dedicated Breast CT Without Contrast
The first prospective comparison of noncontrast dedicated breast CT with screen film mammography was reported in 2008 (15). A prototype scanner was used to image the breasts of ten healthy volunteers and 69 women with Breast Imaging Reporting and Data System (BI-RADS) 4 or 5 (suspicious) lesions. The uncompressed breasts were scanned individually in the pendant position at 80kVp with the mAs chosen to deliver the same mean glandular dose as a two view mammogram. Scans were viewed in stack mode in coronal, sagittal and axial planes on dedicated software (Figure 3).
Figure 3.
The viewing software for DBCT from UCD allows visualization of the breast in stack mode in coronal, sagittal and axial planes. Lesions can be automatically localized in the three planes.
In women with lesions, the DBCT images were compared with mediolateral oblique and craniocaudal mammograms in a nonblinded study. Conspicuity of the lesion in both modalities was subjectively ranked by a single experienced dedicated breast imager. This initial study showed that overall conspicuity of breast lesions on DBCT was equal to that on mammography. There were no significant differences between DBCT and mammography for benign versus malignant lesions or as stratified by breast density; however masses were significantly more conspicuous on DBCT (p=0.002), and calcifications were better seen on mammography (p=0.006).
Updated results from the same trial using dedicated non-contrast DBCT amplifies these findings on 180 lesions (Table 1). Masses were more conspicuous on DBCT (p<0.001), and calcifications were significantly better visualized on mammography (p<0.001).
Table 1.
Conspicuity of Lesions on Non-Contrast Dedicated Breast CT Compared with Mammography***
| Score | ||||
|---|---|---|---|---|
| Category | No. of Breasts | Mean ± SD | Median * | Pvalue |
| All | 180 | 5.7 ± 2.1 | 5.5 (5.4, 5.6) | 0.20 |
| Lesion Type | ||||
| Masses | 124 | 4.9 ± 1.5 | 5.0 (4.7, 5.3) | < 0.001 ** |
| Calcification | 56 | 7.6 ± 1.9 | 8.0 (7.2, 8.8) | < 0.001 ** |
| Lesion Diagnosis | ||||
| Malignant | 97 | 5.5 ± 1.9 | 5.5 (5.3, 5.7) | 0.90 |
| Benign | 83 | 6.1 ± 2.2 | 5.5 (5.0, 6.0) | 0.10 |
Number in parentheses are 95% confidence units
Significant
Conspicuity was ranked from 1 to 10. A score of 5.5 indicates equal visualization with DBCT and mammography; a score of less than 5.5 indicates superior visualization with DBCT and a score greater than 5.5 indicates superior visualization with mammography.
The poor visualization of microcalcifications on unenhanced DBCT poses a significant barrier to the use of this modality for breast cancer screening since many cases of DCIS might not be visualized. For this reason the utility of contrast enhancement is now being studied..
Contrast Enhanced Dedicated Breast CT (CE-DBCT)
The use of intravenous contrast in other breast imaging modalities, particularly in MRI, has been successful in improving lesion conspicuity. Similar improvements would be expected in CE-DBCT. Additionally in CT the Hounsfield unit (HU) is directly proportional to attenuation and serves as a tool for quantification of differential capillary permeability between normal and malignant tissues (17–19).
In a pilot study, 46 women with 54 BIRADS 4 or 5 lesions underwent both non-contrast DBCT and CE-DBCT prior to image guided biopsy (20). Lesion conspicuity on CE-DBCT was compared to mediolateral oblique and craniocaudal mammograms. The conspicuity of each lesion was subjectively and independently scored by two radiologists on a continuous scale from −5 to 5, where zero corresponded to equivalence between the two modalities, negative values corresponded to better conspicuity on CE-DBCT and positive values corresponded to better conspicuity on mammography.
All 29 malignant lesions were significantly more conspicuous on CE-DBCT than on mammography (mean conspicuity = −1.45, p<0.001). When divided by lesion type, malignant masses were significantly more conspicuous on CE-DBCT than on mammography (mean conspicuity = −1.84, p<0.001) (Figure 4). The conspicuity of the 7 malignant calcification lesions was slightly better on CE-DBCT than on mammography (mean conspicuity = −0.29, p=0.64), but the difference was not statistically significant. Five of the 7 calcification lesions were pure DCIS (Figure 5).
Figure 4.
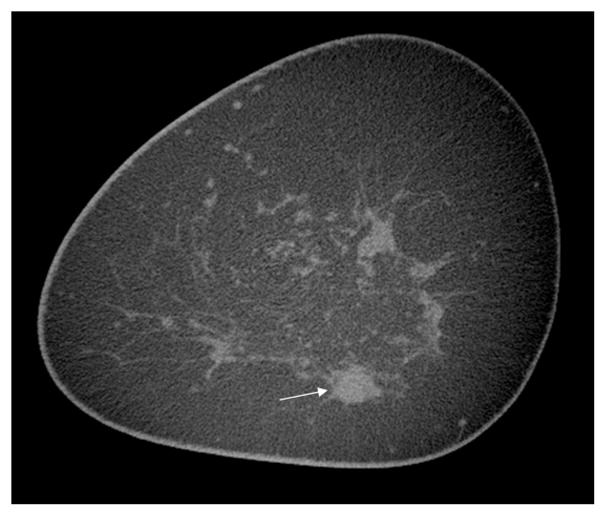


Figure 4a–c Non contrast (a) and contrast-enhanced (b) coronal University of California Davis DBCT scans of an enhancing infiltrating ductal carcinoma (arrows) at 6 o’clock in the left breast, which is partially obscured by superimposed tissue on the corresponding craniocaudal screening mammogram (c). The cancer enhanced by 43.7 HU on DBCT, while the normal glandular tissue at 12 o’clock did not enhance.
Figure 5.


Figure 5a–b Sagittal DBCT scans of DCIS showing a faint group of microcalcifications (arrow) on the University of California Davis non-contrast scan (a) with enhancement of the tumor (arrows) after contrast administration (b). The DCIS enhanced by 60.9 HU.
On CE-DBCT the conspicuity of the 25 benign lesions was similar to that on mammography (mean conspicuity = 0.34, p=0.46); however benign calcifications were significantly better seen on mammography (mean conspicuity=2.33, p<0.01), while benign masses were significantly better visualized on CE-DBCT (mean conspicuity = −1.07, p<0.01). The poor conspicuity of benign calcifications on CE-DBCT suggests the possibility that specificity in diagnosis of indeterminate calcifications may be improved by the use of CE-DBCT, though the numbers in this study are small and further clinical investigation is necessary.
For 52 of the 54 lesions in this pilot study, mean lesion voxel intensity (in Hounsfield units – HU) was measured on pre and post contrast DBCT scans to provide a quantified measurement of enhancement. Malignant lesions enhanced by a mean of 55.9 HU, while benign lesions enhanced by 17.6 HU (p<0.001). The five pure DCIS lesions enhanced by a mean of 59.6 HU, while benign calcifications enhanced by 24.9 HU.
Receiver operator curve (ROC) analysis of the quantitative enhancement of benign and malignant lesions yielded an area under the curve (AUC) of 0.876, suggesting that quantitative enhancement should be useful in predicting malignancy. A large study of the performance of clinical mammography reported an AUC of approximately 0.92 (21), and AUCs of 0.88 have been shown for MRI using both kinetic and morphologic information (22, 23). It seems likely that CE-DBCT could equal or surpass these AUCs if morphologic data are combined with quantitative enhancement metrics.
The use of intravenous contrast in DBCT clearly improves visualization of malignant lesions, including DCIS. Current scanner designs allow imaging of one breast at a time, which is a limitation, however the importance of the delay between contrast injection and imaging in tumor detection and diagnosis remains a research topic. In the pilot study of CE-DBCT described above, the delay between injection of contrast and imaging varied from 52–247 seconds (mean=96 sec), however the delay did not significantly affect lesion enhancement. It is plausible that serial imaging of both breasts with contrast enhanced DBCT will be diagnostically useful.
Patient Perceptions of Dedicated Breast CT
From the patient perspective, DBCT offers an advantage over mammography because breast compression is not currently required. Following both DBCT and CE- DBCT, 209 women were asked to complete a questionnaire regarding their comfort during the procedure rating from 1–10: 1) comfort of their position on the DBCT table ; 2) how difficult it was for them to maintain the breath hold of 16.6 seconds; 3) their overall comfort during DBCT; and 4) to compare their comfort during DBCT to their comfort during mammography. Additional comments about DBCT were also solicited at the end of the questionnaire.
Results (Table 2) showed that many women found the position on the DBCT table somewhat uncomfortable. It was difficult for many to arch their backs so as to push their breasts forward into the scanner for maximum coverage. Additionally many women complained that their necks were uncomfortable during scanning. However, when asked to rate the comfort of DBCT compared to mammography the vast majority preferred DBCT. Ergonomic refinements in the table top may be possible, but it is unlikely that these will be able to significantly decrease the discomfort of positioning. It is also possible for CE-DBCT, if subtraction imaging or kinetics is desired, that some gentle breast compression will eventually be required to limit patient motion.
Table 2.
2 Breast CT Comfort Survey Results In 209 Patients
| Score | ||
|---|---|---|
| Question | Mean ± SD | Median* |
| Position† | 6.6 ± 2.5 | 7 (6.3, 7.7) |
| Breast Hold‡ | 7.3 ± 2.3 | 8 (7.3, 8.7) |
| Comfort⫯ | 8.1 ± 5.0 | 8 (7.1, 8.9) |
| Breast CT vs Mammography↑ | 8.8 ± 2.0 | 10 (10, 10) |
Number in parentheses are the 95% confidence units.
Position refers to comfort of the position on the breast CT table (1 = poor, 10 = excellent).
Breath hold refers to ease of 18.8 second breath hold (1 = very difficult, 10 = not at all difficult).
Comfort refers to the over all comfort of breast CT (1 = very uncomfortable, 10 = extremely comfortable).
Breast CT Verses mammography refers to the comfort during breast CT as compared with comfort during screen-film mammography (1 = comfort was much worse then that of screen-film mammography, 5.5 = comfort was equal to that of screen-film mammography, 10 = breast CT was much more comfortable than screen-film mammography.uite 3100
The Future
The development of DBCT is in its infancy. The scanners currently in use for clinical research studies are prototypes. Reductions in noise and increases in spatial resolution are required to improve visualization of calcifications and to improve overall image quality. Changes in acquisition strategies including a pulsed, rather than a continuous x-ray source, and new detectors with reduced electronic noise and increased frame rates are being considered for the redesign and upgrading of the UC Davis scanners. Even with the above improvements and/or with reduced kVp used by some systems (24), it will be challenging for non-contrast DBCT to equal mammography in the depiction and characterization of microcalcifications.
An additional challenge for DBCT is coverage of the entire breast including the axillary tail extending to the chest wall. Refinements in table design have increased such coverage from the previous report in which chest wall musculature was visualized in only 17% of scanned patients (15), but further improvements are still required. X-ray tubes with a more compact housing design and detectors with smaller bezels (the frame surrounding the detector) are in development and will allow greater coverage of the posterior breast.
DBCT may not become a stand-alone replacement for screening mammography in the general population, however DBCT may play an important role in breast cancer imaging. If DBCT is used as a screening modality in conjunction with mammography, as is proposed for DBT, it is likely that the three-dimensional and multiplanar capabilities of DBCT would lead to a reduction in the number of patient recalls for summation artifacts and could increase sensitivity for masses (Figures 6, 7). Alternatively, DBCT could be used to analyze and validate findings suspected from a screening mammogram since a single scan will create an isotropic image data set of the breast and produce individual scans in the x, y and z directions. Regions of interest can be placed on any slice and be located on the full image as well as the corresponding slices in the other two orthogonal planes.
Figure 6.




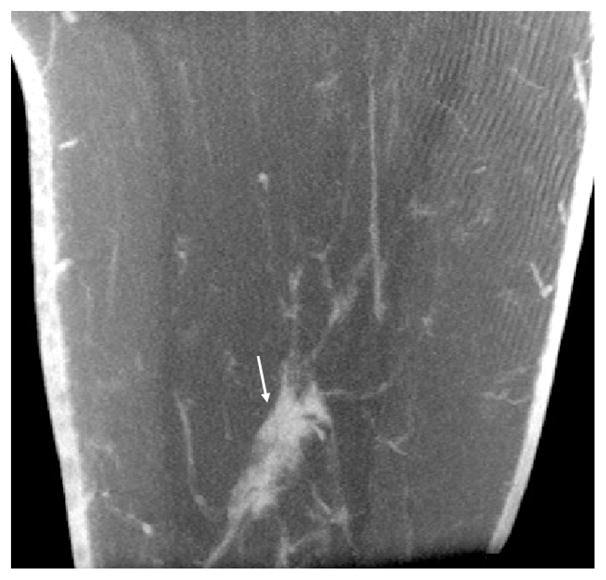
Figure 6a–e Craniocaudal (a) and mediolateral oblique (b) mammograms showing a spiculated mass (arrows), ultimately diagnosed as infiltrating ductal carcinoma at the 6 o’clock position in the right breast. Coronal (c), sagittal (d) and axial (e) DBCT scans from the Koning CBCT System performed on the same patient show the mass (arrows) without superimposed tissue.
Figure 7.



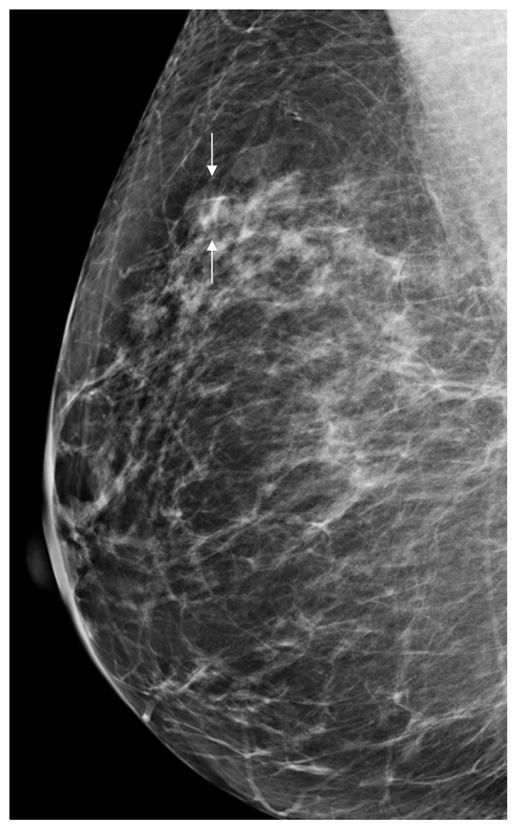
Figure 7a–d Axial (a) and sagittal (b) DBCT scans done without contrast demonstrate a fibroadenoma (arrows), which is not obscured by the overlying glandular tissue as it is on the corresponding craniocaudal (c) and mediolateral oblique (d) mammograms.
Initial work has shown that malignant lesions are significantly more conspicuous on CE-DBCT compared to mammography and DBCT. DCIS, manifested by microcalcifications alone, clearly enhances on CE-DBCT, although patient numbers are small and further study is necessary to determine whether enhancement is related to the nuclear grade of DCIS, as is now being postulated for DCIS enhancement in breast MRI (25).Quantification of lesion enhancement will likely be helpful in improving both sensitivity and specificity of CE-DBCT which could conceivably then replace breast MRI both in screening of high risk women and in the assessment of recently diagnosed cancers (Figure 8). The improved spatial resolution of CE-DBCT over MRI and similar vascular information with contrast, may lead to greater accuracy of CE-DBCT over breast MRI. There are also no metal precautions or issues with claustrophobia. It is likely that both equipment and exam costs for CE-DBCT will be lower than for MRI and, theoretically, these units can be easily sited in breast imaging centers. Patient throughput will be faster with CE-DBCT due to the short scan time of 10–16 seconds.
Figure 8.


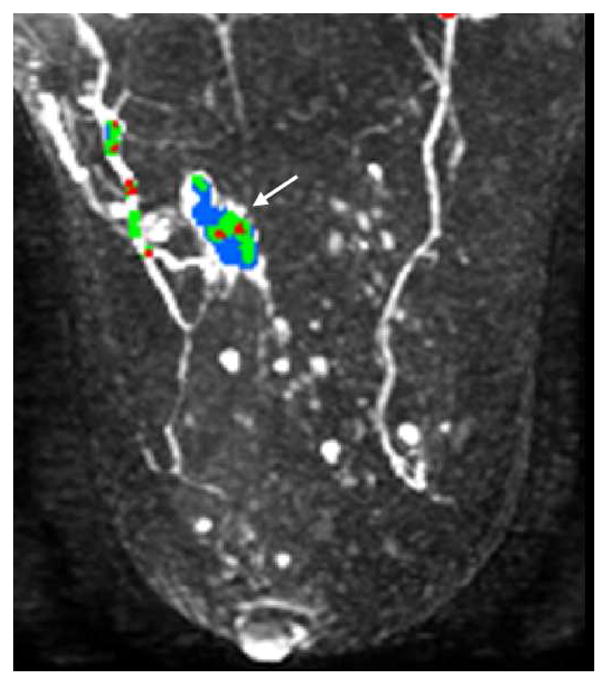
Figure 8a–c Axial contrast enhanced DBCT (a) with corresponding axial T1 subtraction breast MR (b) and maximum intensity projection MR with angiogenesis color overlay (c) of an infiltrating ductal carcinoma (arrows) of the left breast. The DBCT is comparable to the MR.
The DBCT platform is ideal for integration with other modalities useful in breast cancer detection, diagnosis and treatment. An integrated robotic biopsy device is under development and there is potential for incorporation of new therapeutic interventions, such as radiofrequency ablation, cryoablation or high-intensity focused ultrasound. DBCT is likely to be useful in external beam radiation therapy of the breast, since the pendant position will allow more reproducible positioning of the breast. Preliminary work has already shown that a relatively low energy x-ray source (such as 320 kVp), rather than the typical 6 MVp linear accelerator, can be used to deliver highly conformal dose distributions for partial breast irradiation, as well as the homogeneous dose distributions of whole breast irradiation. In addition, a small kVp-based radiation therapy system can be placed above ground in a leaded x-ray room, which would be a very cost effective solution for breast radiation therapy.
Molecular breast imaging offers significant potential for diagnosis and therapeutic evaluation of breast tumors by providing physiologic information. Today such techniques are based on radionuclides and include SPECT and PET. Dedicated breast SPECT and PET systems have each been integrated with DBCT systems (26, 27) to provide high resolution, co-registered anatomic and physiologic information.
In the only clinical study reported to date, four patients with mammographic findings that were highly suspicious for breast cancer, underwent dedicated breast PET/CT on a prototype unit (28). Among the four patients, three had invasive cancers, ranging from 9 mm to 25 mm in diameter and one had a 10mm pure DCIS. All of the invasive cancers were positive on PET, but on DBCT the invasive cancers were seen in only two patients (Figure 9). In the third patient the cancer was close to the chest wall and was not seen as it was out of the DBCT field of view. One patient with an invasive cancer also had an extensive intraductal component (EIC), which was well visualized on dedicated breast PET/CT; detailed histologic correlation of the mastectomy specimen with the breast PET/CT in that patient demonstrated excellent comparability. The one case of pure DCIS, seen as microcalcifications on mammography, was seen on the DBCT, but not on the PET images, likely due to patient motion. These are preliminary results, and although technical challenges remain, they appear promising.
Figure 9.
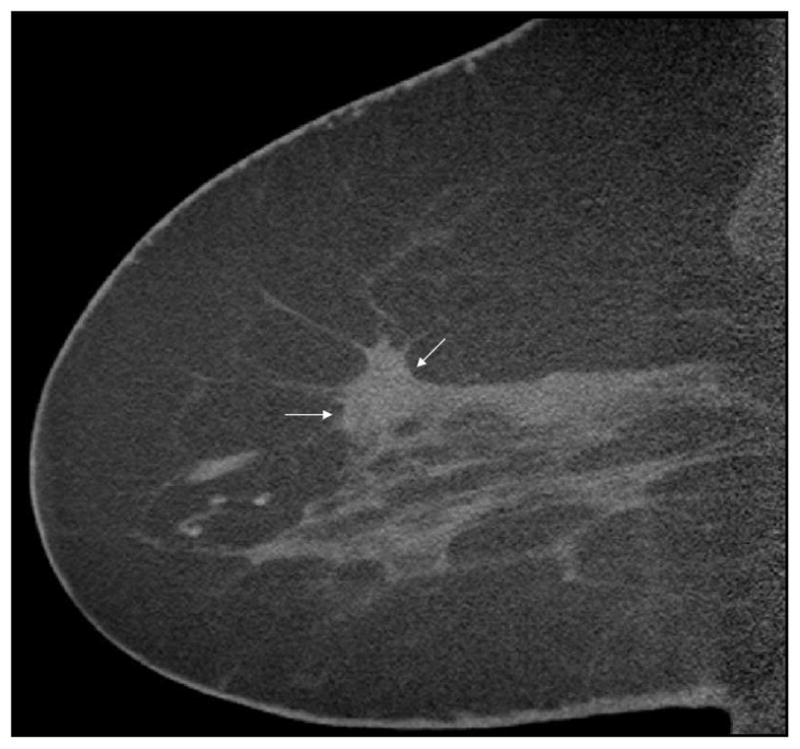


Figure 9a–c Non-contrast sagittal DBCT (a) showing a spiculated mass (arrows) partially obscured by surrounding dense tissue. Contrast subtraction sagittal scan (b), and fused PET/DBCT (c) of the same patient showing two areas of focal uptake on the PET/DBCT and two enhancing lesions (arrows), measuring 13 mm and 9 mm, on the subtraction image. Both were infiltrating ductal carcinoma; the second more posterior cancer was not appreciated initially on the corresponding screening mammogram (not shown). Reprinted with permission from: Bowen SL, Wu Y, Chaudhari AJ, et al. Initial characterization of a dedicated breast PET/CT scanner during human imaging. J Nucl Med 2009 Sept;50(9):1401–1408.
The improved spatial resolution of dedicated breast PET/CT compared with whole body PET/CT (3.7mm vs 6.4mm average full width at half maximum) has the potential to allow detection, diagnosis and evaluation of smaller lesions as well as more accurate local staging of cancers (28). It is also anticipated that quantitative high-resolution PET may play an important enabling role in monitoring response to neoadjuvant therapy. If CE-BCT is performed in conjunction with dedicated breast PET/CT, sensitivity and specificity of the system should improve further. DBCT integrated with PET should find increased utility as new breast cancer specific molecular imaging agents are discovered.
Further clinical comparison of DBCT with other established modalities such as MRI and with emerging technologies including DBT, SDM, 3D/4D ultrasound, ultrasound elastography and radionuclide imaging will be required in order to determine the optimal, most cost-effective strategies for the use of DBCT. In the next decade it is likely that we will move from a standardized approach to breast cancer screening into a more individualized approach based on such factors as risk and breast density. DBCT is likely to play a significant role in breast imaging as this paradigm shift occurs.
Summary
DBCT is a burgeoning technology that has many advantages over current breast imaging systems. Three- dimensional visualization of the breast mitigates the limiting effects of superimposition noted with mammography. Post-processing capabilities will allow application of advanced technologies, such as creation of MIP and subtraction images, as well as the use of both computer-aided detection and possible computer-aided diagnosis algorithms. Excellent morphologic detail and soft tissue contrast can be achieved, due in part to the isotropic image data that DBCT produces. The expected cost should be more reasonable than MRI. At present, since the breast is not compressed, patients find it more comfortable than mammography. Physiologic information can be obtained when intravenous contrast material is used and/or when DBCT is combined with SPECT or positron emission tomography (PET). DBCT provides an excellent platform for multimodality systems including integration with interventional and therapeutic procedures. With a slightly altered design, the DBCT platform may also be useful for external beam radiation with image guidance.
Acknowledgments
This was supported, in part, by Grant No. R01 EB002138 from the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith RA, Duffy SW, Gabe R, et al. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am. 2004 Sep;42(5):793–806. doi: 10.1016/j.rcl.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Performance measures for 3,603,832 screening mammography studies from 1996 to 2006 by age. Rockville, MD: National Cancer Institute; 2008. NCI-funded breast cancer surveillance consortium co-operative agreement. [Google Scholar]
- 3.Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003 Feb;138(3):168–175. doi: 10.7326/0003-4819-138-3-200302040-00008. [DOI] [PubMed] [Google Scholar]
- 4.Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US and MR imaging in pre-operative assessment of breast cancer. Radiology. 2004 Dec;233:830–849. doi: 10.1148/radiol.2333031484. [DOI] [PubMed] [Google Scholar]
- 5.Yankaskas BC, Cleveland RJ, Schell MJ, et al. Association of recall rates with sensitivity and positive predictive values of screening mammography. AJR Am J Roentgenol. 2001 Sep;177(3):543–549. doi: 10.2214/ajr.177.3.1770543. [DOI] [PubMed] [Google Scholar]
- 6.Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography in breast-cancer screening. N Engl J Med. 2005 Oct;353(17):1773–1783. doi: 10.1056/NEJMoa052911. [DOI] [PubMed] [Google Scholar]
- 7.Pisano ED, Hendrick RE, Yaffe MJ, et al. Diagnostic accuracy of digital versus film mammography: exploratory analysis of selected population subgroups in DMIST. Radiology. 2008 Feb;246(2):376–383. doi: 10.1148/radiol.2461070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008 May;299(18):2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith AP, Rafferty EA, Niklason L. Clinical performance of breast tomosynthesis as a function of radiologist experience level. LNCS. 2008;5116:61–66. [Google Scholar]
- 10.Getty DJ, D’Orsi CJ, Newell MS, et al. Improved accuracy of lesion detection in breast cancer screening with stereoscopic digital mammography (abstract) RSNA Scientific Assembly and Annual Meeting Program. 2007:381–382. [Google Scholar]
- 11.Boone JM, Nelson TR, Lindfors KK, et al. Dedicated breast CT: Radiation dose and image quality evaluation. Radiology. 2001 Dec;221(3):657–667. doi: 10.1148/radiol.2213010334. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Ning R. Why should breast tumour detection go three dimensional? Phys Med Biol. 2003 Jul;48(14):2217–2228. doi: 10.1088/0031-9155/48/14/312. [DOI] [PubMed] [Google Scholar]
- 13.Boone JM, Breast CT. Its prospect for breast cancer screening and diagnosis. In: Karellas A, Giger ML, editors. 2004 Syllabus: Advances in Breast Imaging Physics, Technology, and Clinical Applications; Oak Park, ILL: Radiol Soc of North Am; 2004. pp. 165–177. [Google Scholar]
- 14.Glick SJ. Breast CT. Annu Rev Biomed Eng. 2007;9:501–526. doi: 10.1146/annurev.bioeng.9.060906.151924. [DOI] [PubMed] [Google Scholar]
- 15.Lindfors KK, Boone JM, Nelson TR, et al. Dedicated breast CT: Initial clinical experience. Radiology. 2008 Mar;246(3):725–733. doi: 10.1148/radiol.2463070410. Epub 2008 Jan 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boone JM, Shah N, Nelson TR. A comprehensive analysis of DgN(CT) coefficients for pendant-geometry cone-beam breast computed tomography. Med Phys. 2004 Feb;31(2):226–235. doi: 10.1118/1.1636571. [DOI] [PubMed] [Google Scholar]
- 17.Dawson P. Functional imaging in CT. Eur J Radiol. 2006;60(3):331–340. doi: 10.1016/j.ejrad.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Miles KA. Tumour angiogenesis and its relation to contrast enhancement on computes tomography: a review. Eur J Radiol. 1999;30(3):198–205. doi: 10.1016/s0720-048x(99)00012-1. [DOI] [PubMed] [Google Scholar]
- 19.Cuenod CA, Fournier L, Balvay D, Guinebretiere JM. Tumor angiogenesis: pathophysiology and implactions for const-enhanced MRI and CT assessment. Abdom Imaging. 2006;31(2):188–193. doi: 10.1007/s00261-005-0386-5. [DOI] [PubMed] [Google Scholar]
- 20.Prionas ND, Lindfors KK, Ray S, et al. Contrast-enhanced dedicated breast computed tomography: Initial clinical experience. Radiology. 2010 doi: 10.1148/radiol.10092311. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen A, Vejborg I, Severinsen N, et al. Performance of clinical mammography: a nationwide study from Denmark. Int J Cancer. 2006;119(1):183–91. doi: 10.1002/ijc.21811. [DOI] [PubMed] [Google Scholar]
- 22.Warren RM, Thompson D, Pointon LJ, et al. Evaluation of a prospective scoring system designed for a multicenter breast MR imaging screening study. Radiology. 2006;239(3):677–85. doi: 10.1148/radiol.2393042007. [DOI] [PubMed] [Google Scholar]
- 23.Schnall MD, Blume J, Bluemke DA, et al. Diagnostic architectural and dynamic features at breast MR imaging: multicenter study. Radiology. 2006;238(1):42–53. doi: 10.1148/radiol.2381042117. [DOI] [PubMed] [Google Scholar]
- 24.Chen B, Ning R. Cone-beam volume CT breast imaging: Feasibility study. Med Phys. 2002 May;29(5):755–770. doi: 10.1118/1.1461843. [DOI] [PubMed] [Google Scholar]
- 25.Kuhl CK. Why do purely intraductal cancers enhance on breast MR images? Radiology. 2009;253(2):281–87. doi: 10.1148/radiol.2532091401. [DOI] [PubMed] [Google Scholar]
- 26.Wu Y, Bowen SL, Yang K, et al. PET characteristics of a dedicated breast PET/CT scanner prototype. Phys Med Biol. 2009 Jul;54(13):4273–4287. doi: 10.1088/0031-9155/54/13/020. Epub 2009 Jun 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKinley RL, Tornai MP, Brzymialkiewicz C, et al. Analysis of a novel offset cone-beam computed mammotomography system geometry for accommodating various breast sizes. Phys Med. 2006;21 (Suppl 1):48–55. doi: 10.1016/S1120-1797(06)80024-4. [DOI] [PubMed] [Google Scholar]
- 28.Bowen SL, Wu Y, Chaudhari AJ, et al. Initial characterization of a dedicated breast PET/CT scanner during human imaging. J Nucl Med. 2009 Sep;50(9):1401–1408. doi: 10.2967/jnumed.109.064428. [DOI] [PMC free article] [PubMed] [Google Scholar]



