Abstract
Endometriosis is a disease common in women that is defined by abnormal extrauteral growths of uterine endometrial tissue and associated with severe pain. Partly because how the abnormal growths become associated with pain is poorly understood, the pain is difficult to alleviate without resorting to hormones or surgery, which often produce intolerable side effects or fail to help. Recent studies in a rat model and women showed that sensory and sympathetic nerve fibers sprout branches to innervate the abnormal growths. This situation, together with knowledge that the endocannabinoid system is involved in uterine function and dysfunction and that exogenous cannabinoids were once used to alleviate endometriosis-associated pain, suggests that the endocannabinoid system is involved in both endometriosis and its associated pain. Here, using a rat model, we found that CB1 cannabinoid receptors are expressed on both the somata and fibers of both the sensory and sympathetic neurons that innervate endometriosis’s abnormal growths. We further found that CB1 receptor agonists decrease, whereas CB1 receptor antagonists increase, endometriosis-associated hyperalgesia. Together these findings suggest that the endocannabinoid system contributes to mechanisms underlying both the peripheral innervation of the abnormal growths and the pain associated with endometriosis, thereby providing a novel approach for the development of badly-needed new treatments.
Keywords: visceral pain, neural sprouting, neuroplasticity, neurovascular, transplant, sensory-sympathetic coupling
1. Introduction
Endometriosis, a disease common in women of childbearing age, is defined by abnormal growths of uterine endometrial tissue outside the uterus. Symptoms include dysmenorrhea, dyspareunia (vaginal hyperalgesia), chronic pelvic/abdominal musculoskeletal and other types of severe pain that are often difficult to alleviate without resorting either to hormonal treatments that produce often-intolerable side effects of hypoestrogenicity or to surgery that frequently fails to help [16,21,27,16,48]. New treatment strategies are badly needed. Although it is recognized that both inflammatory and neuropathic mechanisms contribute to endometriosis-associated pains, progress in developing new treatment strategies is impeded by a poor understanding of how the growths, which do not correlate with the pains, contribute to them [16,21,27,48].
A rat model of surgically-induced endometriosis (ENDO), in which ectopic uterine cysts form in the abdomen [51], develops pain symptoms similar to those of women, including vaginal hyperalgesia (10) and vaginally-referred abdominal muscle hyperalgesia [38]. The ENDO model’s surgical control (shamENDO) develops neither cysts nor pain symptoms [10,38]. The ectopic growths in both the rat model and in women recruit their own sensory and sympathetic nerve supply [4,5]. This innervation in the rat model, diagrammed in Fig. 1b, consists of branches of axons that sprout from pre-existing sensory neurons whose cell bodies are located in thoracic dorsal root ganglia (DRG) and from sympathetic neurons whose cell bodies are located in the coeliac ganglion (CG). Findings from the rat model and clinical studies strongly support the conclusion that this innervation contributes to endometriosis-associated pain [21,45,48].
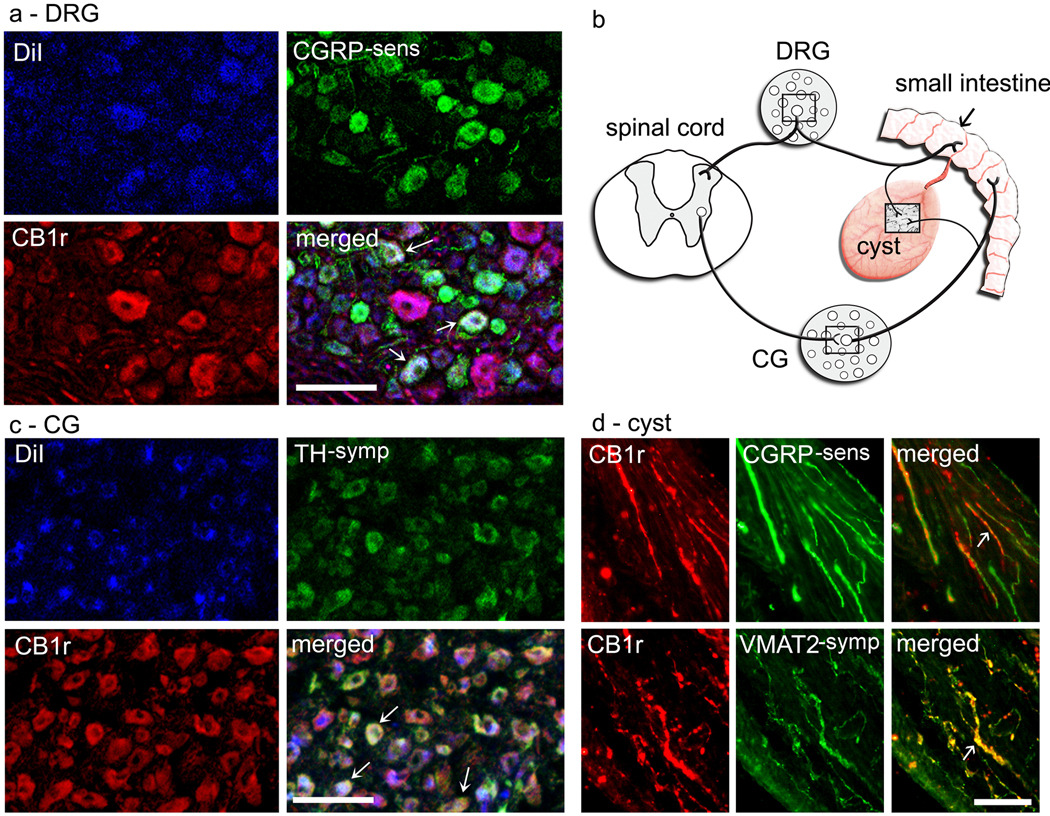
Figure 1.
CB1 co-localization on sensory and sympathetic axons in ectopic endometrial cysts, and on cyst-innervating sensory and post-ganglionic sympathetic ganglion cells in thoracic dorsal root ganglion (DRG) and coeliac ganglion (CG), respectively. Boxed areas in b show approximate locations of photomicrographs in the other panels. Panel a shows cyst-innervating neurons in a T10 DRG retrogradely-labeled with Dil dye (blue), some of which co-label with both CB1 (red) and sensory-calcitonin gene related peptide (CGRP-sens; green) antibodies. Tripe-labeled neurons appear white. Panel c shows cyst-innervating neurons in a CG, some of which co-label with both CB1 (red) and sympathetic-tyrosine hydroxylase (TH-symp; green) antibodies. Tripe-labeled neurons appear white. Panel d shows CB1-labeled (red) either CGRP-sens or vesicular monoamine transporter 2-sympathetic (VMAT2-symp)-labeled fibers (both green) in the cyst. Double-labeled fibers appear yellow. Arrows in all merged images point to examples of either tripe-labeled ganglion cells (panels a and c) or double-labeled axons (panel d). See Methods for details of image acquisition and preparation used to generate this figure. Calibration bars, 100 µm.
The CB1 cannabinoid receptor protein (CB1) is found in the rodent uterus and blastocyst where the receptor contributes to normal fetal implantation and implantation failure [41]. These findings implicate involvement of the endocannabinoid system in reproductive function and dysfunction. The endocannabinoid system plays a key role in pain mechanisms [42], and, previously, cannabinoids were long used by women to alleviate dysmenorrhea [45].
Together the findings suggest that the endocannabinoid system is involved in endometriosis and its associated pain via CB1 receptors and innervation of the ectopic growths. Using the rat model, we performed a combination of immunohistochemical and pharmacological studies to test this hypothesis and assess the endocannabinoid system’s potential as a target for new therapies. Studies were done when the rat was in proestrus, the estrous stage when hyperalgesia in the ENDO model is most severe [10,45,38].
2. Materials and methods
2.1. Animals and vaginal cytology
Adult female Sprague-Dawley rats (Charles River) were used. They weighed 175–200g at study start and 300–350g when euthanized. They were maintained on a 12-h light/dark cycle (lights on 07:00), housed individually in a temperature-controlled room (22.2°C) in plastic cages lined with wood chip bedding, with ad libitum access to Purina rat chow and water. Reproductive status was determined by histological examination of cells extracted by daily vaginal lavage done ~2h after lights on [3]. All rats cycled regularly (4-day cycle) during the study. Assessments were done when the rat was in proestrus (traditional nomenclature; [3]) 3 – 8 h after lights on. The studies were approved by Florida State University’s Animal Care and Use Committee and adhered to the guidelines of the Committee for Research and Ethical Issues of IASP [53].
2.2. Endometriosis (ENDO) and control (shamENDO) surgeries
Surgery was done under aseptic precautions using methods developed by Vernon and Wilson [51]. Rats in diestrus were anesthetized with a ketamine hydrochloride and xylazine mixture (K/X; 73 mg/kg and 8.8 mg/kg, respectively, i.p.) and put on a heating pad to maintain body temperature ~37°C. A midline incision was made to expose the uterus. A ~l-cm segment of left uterine horn and associated fat was removed and put in warm sterile saline. Four pieces of uterine horn (~2 mm × 2 mm) or, for shamENDO, four similarly-sized pieces of fat were cut from this segment. Using 4.0 nylon sutures, the pieces were sewn around alternate cascade mesenteric arteries starting from the caecum, and the wound closed in layers. Postoperative recovery was uneventful; regular estrous cyclicity resumed within a few days. All assessments were done > 6 wks after the surgery; i.e., when ENDO-induced hyperalgesia is fully-developed and stable [10,45,38].
2.3. Sample harvesting and tissue preparation for immunohistochemical studies
On the day of euthanization, each rat (in proestrus, 6 – 10 wks after ENDO surgery) was anesthetized with urethane (1.2 g/kg, i.p.) and perfused transcardially with 4% paraformaldehyde in 0.1M phosphate buffer. The cysts, a 1-cm section of the mid-right (healthy eutopic) uterine horn, dorsal root ganglia (DRG) from T3-S1, and the coeliac ganglion (CG) were harvested and post-fixed in the same fixative for 4h. Samples were cryoprotected in 30% sucrose and stored at −80°C until sectioned. Cysts were embedded in Histo Prep medium (Fisher Scientific), cut serially in 20µm-thick sections on a cryostat, and thaw mounted on subbed slides.
2.4. Single CB1 immunostaining of cysts
The CB1 receptor antibody was raised in rabbits using a glutathione-S-transferase fusion protein containing the last fifteen amino acids of rat CB1. This same fusion protein was used for affinity purification (details in [6]). The specificity of this antibody has been demonstrated by a lack of immunoreactivity in CB1 receptor knockout mice [6]. Antigen was retrieved in 10 mM sodium citrate (pH 9.0), 80°C for 5 min. Sections were quenched (l% NaHB4, 10 min; 0.3% H2O2, 15 min), then blocked in 0.3% Triton X-100 in 0.05 M Tris-NaCl with 10% normal goat serum (NGS) and avidin (2 drops/ml), 1 h. Sections were incubated with rabbit anti-CB1 primary antibody (1:3000) in 0.3% Triton X-100 in 0.05 M Tris-NaCl, including 2% NGS and biotin (2 drops/ml) at 4°C for 48 h. Sections were incubated in biotinylated goat anti-rabbit IgG (1:300; Vector, at room temperature (RT) for 2 h, then incubated in avidin-biotin-peroxidase complex (Elite kit, Vector) for 1.5 h. Staining was visualized with 3,3’-diaminobenzidine (DAB kit, Vector). Controls were omission of primary or secondary antibody and absorption of primary antiserum with its respective antigen prior to use. There was no labeling in control sections.
2.5. Quantification of CB1-positive fibers in cysts and uterus
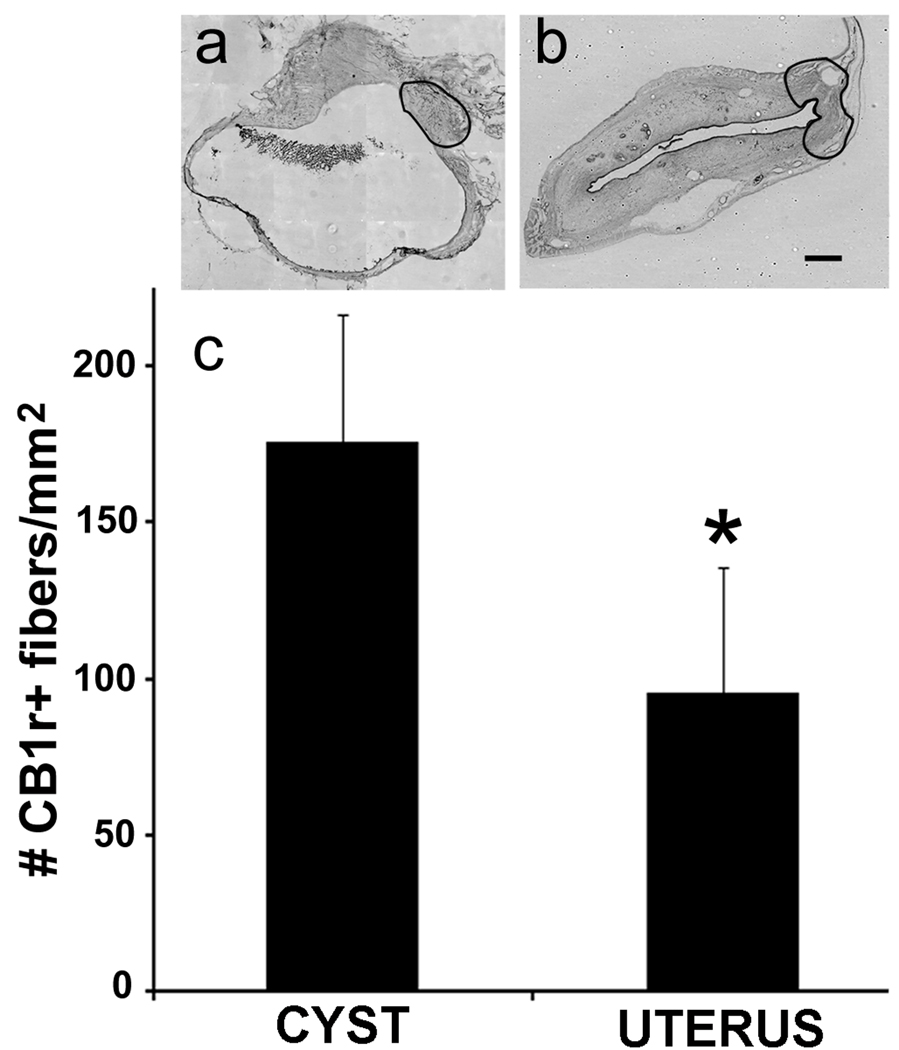
Sections of cysts and uterus (n = 8) harvested from the same rats were analyzed. An image of an entire DAB-stained section through the middle of each cyst or mid-uterine horn was captured with an Optronics Microfire camera and Neurolucida software (MBF Bioscience). Using a protocol described previously [52], a region in each section around the hilus of the cyst or an equivalent region of the uterus was demarcated (Fig. 2a, b), and its area calculated with the Stereo Investigator program. Two observers, blinded to group and consistent (r > 85%) independently counted nerve fibers in this area using criteria described elsewhere [52]. After decoding, data were analyzed with unpaired Student’s t-tests.
Figure 2.
Density of CB1-positive fibers is greater in the cysts and uterine horn. The regions outlined in the photomicrographs (a, b) show the approximate area in which counts were made in each tissue (i.e., the hilus of the cyst; the region in the uterine horn at the entrance of the uterine artery). Note that the size of this area did not differ between the two tissues (P = 0.89, unpaired Student’s t-test). For the graph in c, *, P = 0.024; unpaired Student’s t-test. Data shown as means and s.e.m. Calibration bar = 0.5 mm for both photomicrographs.
2.6. Double-immunostaining of cysts for CB1 and either calcitonin gene related peptide (CGRP) or vesicular monoamine transporter 2 (VMAT2)
Sections were immunostained with primary antibodies for CB1 (rabbit CB1-L15; from [6]) and either rabbit CGRP or rabbit VMAT2 (Chemicon: CGRP, AB5920, polyclonal; VMAT2, AB1598, raised against 13 amino acid C-terminal domain). Sections were washed in phosphate buffered saline (PBS), treated with Histo-VT-one antigen retrieval reagent (Nacalai) for 20min at 70°C, then washed in PBS and quenched in 0.3% H2O2 in PBS for 1h. First blocking was in 5% horse serum (HS) in 0.3% PBS-Tween-20 (PBST) for 1h at RT. Incubation in CB1 primary antibody (1:10,000) was done in 2% HS for 2h at RT, then overnight at 4°C. Sections were washed in 0.1% PBST and incubated in ImmPress (Vector) secondary antibody for 30 min at RT. After washing in 0.1%PBST then PBS, amplified signals were generated with Perkin Elmer Cy3 Tyramide Signal Amplification substrate (Perkin Elmer) at 1:50 in manufacturer’s buffer for 10min. Sections were washed, blocked in normal goat serum (NGS) and incubated with either CGRP or VMAT rabbit primary antibody (1:4,000 dilution each) overnight in 0.3% PBST containing 2% NGS. After washing in PBST, sections were incubated in Cy2 Goat anti-rabbit secondary antibody (1:400, Jackson Immuno) for 2h, washed and mounted (ProLong, Invitrogen) for fluorescent image acquisition. Controls were omission of either primary antibody (no labeling observed), and assurance that CB1 fluorescence was visible only after amplification. A semi-quantitative assessment of CB1 co-labeling with either CGRP (sensory fibers) or VMAT2 (sympathetic fibers) was done on 6 sections from 6 rats. Double-labeling was characterized as "all (> ~75%)," "many (~75-50 %)," "few (~50-25 %)," or "none."
2.7. Retrograde labeling of ganglion cells in dorsal root ganglia (DRG) and coeliac ganglia (CG)
Six to 10 wks after ENDO surgery, 5 rats were anesthetized with K/X. Under aseptic conditions, the cysts were localized and isolated from surrounding tissue. Crystals of the neurotracer Dil (1.2 – 2 mg) were put inside each cyst through a small incision in its wall. The incision was closed with a suture, and rats recovered for ~2 wk before euthanization (in proestrus) and tissue harvesting. Cryostat-cut sections were first analyzed by epi-fluorescent microscopy to select sections containing retrogradely-identified ganglion cells labeled adequately for later confocal image acquisition to examine triple labeling with Dil and antibodies specific for sensory and sympathetic fibers, described next.
2.8. Immunohistochemical processing of DRGs and CGs
DRGs containing ganglion cells retrogradely-labeled with Dil were double-immunostained using primary antibodies for CB1 and CGRP (specified in Sections 2.4 and 2.6). CGs containing ganglion cells retrogradely-labeled with Dil were double-immunostained using primary antibodies for CB1 and for sympathetic neurons (rabbit a-tyrosine hydroxylase; TH; Chemicon, AB152, polyclonal, purified, SDS-denatured TH was used as the immunogen). Immunohistochemistry was done as described above with the following modifications: amplified signals for CB1 were generated with Perkin Elmer Cy5 Tyramide Signal Amplification substrate at 1:500 in amplification buffer for 15min. After washing and subsequent blocking in 5% NGS, sections were incubated with either CGRP (for DRGs) or TH (for CGs) rabbit primary antibody (1:4,000 dilution each) overnight in 0.3% PBST/2% NGS. After washing in PBST, sections were incubated in Cy2 Goat anti-rabbit secondary antibody (1:400, Jackson Immuno) for 2h, washed and mounted (ProLong) for confocal image acquisition. Controls were: (a) omission of CB1 antibody followed by TSA amplification and subsequent verification that the CB1 antibody signal could only be detected when amplified, (b) omission of either second primary antibody (CGRP/TH), and (c) omission of Cy2 secondary antibody.
Confocal images were captured using a Leica TCS SP2 AOBS laser confocal microscope. Monochromatic lasers at minimal excitement (Cy2 = 488 nm; Dil = 543 nm, Cy5 = 647 nm) and optimal photomultiplier settings for each of the three fluorescent dyes were used. Image stacks of 10 images at 2-µm intervals were acquired for each dye. Averaged overlays of the three separate captures were generated with Leica LCS suite software and exported for contrast and brightness correction in Adobe Photoshop, v. 6 (Adobe Systems). For clarity in Fig. 1, the original dye colors were changed using Adobe Photoshop CS4 Extended (http://www.microscopyu.com/articles/confocal/threecolorconfocal.html). Grey scale image data from each dye capture was placed into a single channel of a Red-Green-Blue (RGB) image file. Image capture from Dil (originally red) was placed into the Blue channel, showing only blue. Image capture from CB1 (originally magenta) was placed into the Red channel, showing only red. Image captures from CGRP and TH (originally green) were placed into the Green channel; showing only green. The three differently-colored images were then aligned and merged, and the contrast and brightness of the merged images globally adjusted.
2.9. Assessment of triple-labeled ganglion cells in DRG and CG
T9 or T10 DRGs and CGs from four rats were chosen based on image quality of their retrograde labeling and immunohistochemical labeling with two antibodies. Singe sections were randomly selected from the middle of each DRG or CG. For each section, the original separate confocal images of fluorescent labeling for each of the three markers in (Dil, CB1 and either CGRP or TH) were coded and quantified using the Stereo Investigator program from MBF Bioscience. The three images from each section were overlaid and counted individually, allowing the experimenter to count neurons labeled with CB1, Dil, and either TH (in CGs) or CGRP (in DRGs), and then the double- and triple-labeled cells. The area and diameter of each counted cell in the DRGs was measured using the same program. Numbers of cells labeled by CB1, Dil, and either TH or CGRP within each ganglion, and the cell sizes were exported to spreadsheets to calculate the number of single-, double-, and triple-labeled DRG and CG cells, and the average sizes of the differently-labeled DRG cells.
2.10. Studies of visceromotor reflex (VMR) to vaginal distention, general
Before initiating VMR measures, and > 10 wks after ENDO surgery, each rat was acclimatized to the testing box (a clear acrylic chamber 65 mm wide × 75 mm high × 220 mm long) and to receiving vaginal distention in the box. Rats were put in the box for 10 min daily for 4–5 days. During the last 2–3 days of this period, a distendable latex balloon (8 mm long by 1.5 mm wide uninflated) was inserted in the mid-vaginal canal and distended in 0.05 ml increments over ~1-min period via a hand-held 1.0-ml syringe filed with water to a 1.0 ml maximum unless the rat struggled. This distention stimulus was identical to that used in previous studies of vaginal nociception in awake rats [10,45]. Note that in these earlier behavioral studies, we found that the probability of the rat’s making escape responses increases as the distention volume increases. Most naive rats begin making responses when distention volume reaches 0.35 ml; the probability of escape increases to 100% by 0.85–1.0 ml of distention [10,45].
2.11. Electrode implantation; recording electromyographic (EMG) activity during vaginal distention
Each fully-acclimated rat was anesthetized with K/X. Under aseptic surgical conditions, sterilized Teflon-coated stainless steel wires (AS633, Cooner Wire) were threaded into the left external abdominal oblique muscle and exteriorized at the back of the neck. After 7 to 10-days recovery, the rat was put in the testing box and its electrodes connected to measure EMG activity. EMG signals were amplified, filtered (low, 3 kHz; high, 10 Hz), converted to digital data using a Micro 1401 processor and sampled at 1 kHz using Spike2 software (Cambridge Electronics Design). The balloon was inserted in the vaginal canal, and connected via a catheter to a pressure transducer (COBE Cardiovascular) and to a water-filled syringe connected to a syringe pump. After a stable baseline EMG activity was recorded, the balloon was inflated at a steady rate (0.3 ml/min). The maximum distention was either the volume that evoked enough EMG activity so that a threshold could later be clearly defined offline or a top volume of 0.825 ml. (The threshold volume; i.e., the VMRth, is described below in the data analysis section.) Note that if the rat struggled or tried to remove the balloon, the balloon was immediately deflated.
2.12. Drug treatment
Before drugs were delivered, the VMR was measured twice (10–15 min interval). The drug solution was then injected i.p. Thirty minutes later, the VMR was recorded again. In the WIN2, dose-response study (Fig. 3b), rats were treated with different doses (1 mg/kg, 3 mg/kg) of (R)-(+)-WIN 55,212-2 mesylate salt (WIN2) using a within-rat, cross-over design. The interval between doses was 4 days. To determine if this dosing scheme produced tolerance, the effects of single and multiple doses were compared. As seen in Fig. 4, this dosing scheme did not produce tolerance. In the antagonist study (Fig. 3c), rats were treated with: (a) WIN2 (3mg/kg), (b) WIN2 (3mg/kg) combined with the CB1 antagonist (AM251, 3 mg/kg) or with the CB2 antagonist (AM630, 1 mg/kg), (c) the antagonists alone (same doses), or (d) vehicle alone. Each rat was tested 2 to 6 times with a different treatment each time, at intervals greater than 4 days.
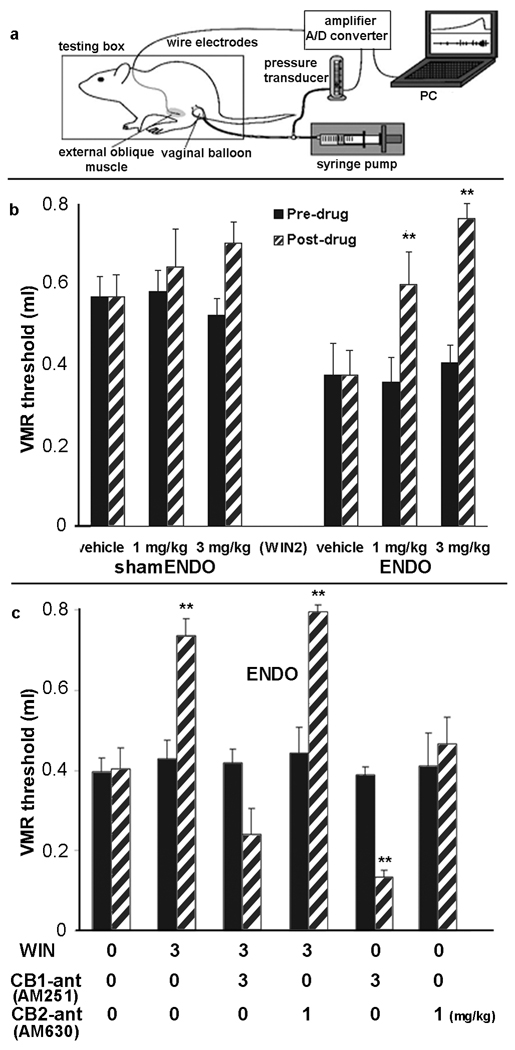
Figure 3.
Effects of WIN2 (CB1/CB2 agonist), AM 251 (CB1 antagonist), AM 630 (CB2 antagonist) on the visceromotor reflex threshold (VMRth) to vaginal distention. The diagram in a shows the testing set-up. The graph in b compares the effect of 2 doses of WIN2 on the VMRth in shamENDO and ENDO rats. Comparing only the back bars shows that the pre-drug VMRth was significantly greater in shamENDO than in ENDO rats; P = 0.00025, unpaired Student’s t-test, which confirms earlier studies that ENDO produces referred muscle hyperalgesia [38]. In the shamENDO group, WIN2 had no significant affects; ANOVA (P = 0.353). In contrast, in the ENDO group, both doses of WIN2 increased the VMRth significantly. **P < 0.01 compared with pre-drug; ANOVA followed by post-hoc paired Student’s t-tests. Thus, WIN2 alleviates referred muscle hyperalgesia in the ENDO group. The graph in c illustrates, in ENDO rats, the effects of WIN2 when combined with antagonists AM 251 (CB1-antagonist; CB1 ant) and AM630 (CB2 antagonist; CB2-ant) and with the antagonists delivered alone. **P < 0.008 compared with pre-drug; ANOVA followed by post-hoc Student’s t-tests, with alpha set at 0.008 (Bonferroni correction). The CB1, but not the CB2 antagonist prevented the effect of WIN2, which indicates that WIN2 acts via the CB1 receptor. The CB1, not the CB2, antagonist alone significantly reduced the VMRth (increases nociception), indicating that endocannabinoids normally suppress endometriosis-induced hyperalgesia. Data shown as means and ± s.e.m.
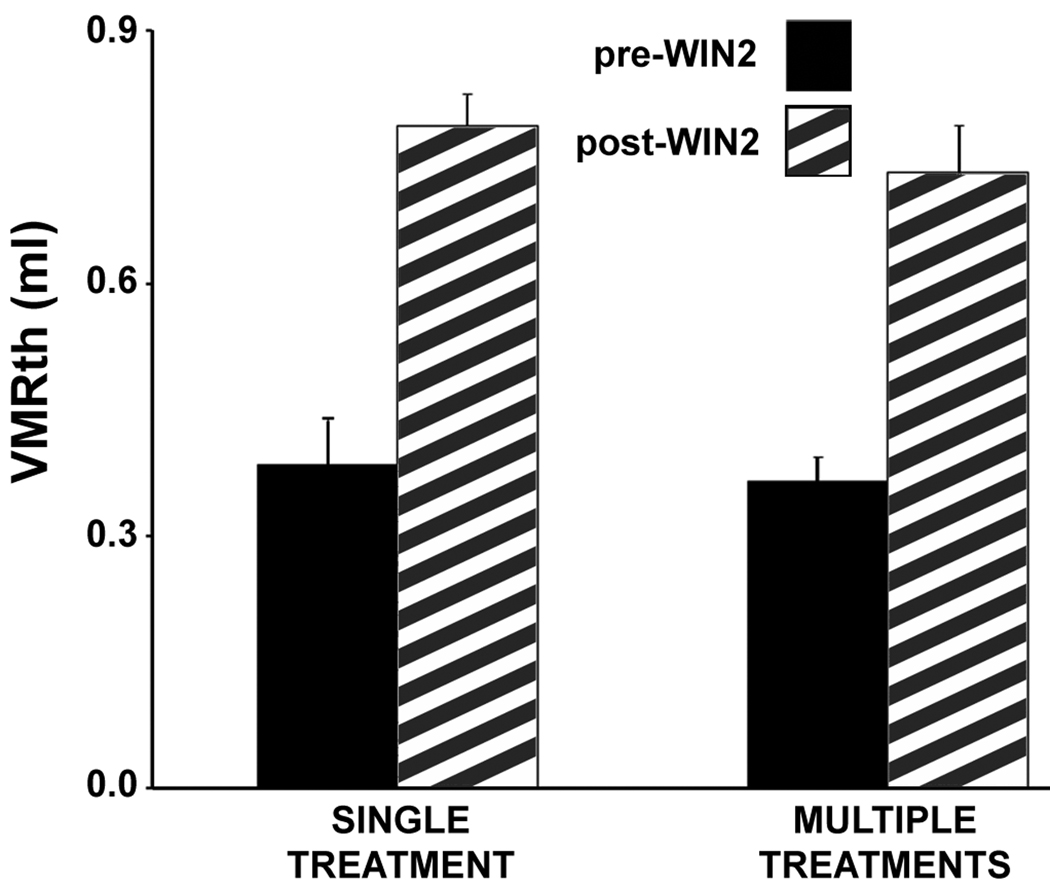
Figure 4.
Lack of tolerance to repeated administration of WIN2 over 8 days. The graph compares the effect on the VMRth of a single treatment of WIN2 (3 mg/kg) with multiple treatments in ENDO rats. In the multiple group, WIN2 was given three times at four-day intervals. No values between single-and multiply-dosed groups differed significantly; i.e., pre drug (P = 0.775), post-drug (P = 0.437), or the change in VMRth from pre to post drug (P = 0.711, not shown on graph); Students t-tests. Data are shown as means ± s.e.m.
2.13. Drugs and doses
WIN2 and AM 251 trifluoroacetate salt were purchased from Sigma. AM 630 was purchased from Tocris. Drugs were dissolved with 5% dimethyl sulfoxide-distilled water and administered at 2 ml/kg. The doses for WIN2, AM251 and AM630 were chosen based on effective doses in similar studies by others [19,32]. Note that the lower dose of the CB2 antagonist is unlikely to account for the lack of effectiveness of this antagonist in the present studies (Fig. 3c) because others have shown in rats that a pharmacological effect on a visceral organ that had been induced by a selective CB2 agonist was abolished by the same dose of AM 630 used here (1 mg/kg) [33]; i.e., this dose is sufficient to produce a selective CB2 antagonism.
2.14. Data analysis for VMR studies
As previously [38], area-under-the-curve (AUC) values of the VMR were used for statistical analyses. AUCs of the EMG were calculated for every 15-sec period beginning with a baseline period 15 sec before inflation of the balloon. The VMR threshold value (VMRth) was defined as the distention volume that induced twice (200%) the baseline AUC. Changes in VMRth values from before to after drug administration were then calculated.
SPSS/PASW version 17 software (IBM) was used for statistical analyses. For the two WIN2-dosing studies (ENDO group, shamENDO group, Fig. 3b), the data were analyzed using two-way ANOVAs. The ENDO group’s ANOVA was significant [F(5,24) = 6.928, P = 0.000], with significant effects of dose (P = 0.010), pre-post (P = 0.001), and dose/pre-post interactions (P = 0.027). The ANOVA was therefore followed by post-hoc Student’s t-tests for pre-drug versus post-drug effects, significance set at 0.05. The shamENDO group’s ANOVA was not significant [F(5,24) = 1.168, P = 0.353], and therefore not followed by post-hoc tests. For the antagonist study (Fig. 3c), the two-way ANOVA was significant [F(11,48) = 13.010, P = 0.000], with significant effects of treatment (P = 0.000) and treatment/pre-post interaction (P = 0.000). This ANOVA was therefore followed by Student’s t-tests, with significance set at 0.008 (Bonferroni correction).
3. Results
3.1. CB1 receptors are located on sympathetic and sensory fibers that innervate the ectopic growths
If the endocannabinoid system is involved in endometriosis and its associated pain, then CB1 receptors should be located on the axonal fibers innervating the cysts. With double-labeling fluorescence immunohistochemistry, most (> 75%) sympathetic fibers (vesicular monoamine transporter 2, VMAT2-positive) and many (50 – 75%) sensory fibers (calcitonin gene related peptide, CGRP-positive) in the cysts co-labeled with an antibody for CB1 receptors (CB1; [6]) (Fig. 1d). The fibers were associated mainly with blood vessels and muscle fibers in the walls of the cysts, and were seen less often in the endometrial layer. Although some CB1-labeled fibers were observed in the eutopic uterus of the same rats, notably, the density of the fibers was significantly higher in the cysts than uterus (Fig. 2).
3.2. CB1 receptors are located on neuronal somata from which axons sprout to innervate the ectopic growths
Support for CB1 involvement in endometriosis-associated pain would be strengthened by evidence that CB1 receptors are located on the somata of sensory and sympathetic neurons that innervate the cysts. Results of tripe-labeling fluorescence immunohistochemical experiments examining neuronal somata were consistent with the fiber labeling. For cyst-projecting CG neurons (identified by the retrogradely-labeled fluorescent marker Dil), 52% co-labeled with CB1, many of which (68%) were additionally labeled with an antibody identifying them as sympathetic neurons (tyrosine hydroxylase, TH). For Dil-identified cyst-projecting DRG neurons, 47% were labeled with the CB1, some of which (24%) were also CGRP-labeled. Overall, cyst-projecting CB1-positive DRG neurons were small to medium-sized (diameter range and median:, 22.9 – 41.5 µm; 33.6 µm). Those DRG neurons that were additionally-labeled by CGRP were mainly small (diameter range and median: 22.9 – 30.4 µm; 27.7 µm). These sizes are within ranges reported by others for DRG neurons containing either CB1 [20,46] or CGRP [31].
3.3. Pharmacological studies
Support for the involvement of CB1 receptors in endometriosis-associated pain and for the endocannabinoid system as being a potential treatment target would be strengthened by evidence that exogenous cannabinoid agents reduce ENDO-induced hyperalgesia. Three studies were done to address this issue by testing the effects of cannabinoid agents on referred nociception in ENDO or shamENDO rats. Referred nociception was measured by distending the vaginal canal and recording the volume threshold at which electrical activity occurred in the external abdominal oblique musculature (Fig. 3a) [38]. This muscle activity is called the visceromotor reflex (VMR; [39]) and its threshold, the VMRth.
3.4. WIN 55212-2 (WIN2), a CB1/CB2 agonist, alleviates referred muscle hyperalgesia in ENDO rats
The effects of different doses of the CB1/CB2 agonist, WIN 55212-2 (WIN2) [22], on vaginal distention-induced referred abdominal muscle nociception in conscious ENDO and shamENDO rats were tested using a within-rat cross-over design. The VMRth was significantly lower in ENDO than shamENDO rats, confirming the existence of vaginally-referred muscle hyperalgesia in unanesthetized ENDO rats [38]. WIN2 treatment had no significant effect on shamENDO rats, but significantly increased the VMRth in ENDO rats (Fig. 3b). No tolerance was apparent after multiple treatments (Fig. 4).
3.5. WIN2 alleviates referred muscle hyperalgesia in ENDO rats in a CB1 receptor-dependent manner
To determine which receptor was involved in the alleviation of hyperalgesia, we tested the effects on the VMRth of combining WIN2 with a CB1 or CB2 receptor antagonist (AM 251 or AM 630, respectively; [22]). Co-administration of AM 251, but not of AM 630, with WIN2 prevented WIN2’s reduction of ENDO-induced hyperalgesia (Fig. 3c).
3.6. The endocannabinoid system is normally engaged in ENDO’s induction of vaginal hyperalgesia
To examine the potential role of endogenous cannabinoids in ENDO-induced vaginal hyperalgesia, the CB1 or CB2 receptor antagonists were given alone to ENDO rats. The CB1 (AM 251), but not the CB2 (AM 630) antagonist, increased the referred hyperalgesia (i.e., decreased the VMRth; Fig. 3c). Because AM251 competitively inhibits endocannabinoid activation of CB1 receptors [18], this finding, together with the immunohistochemical results above, suggests that endocannabinoids tonically suppress ENDO-induced hyperalgesia.
4. Discussion
4.1. Ectopic growths, their innervation, and CB1 receptors
We used a rat model to test the hypothesis that the endocannabinoid system contributes via the nervous system to both the ectopic growths that define endometriosis and the hyperalgesia associated with it. Two findings support the first part of this hypothesis, endocannabinoid involvement in innervation of the growths. First, CB1 receptors were abundantly found not only on sensory and sympathetic fibers that had sprouted to innervate the ectopic growths (cysts), but also on retrogradely-identified somata of DRG and CG neurons from which the sprouted fibers originate. This finding indicates that CB1 receptors are strategically located for endogenous cannabinoids to influence functioning of cyst-innervating neurons. Second, CB1 labeling was significantly denser in the cysts than in the eutopic uterus. This finding indicates an upregulation of CB1 receptors in the ectopic uterine tissue relative to the healthy uterine tissue, which suggests that the endocannabinoid system is involved in the development and functioning of the abnormal sprouted innervation.
Another relevant aspect of our findings is that both sensory and sympathetic fibers invade the ectopic growths [4], creating direct two-way communication between the growths and the central nervous system. The fact that both types of fibers and their cell bodies are invested with CB1 receptors suggests that the endocannabinoid system contributes to coordinating and maintaining this communication. Furthermore, the physical proximity between sensory and sympathetic fibers within the cysts suggests a functional coupling between them [28,36]. The investment of these peripheral axons with CB1 receptors further suggests that the endocannabinoid system can regulate this coupling.
4.2. CB1 involvement in ENDO-associated vaginal hyperalgesia
Two findings support the second part of our hypothesis, endocannabinoid system involvement in ENDO-associated vaginal hyperalgesia via CB1, which in turn suggests a new approach to alleviate endometriosis-associated pain. First, treating ENDO rats with a CB1 (AM251) but not a CB2 (AM630) receptor antagonist increased the rats' ENDO-induced referred hyperalgesia, suggesting that CB1 but not CB2 receptors normally act to reduce the hyperalgesia. Because AM251 can act as an inverse agonist, however, further support for endocannabinoid involvement would come from testing drugs that increase endocannabinoid levels such as fatty acid amide hydrolase or monoacylglycerol lipase inhibitors [22]. Second, treatment of ENDO rats with a CB1/CB2 receptor agonist alleviated the ENDO-induced hyperalgesia in a CB1 receptor-dependent manner. Importantly, rats that were multiply-dosed with the CB1/CB2 agonist did not show tolerance, suggesting that repeated treatment with cannabinoids would continue to alleviate ENDO-induced hyperalgesia without losing efficacy.
4.3. Mechanisms underlying treatment efficacy in ENDO-associated pains and their potential association with the endocannabinoid system
The finding here that a cannabinoid agent alleviates hyperalgesia in a rat model of endometriosis is consistent with the known efficacy of cannabinoid agents in women with endometriosis [45] and supports a growing body of previous work in numerous contexts demonstrating the considerable potential of the endocannabinoid system as a target for the development of new therapies to alleviate pain [14,17].
Although mechanisms by which the endocannabinoid system and exogenous cannabinoid agents act to influence pain are under intense study [14,17], little is known in the specific context of endometriosis-associated pain. One obvious possibility in the rat model, and perhaps women, is that exogenous cannabinoid agents act directly on the sensitized peripheral nociceptive afferents, as demonstrated in other inflammatory and neuropathic models using CB1 knockout mice [1].
Other possibilities relate to mechanisms underlying the efficacy of clinical treatments currently used to alleviate endometriosis-associated pain. As reviewed by Giudice [16], in addition to surgical removal of the ectopic growths, medical treatments effective for some women include a variety of agents targeted on “minimizing inflammation” (e.g., non-steroidal anti-inflammatory drugs, NSAIDs). Other more reliable medical treatments are targeted on “interrupting or suppressing cyclic ovarian hormone production, inhibiting the action and synthesis of estradiol, and reducing or eliminating menses” (e.g., progestins, GnRH agonists, aromatase inhibitors, Danazol, and combined oral contraceptives).
Regarding NSAIDs, some, like naproxen, are of debatable efficacy in endometriosis-associated pain (e.g., 2,26). It is generally concluded, however, that the efficacy of NSAIDs likely depends on their influence on the inflammatory environment of the ectopic growths or peritoneal fluid [30,21,48]. For example, one purported mechanism of endometriosis-associated dysmenorrhea involves increased peritoneal fluid levels of prostaglandins and many other pro-inflammatory molecules [21,29]; effective NSAIDs would reduce these molecules and thus be acting peripherally.
On the other hand, there is a well-recognized extensive and complex association between the endocannabinoid and inflammatory systems. It is therefore possible that some NSAIDs might act to reduce endometriosis-associated pain via the endocannabinoid system. For example, COX-2 converts the endocannabinoid 2-arachidonoylglycerol (2-AG) into prostaglandin E2 glycerol ester (PGE2-G), which is pro-nociceptive because, when administered into the footpad, it induces mechanical allodynia and thermal hyperalgesia [23]. Thus, specific COX-2 inhibitors, which alleviate endometriosis-associated pain [12], could, as has recently been shown, act centrally to reduce hyperalgesia by preventing 2-AG breakdown into pro-nociceptive molecules [7,47,50].
Regarding hormonal therapies, mechanisms by which they alleviate endometriosis-associated pain are poorly understood [16,48]. One mechanism could involve opioid receptors. Matsuzaki and colleagues [34] found that muopioid receptors exist in sampels of deep-infiltrating endometriosis (associated with severe pain). However, this group also found that while treatment with a GnRH agonist or oral progestin eliminated the receptors, their function is unclear. Furthermore, there is a paucity of clinical data regarding the efficacy of opioids in treating endometriosis-associated pain. [16,27,48]. A second mechanism could involve the influence of hormonal therapies on the complex association between prostaglandins and estradiol. For example, PGE2 increases estradiol concentrations in ectopic growths [9], which could increase sprouting of nociceptors into them [11], but this possibility has not yet been studied directly in the context of pain.
A third mechanism could involve a direct influence of hormonal therapy on the ectopic growths’ innervation. In the rat model, the proestrous-to-estrous stage reduction in both plasma estradiol and ENDO-associated hyperalgesia is in turn associated with a reduction in both sympathetic fiber density and growth factors within the ectopic growths [52]. This finding suggests that hormonal therapy-induced hypoestrogenicity could act directly on the sympathetic-sensory coupling mechanism discussed in section 4.1. Consistent with this possibility are recent findings from a comprehensive clinical review that hormonal therapy is related to a reduction in nerve fiber density in ectopic endometrial growths [37].
Finally, studies regarding the association between hormonal or reproductive status and the endocannabinoid system are in their infancy; most focus, not on pain, but on healthy reproductive function [e.g., 49]. Even less is known regarding interactions between reproductive/hormonal status and the potential efficacy of cannabinoid agents for pain. Results so far are inconclusive. Kalbasi and colleagues [25] found that WIN2’s efficacy on the thermal tail flick test in mice is reduced by estradiol, whereas Craft and Leitl [13] found that Δ9-tetrahydrocannabinol’s efficacy on tail withdrawal and paw pressure tests in rats is enhanced by estradiol. In contrast, two other rodent studies suggest that hormonal therapies in endometriosis might affect how cannabinoids influence central pain mechanisms; both studies observed ovarian cyclical changes in cannabinoid receptor density or endocannabinoid content in brain areas potentially associated with nociception, [8,44].
4.4. Endocannabinoid involvement in neurovascular coordination
Previous findings from our laboratory suggest that the innervation of ectopic uterine growths by sensory and sympathetic fibers in the rat model likely derives from axonal branches that sprout from innervated blood vessels that are themselves simultaneously branching to vascularize the abnormal tissue as it grows [4]. Investment of the sprouted fibers with CB1 receptors supports the suggestion that the endocannabinoid system is involved in this neurovascular coordination [43], which has implications for conditions other than endometriosis. Thus, such a process could be common to other conditions that similarly involve growth of pathological tissue (e.g., benign or malignant tumors) or in which tissue is transplanted. Indeed, evidence is accumulating for endocannabinoid involvement in some of these conditions [15,24,40], but association of this involvement with neurovascular coordination has not yet been considered or studied.
4.5. Summary and conclusions
These studies in a rat model of endometriosis provide evidence that endocannabinoids might regulate the innervation of the disease’s abnormal growths and that exogenous cannabinoid agents can be effective in reducing endometriosis symptoms. The fact that CB1 receptor expression is greater in the cysts than healthy uterus from the same rats suggests that treatments to activate CB1 receptors (either directly by CB1 agonists or indirectly by increasing relevant endocannabinoid levels) could be developed with minimal effects on uterine function. Although the rat model parallels many aspects of endometriosis in women, there are of course significant differences [48]. However, when considered together with the past history of successful use of cannabinoids for alleviation of gynecological pains [45], and insofar as findings in rats can model mechanisms of endometriosis-related signs and symptoms, the present results suggest that approaches targeted at the endocannbinoid system represent a promising new direction for developing badly-needed new treatments for pain suffered by women with endometriosis.
ACKNOWLEDGEMENTS
We thank Charles Badland for help with Figure 1 and John Chalcraft for help with all figures. This work was supported by National Institutes of Health Grants NS011892 (to K. J. B.) and DA011322 and DA021696 (to K. Mackie).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen C, Hopewell S, Prentice A, Gregory D. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev. 2009;(2) doi: 10.1002/14651858.CD004753.pub3. CD004753. [DOI] [PubMed] [Google Scholar]
- 3.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 4.Berkley KJ, Dmitrieva N, Curtis KS, Papka RE. Innervation of ectopic endometrium in a rat model of endometriosis. Proc Natl Acad Sci USA. 2004;101:11094–11098. doi: 10.1073/pnas.0403663101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308:1587–1589. doi: 10.1126/science.1111445. [DOI] [PubMed] [Google Scholar]
- 6.Bodor AL, Katona I, Nyíri G, Mackie K, Ledent C, Hájos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradshaw H. CB1-induced side effects of specific COX-2 inhibitors: a feature, not a bug. Pain. 2010;148:5. doi: 10.1016/j.pain.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Bradshaw HB, Rimmerman N, Krey JF, Waker JM. Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am J Physiol Regul Integr Comp Physiol. 2006;291:R349–R358. doi: 10.1152/ajpregu.00933.2005. [DOI] [PubMed] [Google Scholar]
- 9.Bulun SE, Utsunomiya H, Lin Z, Yin P, Cheng YH, Pavone ME, Tokunaga H, Trukhacheva E, Attar E, Gurates B, Milad MP, Confino E, Su E, Reierstad S, Xue Q. Steroidogenic factor-1 and endometriosis. Mol Cell Endocrinol. 2009;300:104–108. doi: 10.1016/j.mce.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Cason AM, Samuelsen CL, Berkley KJ. Estrous changes in vaginal nociception in a rat model of endometriosis. Horm Behav. 2003;44:123–131. doi: 10.1016/s0018-506x(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 11.Chakrabarty A, Blacklock A, Svojanovsky S, Smith PG. Estrogen elicits dorsal root ganglion axon sprouting via a renin-angiotensin system. Endocrinology. 2008;149:3452–3460. doi: 10.1210/en.2008-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cobellis L, Razzi S, De Simone S, Sartini A, Fava A, Danero S, Gioffrè W, Mazzini M, Petraglia F. The treatment with a COX-2 specific inhibitor is effective in the management of pain related to endometriosis. Eur J Obstet Gynecol Reprod Biol. 2004;116:100–102. doi: 10.1016/j.ejogrb.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Craft RM, Leitl MD. Gonadal hormone modulation of the behavioral effects of Delta9-trahydrocannabinol in male and female rats. Eur J Pharmacol. 2008;578:37–42. doi: 10.1016/j.ejphar.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Elikkottil J, Gupta P, Gupta K. The analgesic potential of cannabinoids. J Opioid Manag. 2009;5:341–357. [PMC free article] [PubMed] [Google Scholar]
- 15.Freimuth N, Ramer R, Hinz B. Antitumorigenic effects of cannabinoids beyond apoptosis. J Pharmacol Exp Ther. 2010;332:336–344. doi: 10.1124/jpet.109.157735. [DOI] [PubMed] [Google Scholar]
- 16.Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS Neurol Disord Drug Targets. 2009;8:403–421. doi: 10.2174/187152709789824660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haj-Dahmane S, Shen RY. Endocannabinoids suppress excitatory synaptic transmission to dorsal raphe serotonin neurons through the activation of presynaptic CB1 receptors. J Pharmacol Exp Ther. 2009;331:186–196. doi: 10.1124/jpet.109.153858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hama A, Sagen J. Antinociceptive effect of cannabinoid agonist WIN 55,212-2 in rats with a spinal cord injury. Exp Neurol. 2007;204:454–457. doi: 10.1016/j.expneurol.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hohmann AG, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience. 1999;90:923–931. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- 21.Howard FM. Endometriosis and mechanisms of pelvic pain. J Minim Invasive Gynecol. 2009;16:540–550. doi: 10.1016/j.jmig.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Howett AC, Barth F, Bonner TI, Cabral G, Caselas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 23.Hu SS, Bradshaw HB, Chen JS, Tan B, Walker JM. Prostaglandin E2 glycerol ester, an endogenous COX-2 metabolite of 2-arachidonoylglycerol, induces hyperalgesia and modulates NFkappaB activity. Br J Pharmacol. 2008;153:1538–1549. doi: 10.1038/bjp.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones S, Howl J. Cannabinoid receptor systems: therapeutic targets for tumour intervention. Expert Opin Ther Targets. 2003;7:749–758. doi: 10.1517/14728222.7.6.749. [DOI] [PubMed] [Google Scholar]
- 25.Kalbasi Anaraki D, Sianati S, Sadeghi M, Ghasemi M, Paydar MJ, Ejtemaei Mehr S, Dehpour AR. Modulation by female sex hormones of the cannabinoid-induced catalepsy and analgesia in ovariectomized mice. Eur J Pharmacol. 2008;586:189–196. doi: 10.1016/j.ejphar.2008.02.055. [DOI] [PubMed] [Google Scholar]
- 26.Kauppila A, Ronnberg L. Naproxen sodium in dysmenorrhea secondary to endometriosis. Obstet Gynecol. 1985;65:379–383. [PubMed] [Google Scholar]
- 27.Kennedy S, Bergqvist A, Chapron C, D'Hooghe T, Dunselman G, Greb R, Hummelshoj L, Prentice A, Saridogan E. ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20:2698–2704. doi: 10.1093/humrep/dei135. [DOI] [PubMed] [Google Scholar]
- 28.Kessler JA, Bell WO, Black IB. Interactions between the sympathetic and sensory innervation of the iris. J Neurosci. 1983;3:1301–1307. doi: 10.1523/JNEUROSCI.03-06-01301.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koike H, Egawa H, Ohtsuka T, Yamaguchi M, Ikenoue T, Mori N. Correlation between dysmenorrheic severity and prostaglandin production in women with endometriosis. Prostaglandins Leukot Essent Fatty Acids. 1992;46:133–137. doi: 10.1016/0952-3278(92)90219-9. [DOI] [PubMed] [Google Scholar]
- 30.Kyama CM, Mihalyi A, Simsa P, Falconer H, Fulop V, Mwenda JM, Peeraer K, Tomassetti C, Meuleman C, D'Hooghe TM. Role of cytokines in the endometrial-peritoneal cross-talk and development of endometriosis. Front Biosci (Elite Ed) 2009;1:444–454. doi: 10.2741/e40. [DOI] [PubMed] [Google Scholar]
- 31.Lawson SN, McCarthy PW, Prabhakar E. Eectrophysiological properties of neurones with CGRP-like immunoreactivity in rat dorsal root ganglia. J Comp Neurol. 1996;365:355–366. doi: 10.1002/(SICI)1096-9861(19960212)365:3<355::AID-CNE2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 32.Liang YC, Huang CC, Hsu KS. The synthetic cannabinoids attenuate allodynia and hyperalgesia in a rat model of trigeminal neuropathic pain. Neuropharmacology. 2007;53:169–177. doi: 10.1016/j.neuropharm.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Mathison R, Ho W, Pittman QJ, Davison JS, Sharkey KA. Effects of cannabinoid receptor-2 activation on accelerated gastrointestinal transit in lipopolysaccharide-treated rats. Br J Pharmacol. 2004;142:1247–1254. doi: 10.1038/sj.bjp.0705889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuzaki S, Canis M, Pouly JL, Botchorishvili R, Déchelotte PJ, Mage G. Both GnRH agonist and continuous oral progestin treatments reduce the expression of the tyrosine kinase receptor B and mu-opioid receptor in deep infiltrating endometriosis. Hum Reprod. 2007;22:124–128. doi: 10.1093/humrep/del368. [DOI] [PubMed] [Google Scholar]
- 35.McAllister SL, McGinty KA, Resuehr D, Berkley KJ. Endometriosis-induced vaginal hyperalgesia in the rat: role of the ectopic growths and their innervation. Pain. 2009;147:255–264. doi: 10.1016/j.pain.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMahon SB. Mechanisms of sympathetic pain. Br Med Bull. 1991;47:584–600. doi: 10.1093/oxfordjournals.bmb.a072494. [DOI] [PubMed] [Google Scholar]
- 37.Medina MG, Lebovic DI. Endometriosis-associated nerve fibers and pain. Acta Obstet Gynecol Scand. 2009;88:968–975. doi: 10.1080/00016340903176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagabukuro H, Berkley KJ. Influence of endometriosis on visceromotor and cardiovascular responses induced by vaginal distention in the rat. Pain. 2007;132 Suppl 1:S96–S103. doi: 10.1016/j.pain.2007.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- 40.Pacher P, Haskó G. Endocannabinoids and cannabinoid receptors in ischaemia-reperfusion injury and preconditioning. Br J Pharmacol. 2008;153:252–262. doi: 10.1038/sj.bjp.0707582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paria BC, Wang H, Dey K. Endocannabinoid signaling in synchronizing embryo development and uterine receptivity for implantation. Chem Phys Lipids. 2002;121:201–210. doi: 10.1016/s0009-3084(02)00156-1. [DOI] [PubMed] [Google Scholar]
- 42.Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics. 2009;6:713–737. doi: 10.1016/j.nurt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ralevic V, Kendall DA. Cannabinoid modulation of perivascular sympathetic and sensory neurotransmission. Curr Vasc Pharmacol. 2009;7:15–25. doi: 10.2174/157016109787354114. [DOI] [PubMed] [Google Scholar]
- 44.Rodríguez de Fonseca F, Cebeira M, Ramos JA, Martín M, Fernández-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. 1994;54:159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- 45.Russo E. Cannabis treatments in obstetrics and gynecology: a historical review. J Cannabis Ther. 2002;2:5–34. [Google Scholar]
- 46.Sañudo-Peña MC, Strangman NM, Mackie K, Walker JM, Tsou K. CB1 receptor localization in rat spinal cord and roots, dorsal root ganglion, and peripheral nerve. Zhongguo Yao Li Xue Bao. 1999;20:1115–1120. [PubMed] [Google Scholar]
- 47.Staniaszek LE, Norris LM, Kendall DA, Barrett DA, Chapman V. Effects of COX-2 inhibition on spinal nociception: the role of endocannabinoids. Br J Pharmacol. 2010;160:669–676. doi: 10.1111/j.1476-5381.2010.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Human Reproduction Update. doi: 10.1093/humupd/dmq050. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor AH, Amoako AA, Bambang K, Karasu T, Gebeh A, Lam PM, Marzcylo TH, Konje JC. Endocannabinoids and pregnancy. Clin Chim Acta. 2010;411:921–930. doi: 10.1016/j.cca.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Telleria-Diaz A, Schmidt M, Kreusch S, Neubert AK, Schache F, Vazquez E, Vanegas H, Schaible HG, Ebersberger A. Spinal antinociceptive effects of cyclooxygenase inhibition during inflammation: Involvement of prostaglandins and endocannabinoids. Pain. 2010;148:26–35. doi: 10.1016/j.pain.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 51.Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985;44:684–694. [PubMed] [Google Scholar]
- 52.Zhang G, Dmitrieva N, Liu Y, McGinty KA, Berkley KJ. Endometriosis as a neurovascular condition: estrous variations in innervation, vascularization, and growth factor content of ectopic endometrial cysts in the rat. Am J Physiol Regul Integr Comp Physiol. 2008;294:R162–R171. doi: 10.1152/ajpregu.00649.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]