Abstract
D-aspartate (D-Asp) is an endogenous molecule that is often detected in central nervous system and endocrine tissues. Using capillary electrophoresis and a variety of radionuclide detection techniques, we examine the synthesis, release, and uptake/accumulation of D-Asp in the central nervous system of the marine mollusk Aplysia californica. We observe the preferential synthesis and accumulation of D-Asp over L-aspartate (L-Asp) in neuron-containing ganglia compared to surrounding sheath tissues. Little conversion of D-Asp to L-Asp is detected. The Ca2+ ionophore ionomycin and elevated extracellular potassium stimulates release of D-Asp from the cerebral ganglia. Lastly, radioactive D-Asp in the extracellular media is efficiently taken up and accumulated by individual F-cluster neurons. These observations point to a role for D-Asp in cell-to-cell signaling with many characteristics similar to classical transmitters.
Keywords: D-amino acids, D-aspartate, Aplysia californica, neurotransmitter, hormone
Introduction
It had been long presumed that amino acids, so vital to organismal function, predominantly occurred in the L-form—especially in higher animals. This view persisted for decades, even though a variety of D-amino acids (DAAs) were reported since the 1930s in mammalian tissues (reviewed in (Berg 1953)), intriguing many when they were observed in human tumors (Arnow & Opsahl 1939, Lipmann et al. 1940). Hundreds of studies have now documented the widespread occurrence of DAAs in animals. Nevertheless, D-amino acid function in higher organisms remained enigmatic until fairly recently, when the role of D-serine (D-Ser) as a co-agonist of the NMDA receptor (Kleckner & Dingledine 1988, Mothet et al. 2000), and its participation in gliotransmission (Schell et al. 1995), were established. These observations, combined with additional available experimental evidence, support the likelihood that other endogenous DAAs also have physiological roles, specifically, D-aspartate (D-Asp) (Miao et al. 2006, D’Aniello 2007), D-alanine (Hashimoto et al. 1992, Morikawa et al. 2008), D-glutamate (D-Glu) (Mangas et al. 2007, Huang et al. 2009), and D-proline (Morikawa et al. 2007).
Of these, D-Asp is one of most studied endogenous DAAs in Metazoan (D’Aniello et al. 1995a, Hashimoto & Oka 1997, Di Fiore et al. 1998, Nakatsuka et al. 2001, D’Aniello et al. 2003, Miao et al. 2006, D’Aniello 2007, Morikawa et al. 2007, Huang et al. 2009). It has been detected in a wide variety of species and is present in the nervous system of both vertebrates and invertebrates. For example, within mollusks, free D-Asp has been found in the nervous tissue of the Cephalopoda species Octopus vulgaris, Loligo vulgaris, and Sepia officinalis (D’Aniello et al. 1995b), in the buccal ganglia of the opistobranch mollusk Aplysia fasciata (D’Aniello et al. 1993a), in several ganglia of Aplysia californica (Zhao & Liu 2001, Miao et al. 2005, Miao et al. 2006) and Aplysia limacina (Spinelli et al. 2005), as well as in the reproductive glands of O. vulgaris (D’Aniello et al. 1995a, D’Aniello et al. 1995b).
Interestingly, D-Asp appears at relatively high levels during embryonic development throughout Metazoan, but the concentration decreases to much lower levels in adult animals. In vertebrates, high D-Asp concentrations occur in the developing retina and CNS of chickens (Neidle & Dunlop 1990), rats (Schell et al. 1997, Sakai et al. 1998), lizards (Assisi et al. 2001), frogs (Di Fiore et al. 1998), and humans (Hashimoto et al. 1993a). D-Asp drops to trace levels in most animal tissues after maturation, exceptions being specific endocrine and exocrine systems. In adult rats for example, D-Asp has been measured in significant quantities in the testes and in the adrenal and pineal glands (Hashimoto et al. 1995, Hamase et al. 1997), with concentrations in these tissues reaching up to 40% of the free Asp.
Despite these reported observations, the functions of D-Asp are not established; however, progress is being made. Indirect evidence demonstrates that changes in D-Asp levels are correlated with disease state (Fisher et al. 1994) and similar to D-Ser, it is found in mammalian neurons during development (Hashimoto et al. 1993b, Fisher et al. 1994). In an exciting recent report, Kim et al. (Kim et al. 2010) showed that D-Asp is involved in adult mouse neurogenesis, playing a role as a trophic factor influencing dendritic development. Clearly, more studies are required to understand its role in the adult animal.
We and others have hypothesized that D-Asp may function as a neurotransmitter or hormone (Schell et al. 1997, Spinelli et al. 2005, Miao et al. 2006). However, confirmation of a physiological role for D-Asp as a signaling molecule requires that several crucial characteristics be demonstrated: presence in a presynaptic neuron, activity-dependent release, generation of an effect in the postsynaptic cell via specific cellular mechanisms, and inactivation of the molecular signal by uptake or catabolism. To examine the function of D-Asp in morphologically documented neuronal networks, we have selected the well-characterized neurobiological model, Aplysia californica. For many years, this marine slug has been used in studies linking neurochemical pathways to function, and thus, it is an excellent choice for our current investigation. In our prior analyses of the A. californica CNS, we observed significant quantities of D-Asp in the C-, F-, and G-clusters of the cerebral ganglia, with over 85% of the free Asp present in the insulin-producing neurons of the F-cluster being in the D-form (Miao et al. 2006). D-Asp is actively transported along the pleural-abdominal connective in a colchicine-dependent manner and induces electrophysiological responses in several neurons. These observations, along with the results of a single cell study (Miao et al. 2005), demonstrate that D-Asp is present in A. californica neurons and may play a role in cell-to-cell signaling.
Here we continue these earlier investigations by studying the biosynthesis, uptake/accumulation and stimulated release of D-Asp. Briefly, by using radiolabeled [14C]-Asp, we are able to determine the site of D-Asp synthesis, as well as observe whether the reverse synthesis of L-Asp from D-Asp takes place. We examine both L-Asp and D-Asp uptake/accumulation in the cerebral ganglion and the pleural ganglia, as well as in individual F- and B-cluster neurons. Our findings show that the characteristics of L-Asp to D-Asp conversion, D-Asp cellular uptake/accumulation, and stimulation-dependent release are consistent with D-Asp being involved in neurotransmission and/or neuromodulation within the A. californica nervous system.
Experimental
Reagents and solutions
We prepared 500 mL of 50 mM borate buffer (pH 9.4) by dissolving 2.38 g of sodium borate (Na2B4O7·10H2O) (Sigma–Aldrich, St. Louis, MO, USA) in 456 mL of ultrapure de-ionized (DI) water (Milli-Q Ultrapure Water Filtration Systems; Millipore, Bedford, MA, USA) and mixing with ~44 mL of 0.2 M NaOH; this solution was used in sample preparations and as a sheath flow buffer, unless otherwise noted. The separation buffer solution consisted of 20 mM of β-cyclodextrin (β-CD) and 50 mM of sodium dodecyl sulfate (SDS) in 50 mM of borate buffer (pH 9.4) and 15% methanol (V/V). Naphathalene-2, 3-dicarboxaldehyde (NDA) was from Molecular Probes (Eugene, OR, USA). As indicated in several cases, a citric acid sheath buffer (25 mM, pH 2.5) was used that was made by dissolving 5.25 g of citric acid monohydrate (C6H8O7·H2O; Sigma–Aldrich) in 1.0 L of ultrapure DI water (ELGA Purelab Ultra water system; USFilter, Lowell, MA, USA), and titrating to pH 2.5 using 0.10 M NaOH. Other reagents were obtained from Sigma–Aldrich at the highest purity available. Solutions were filtered through 0.45 μm Acrodisc syringe filters (Gelman Laboratory, Ann Arbor, MI, USA) before use. Radioisotope-labeled D-Asp and L-Asp were purchased from American Radiolabeled Chemicals (St. Louis, MO, USA) (L-Asp [4-14C], Catalog No. ARC 226; L-Asp [2,3-3H], Catalog No. ARC 0211; and D-Asp [4-14C], Catalog No. ARC 213; D-Asp [2,3-3H], Catalog No. ARC 212). The handling and disposal of [14C]-labeled Asp and [3H]-labeled Asp requires procedures which should be followed as outlined in each institution’s division of environmental health and safety guidelines. Solid-phase extraction beads (~40 μm diameter) were obtained from Waters (Waters Oasis HLB, Milford, MA, USA).
Capillary Electrophoresis
Details of the laboratory-constructed capillary electrophoresis systems with both fluorescence and radionuclide detection are provided in the supplemental section.
Buccal, cerebral and pleural ganglia isolation
A. californica (200–300 g) were obtained from Charles Hollahan (Santa Barbara, CA, USA) and kept in an aquarium containing continuously circulating, aerated and filtered sea water (Instant Ocean, Aquarium Systems Inc., Mentor, OH, USA) at 14–15°C until used. Animals were anesthetized by injection of isotonic MgCl2 (~30 to ~50% of body weight) into the body cavity. The cerebral and buccal ganglia were dissected and placed in artificial sea water (ASW) containing (in mM): 460 NaCl, 10 KCl, 10 CaCl2, 22 MgCl2, 6 MgSO4, and 10 HEPES (pH 7.8), or in ASW-antibiotic solution: ASW supplemented with 100 units/mL penicillin G, 100 μg/mL streptomycin, and 100 μg/mL gentamicin (pH 7.8). To improve isolation of cellular clusters and individual neurons, the ganglion sheaths were digested enzymatically by incubating the ganglia in 1% protease (Type IX: Bacterial; Sigma–Aldrich) ASW-antibiotic solution at 34 °C for 1–2 h depending on animal size and season. Next, the ganglia were washed in fresh ASW. For several measurements, the total protein has been determined as described in the supplemental section to correct for animal to animal variations in the ganglia.
D-Asp biosynthesis
Sample preparation
For the D-Asp biosynthesis study in intact cerebral ganglia using radiolabels, whole cerebral ganglia with sheath tissue attached were incubated in 5 μCi/mL of [14C]-L-Asp in ASW. In experiments to investigate regional D-Asp biosynthesis, the cerebral ganglia were separated into two equal segments, consisting of nearly identical left and right hemiganglia, by cutting the sheath tissue and cerebral connective. Left and right pleural ganglia were also used in these experiments. The two hemiganglia and two pleural ganglia were then separated into different experimental vessels, with one hemiganglion and one pleural ganglion placed in 100 μL of ASW containing 5 μCi/mL of [14C]-L-Asp and the other hemiganglion and pleural ganglion from the same animal placed in 100 μL of ASW containing 5 μCi/mL of [14C]-D-Asp for 24 h.
After incubation, the intact ganglia, neuron-containing ganglionic structures and sheath tissue were washed with fresh ASW and stored in separate vials at −20 °C until analysis, which was done within 24 h. After removing from the freezer, the samples were homogenized, 10 μL of 0.1 M HCl added to each vial, sonicated for 5 min, then centrifuged at 15,000 g for 10 min. The supernatant was taken and dried with a stream of N2, redissolved in 30 μL of 50 mM borate buffer (pH 9.4) and derivatized with 10 μL of 17 mM NDA (in methanol) and 10 μL of 30 mM KCN (in DI water). Both NDA and KCN were filtered with 0.45 μm Acrodisc syringe filters. The samples were vortexed for 1 min and incubated for ~45 min at room temperature. Before analysis, 5 μL aliquots of the derivatized samples were spiked with 3 μL of a mixture of 1 μL of 5 mM D-Asp and 1 μL of 6 mM L-Asp, also derivatized with NDA and KCN.
Detection of Asp release
Radionuclide detection of released Asp
The cerebral hemiganglia were dissected as above and deposited into separate incubation vessels, with one hemiganglion placed in 100 μL of ASW containing 5 μCi/mL of [14C]-L-Asp and the other in 100 μL of ASW containing 5 μCi/mL of [14C]-D-Asp. After a 24 h incubation, each ganglion was washed with ASW and individually placed in vials containing 100 μL ASW or 100 μL ASW with 0.1% dimethyl sulfoxide (DMSO) for 20 min. This incubation period (20 min) was required in order to have enough released material for precise releasate detection. Twenty-five microliter aliquots of solution were then obtained from each vial in duplicate (50 μL total for each sample) and deposited into individual 5 mL plastic liquid scintillation vials (Sarstedt Inc., Newton, NC). The vials were filled with Ultima Gold AB liquid scintillation fluid (Packard Instrument Co., Merinden, CT). Fifty microliters of 120 mM KCl in ASW, or 20 μM ionomycin in ASW with 0.1% DMSO were then added to the remaining 50 μL solution in the vials, to make a 60 mM KCl solution or 10 μM ionomycin solution. As ionomycin stock solution contains DMSO, DMSO was added into the ASW control solutions. The hemiganglia were incubated for 20 min and another 25 μL aliquot was taken from each vial in duplicate and deposited into liquid scintillation vials as above. Analysis of these samples was performed without delay by an LS 6500 Multi-purpose Scintillation Counter (Beckman-Coulter, Fullerton, CA), and the total counts of radioactivity were used to calculate the amounts of Asp released from the hemiganglia.
Capillary electrophoresis (CE)-laser induced fluorescence (LIF) cerebral ganglion KCl-stimulated release analysis
The cerebral ganglia were pinned down in a small ~450 μL well. The ganglia were allowed to rest in ASW for > 1.5 h, and then stimulated by increasing the extracellular potassium concentration to 53 mM for 20 min. Aliquots (200 μL) of extraganglionic media were collected before, during and after the KCl stimulation. Three washes of the ganglia were made after the KCl stimulation and before collection of the last sample. Each 200 μL was divided into five 40 μL aliquots in PCR vials and stored frozen until the CE-LIF investigation.
The 40 μL aliquots were dried in the oven overnight and reconstituted with 5 μL water or 5 μL internal standard containing 1 μM D-Asp. The standard allows accurate determination of extracellular D-Asp and L-Asp levels and reduces methodological uncertainties. KCN and naphathalene-2, 3-dicarboxaldehyde (NDA) solutions were added to the reconstituted samples and the reactions allowed to continue for 20 min in the dark to form N-substituted 1-cyanobenz[f]isoindole amino acid (CBI-AA). The large amount of inorganic salts in these samples creates issues with CE when the solutions are concentrated; thus, the salts were removed via extraction. Specifically, HCl solution was added to the reaction mixture to adjust the pH and the resulting neutrally charged CBI-AA was extracted into CH2Cl2. The lower layer was placed into a PCR tube and the extraction process repeated three times. The combined collected organic layer was dried and reconstituted with buffer. The samples were then assayed via CE as described in the supplemental section.
D- and L-Asp uptake/accumulation
Cerebral hemiganglia radioisotope incubation in sodium-free ASW
To study the effect of sodium on D-Asp uptake/accumulation in the cerebral ganglia, individual cerebral hemiganglia were incubated for 24 h in either 100 μL sodium-free ASW, made by replacing the 460 mM NaCl of ASW with 460 mM choline chloride and containing 5 μCi/mL of [14C]-D-Asp solution, or 100 μL of ASW containing 5 μCi/mL of [14C]-D-Asp solution. After the 24 h incubation period, they were taken out of the solution and washed in ASW. Excess ASW surrounding the tissue was removed. The hemiganglia were placed in pre-weighed 5 mL liquid scintillation vials. Weighing the vials before and after tissue deposition allowed determination of the wet mass of the hemiganglia. Analysis by liquid scintillation counting (LSC) was carried out immediately after addition of Ultima Gold AB scintillation liquid to the vials.
Cerebral hemiganglia radioisotope incubation at different temperatures
Eight hemiganglia (from four animals) were individually incubated in an excess of 5 μCi/mL [14C]-D-Asp at either ambient temperature or at ~4 °C (one hemiganglion from each animal in each condition). Two aliquots (1 μL) of incubation solution were removed for measurement by LSC at each time point (0, 0.5, 1, 1.5, 2, 3, 4, and 24 h). All aliquots/timepoints, as well as the hemiganglia collected at 24 h were measured as one set by LSC.
Single-cell radioisotope analysis
After the protease treatment and incubation with radiolabeled Asp enantiomer, 3–5 individual neurons from each F-cluster and 3–5 single neurons from the B-cluster of the cerebral hemiganglia were manually isolated under visual control, and washed individually in fresh ASW. The single neurons, groups of cells, pleural hemiganglia, and remaining cerebral ganglia were placed in individual 5 mL plastic liquid scintillation vials and filled with Ultima Gold AB scintillation fluid. Analysis on all samples was carried out immediately by LSC. In some cases, dual radioactivity measurements were performed as described in the supplemental section so that both tritiated and 14C-labaled Asp could be characterized simultaneously (e.g., 14C-L-Asp and 3H-D-Asp).
Results
D-Asp biosynthesis in cerebral ganglia
Several radioactively labeled Asp enantiomer incubation experiments were carried out to determine if D-Asp is synthesized in the cerebral, pleural, or buccal ganglia of A. californica. Previously, we showed that D-Asp is converted from L-Asp in the cerebral ganglion (Miao et al. 2006). However, it is not clear which structure(s) or cell types (e.g., glia, sheath cells, neurons) are responsible for the synthesis and whether the synthesis is ganglion-specific. Here, we investigated the tissue specificity of D-Asp synthesis to decipher the location of this process and whether it is specific for the L to D conversion. When analyzing D-Asp synthesis with CE-LIF, a concentration-dependent increase of D-Asp (compared to the internal standard L-cysteic acid) in cerebral ganglia was observed (data not shown). Incubation of the buccal ganglia in a similar manner with L-Asp showed little to no increase in D-Asp (data not shown). These experiments suggest that the conversion of L-Asp to D-Asp is ganglion-specific and therefore may occur in neurons due to the similarity in the structure of sheath tissue and glia between the ganglia. Because of the large background presence of D-Asp in the tissues, measuring newly synthesized D-Asp is difficult with CE-LIF; thus, we used a pulse-chase approach using radionuclide detection. Specifically, we measured [14C]-L-Asp conversion to [14C]-D-Asp. Fig. 1 shows an experiment in which cerebral ganglia, including surrounding sheath tissue, were incubated in [14C]-L-Asp; we observed that approximately 40% of the total [14C]-Asp in the sample was converted to the D-form after 24 h.
Fig. 1.
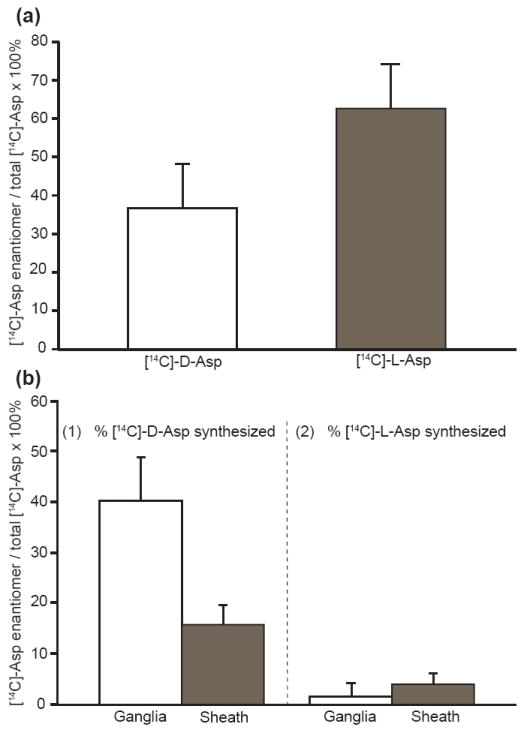
Biosynthesis of D-Asp in a cerebral ganglion of A. californica. (a) Incubation of an intact cerebral ganglion in 5 μCi/mL [14C]-L-Asp, showing final percentages of radiolabeled Asp enantiomers found after ~24 h. (b) Incubation of a desheathed cerebral hemiganglia and related sheath tissue in (1) 5 μCi/mL [14C]-L-Asp and (2) 5 μCi/mL [14C]-D-Asp, showing conversion to enantiomer after ~24 h. A significantly larger conversion of [14C]-L-Asp to [14C]-D-Asp occurs in the cerebral hemiganglion over that of the sheath tissue (n = 3, p <0.01). Error bars represent standard deviations.
To determine the ganglionic region responsible for the conversion, these same tissue experiments were then performed on the areas where the hemiganglia and sheath tissue were separated. In Fig. 1b it can be seen that the majority of that conversion occurs in the neuron-containing ganglia, and little from the sheath tissue (n = 3, p <0.01), which possesses primarily structural elements such as fibers and sheath cells. Small numbers of neuron somata as well as neurites may be present in the sheath. Importantly, the reverse conversion of D- to L-Asp was negligible. These data show that D-Asp is formed from L-Asp in the A. californica desheathed cerebral ganglia, while the reverse conversion of D-Asp to L-Asp is not observed in these tissues. The pleural-pedal ganglion was also tested for biosynthetic activity with radiolabels, but showed no significant conversion of D- to L-Asp or L- to D-Asp (data not shown). These sets of experiments indicate that D-asp is synthesized in specific neuron-containing structures of A. californica.
Secretion of D-Asp from F-cluster cells
Establishing activity-dependent release from neurons is an important criterion for determining if a compound functions as a neurotransmitter and/or neuromodulator. Increase in the extracellular concentration of both endogenous D-Asp and L-Asp was observed upon stimulation of intact cerebral ganglia by elevated potassium ion concentrations in the media surrounding the ganglia (Fig. 2). Interestingly, while Asp-levels increased, the ratio of the enantiomer differed from experiment to experiment (Fig. 2c), with instances when D-Asp (experiment #2) or L-Asp (experiment #3) contributed most to this increase. This variability differs from the range of levels of D- and L-Asp present in cells and tissues and, therefore, may indicate intrinsic regulation of D-Asp release.
Fig. 2.
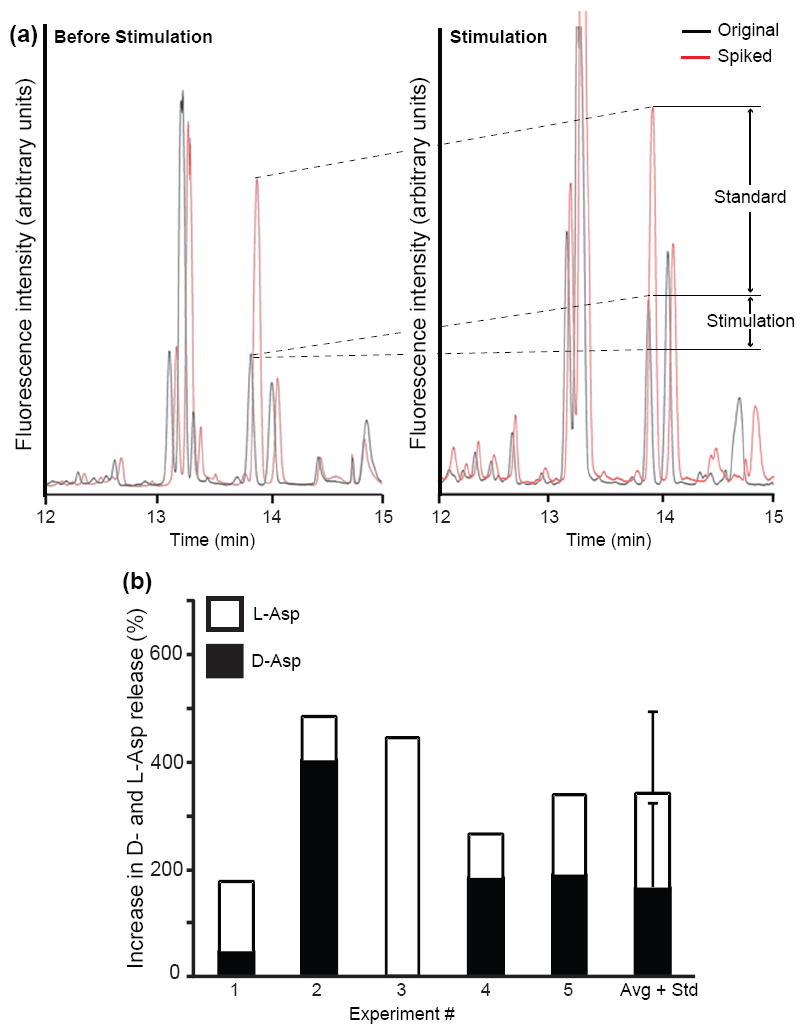
Elevated extracellular concentrations of potassium ions potentiate release of L- and D-Asp from the cerebral ganglia. CE-LIF electropherograms of cerebral ganglion releasate: (a) before KCl stimulation (left) and after KCl stimulation (right). In both cases, the black trace shows the original sample and the red trace shows the sample spiked with 1 μM D-Asp, used to calibrate the signal levels. Arrows indicate the increase in D-Asp signal due to the addition of standard and the change due to potassium ion stimulation (labeled standard and stimulation, respectively). (b) Stacked histograms summarizing results from five experiments, with statistical data placed in the sixth column. For each experiment, the extracellular potassium concentration was increased to 53 mM during the 20 min stimulation.
To further refine the information on D-Asp release, we studied the secretion of D-Asp from two cerebral ganglion neurohemal regions—the anterior tentacular and upper labial nerves—using bead sampling technology (Hatcher et al. 2005, Hatcher & Sweedler 2008) (Fig. 3) with CE-LIF measurements of the collected releasates. Our previous experiments showed that cerebral F-cluster cells have high levels of D-Asp present in their cell body (Miao et al. 2006). Many of these neurons send their processes to the neurohemal areas located at the bases of the anterior tentacular and upper labial nerves, as well as the cerebral commissure (Floyd et al. 1999, Li et al. 2001). Larger Aplysia insulin-producing neuroendocrine cells have their terminals located in the first two areas (Floyd et al. 1999). We detected a small amount of both D- and L-Asp in prestimulation samples, suggesting a small, constitutive secretion rate (Fig. 4). Following stimulation with 10 μM of the calcium ionophore, ionomycin, a marked increase in D-Asp release is detected, suggesting that secretion of the enantiomer into the hemolymph does in fact occur and is, moreover, induced by calcium entry. In one set of experiments, >160 pmol of D-Asp was collected after stimulation with ionomycin from the F-cluster cells. These data support the concept of Ca2+-dependent release of D-Asp from neurons, consistent with vesicular exocytosis of both enantiomers.
Fig. 3.
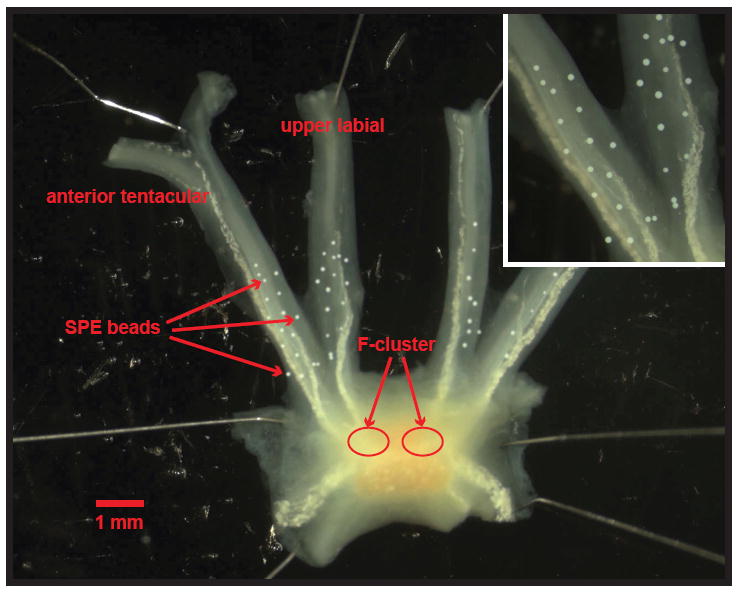
Microphotograph of SPE collection beads placed upon the anterior tentacular and upper labial nerves of a cerebral ganglion from A. californica to collect releasate from neurohemal areas containing terminals of the F-cluster neurons. (Inset) Close-up of the SPE beads on the nerves. Scale bar = 1 mm.
Fig. 4.

CE-LIF electropherograms of F-cluster releasate collected by SPE beads. (a) Releasate from F-cluster cells before stimulation. (b) Releasate from F-cluster cells after chemical stimulation with 10 μM ionomycin. (c) Accuracy of L- and D-Asp signal assignments are determined via standard addition using both enantiomers.
D-Asp versus L-Asp cellular uptake and accumulation
To be considered a neurotransmitter or neuromodulator, after release the compound should be catabolized, or removed from the extracellular space; this reduces the signal, decreases its contribution to the background, and allows uninterrupted signal transmission (Kandel et al. 2000). Signaling molecules are often accumulated in neurons and recycled during neurotransmission / neuromodulation; accumulation represents the balance between release and uptake.
We investigated D-Asp uptake and accumulation by the Aplysia CNS in a series of incubation experiments. Here, cerebral ganglia were incubated in ASW supplemented with [14C]-D-Asp. On average almost 500 pmol of [14C]-D-Asp per milligram of total protein were accumulated by the ganglia (Fig. 5). To pinpoint the cellular origin of D-Asp accumulation, we analyzed individual cells from F- and B-clusters of cerebral ganglia using LSC. The cerebral hemiganglia were incubated in [14C]-D-Asp or [14C]-L-Asp in ASW (pH ~ 7.7) for 24 h and the cells from the two clusters were removed and examined. Fig. 6 shows the relative accumulation of radioactivity in individual neurons. Predictably, the neuroendocrine F-cluster cells show the largest preference for D-Asp, with ~75% (n = 12, p <0.01) of the total Asp accumulated by these cells (Fig. 6a). Total Asp represents cumulative signal from an equal number of incubated and studied cells. Similarly, larger B-cluster neurons preferentially accumulated up to ~62% (n = 9, p <0.01) D-Asp over L-Asp.
Fig. 5.
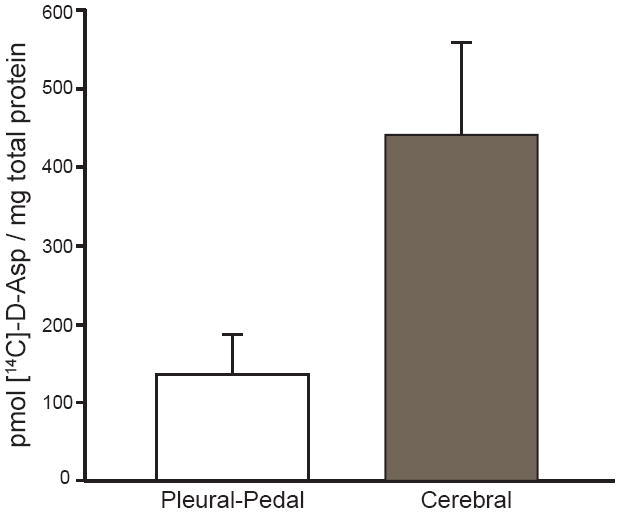
Comparison of [14C]-D-Asp uptake and accumulation per mg total protein between the cerebral and the pleural-pedal ganglia in the A. californica CNS after a 21 h incubation in 5 μCi/mL [14C]-D-Asp. The cerebral ganglia show a greater accumulation of [14C]-D-Asp per mg protein over that of the pleural-pedal ganglia (n = 7, p <0.05). Error bars represent standard deviations.
Fig. 6.
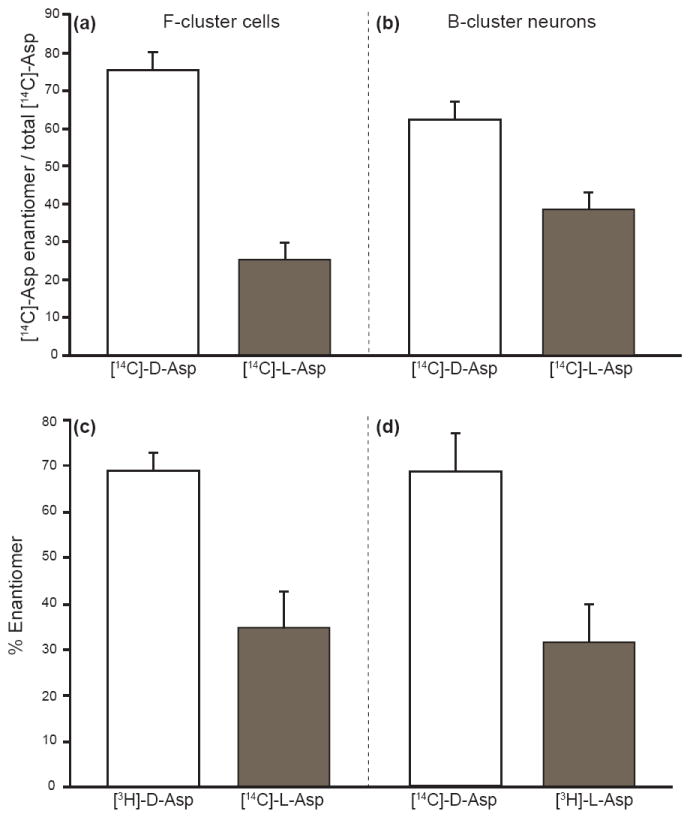
Cellular radiolabeled Asp accumulation by single cells in the cerebral ganglion. (a) Peptidergic F-cluster cells show ~75% of the total Asp accumulated is in the D-form when incubated in L-Asp or D-Asp (n = 12, p<0.01). (b) Larger B-cluster neurons show ~62% of the total Asp taken up is in the D-form (n = 9, p<0.01). Investigating the enantiomer uptake and accumulation preference of cerebral ganglion F-cluster cells incubated in mixtures of (c) 90 μM [3H]-D-Asp and [14C]-L-Asp (n = 4, p <0.01) or (d) 90 μM [14C]-D-Asp and [3H]-L-Asp (n = 5, p <0.01) produced similar results. In both cases the F-cluster cells show an ~68% total preference for D-Asp. Error bars represent standard deviations.
One may ask, does the preference in D-Asp accumulation occur when both enantiomers are present in the extracellular environment and is there any possibility that the radiolabel is responsible for this preference? Using ASW supplemented with mixtures of paired 3H and 14C radiolabeled forms of L-Asp and D-Asp, we investigated preferential accumulation of D-Asp by F-cluster cells. The cerebral ganglia were incubated in equal (by Asp concentration) enantiomeric mixtures of [14C]-D- or L-Asp and [3H]-L- or D-Asp, and the total radioactivity accumulation within the F-cluster cells was determined by using the dual-window capability of LSC. Fig. 6c demonstrates a preference for D-Asp uptake and accumulation by single F-cluster cells, regardless of the radiolabel used or presence of the enantiomer (n = 9, p <0.01). This approach also normalizes cell size variability by ensuring that the comparison of normalized uptake for both enantiomers takes place within a single cell simultaneously.
Both Glu and Asp are actively taken up by excitatory amino acid transporters (EAATs) (Nicholls et al. 2001). The proper functioning of active EAATs is often modulated by temperature and dependent upon the presence of sodium symporters to allow the transfer of the amino acids across a boundary (Kandel et al. 2000). The hypothesis of EAAT involvement in D-Asp uptake is supported by experiments where cerebral hemiganglia were incubated in [14C]-D-Asp solution at either room temperature or 4 °C (Fig. 7a-c). Time-dependent reduction of radioactivity levels in the extraganglionic media, as well as accumulation of the radioactive marker in the hemiganglia (Fig. 7c), were temperature dependent. As is typical for EAAT-dependent amino-acid uptake, lower temperatures resulted in lower uptake/accumulation of [14C]-D-Asp, with the maximum detected at 24 h and a linear increase in accumulation during the first 4 h. If EAATs are indeed responsible for the observed preferential uptake of D-Asp, then Asp transport will depend on sodium levels. As shown in Fig. 7d, the cerebral ganglia [14C]-D-Asp accumulation was significantly reduced when sodium ions were absent from media.
Fig. 7.

Uptake and accumulation of [14C]-D-Asp modulated by temperature and extracellular sodium concentration (a) time course of the reduction of [14C]-D-Asp signal in surrounding cerebral hemiganglia environment kept at different temperatures. (b) Final [14C]-D-Asp signal in the extraganglionic environment after incubation for 24 h. (c) Corresponding accumulation of radioisotope in the hemiganglia. (d) [14C]-D-Asp accumulation in the cerebral ganglia in the presence and absence of sodium ions in the extracellular media. Cerebral ganglia are incubated in 5 uCi/mL [14C]-D-Asp in either ASW or Na+-free ASW solution. [14C]-D-Asp uptake shows sodium-dependence (n = 4, p <0.01). Error bars represent standard deviations, cpm: counts per minute, RT: room temperature.
Discussion
D-Asp biosynthesis
While a racemization mechanism for D-aspartyl residues in peptides has been suggested (Fujii et al. 2002), D-Asp is thought to be formed endogenously from L-Asp via an amino acid racemase, but this process is yet to be completely characterized in animals. D-Asp biosynthesis has been seen in mammalian undifferentiated pheochromocytoma cells analyzed by high performance liquid chromatography (HPLC), D-Asp oxidase digestion and immunocytochemical staining methods (Long et al. 1998). Recently it was reported that a racemase in mice colocalizes with D-Asp (Kim et al. 2010). Asp racemase has also been purified and partially sequenced in the bivalve mollusk Scapharca broughtonii (Watanabe et al. 1998). The enzymatic biosynthesis of D-Asp from L-Asp has been observed in the cerebral ganglion of the ascidian Ciona intestinalis (D’Aniello et al. 2003) and cerebral ganglia of the mollusk A. limacina (Spinelli et al. 2006). Interestingly, evidence suggests that D-Asp is involved in NMDA synthesis, as NMDA has been shown to be endogenously synthesized from D-Asp in rat neuroendocrine glands (D’Aniello et al. 2000).
Our finding of biosynthetic activity occurring in the cerebral ganglia of A. californica indicates that D-Asp is endogenously synthesized from L-Asp at these locations. This synthesis can be performed by homologous amino acid racemases, which have been reported in rat brain (Wolosker et al. 2000) and in mollusks (Shibata et al. 2003). Recently, we reported a preliminary characterization of a neuronal A. californica Asp racemase (Wang et al. 2009). Our results shown here demonstrate that the formation of L-Asp from D-Asp is unlikely. The non-racemic and unidirectional synthesis of D-Asp from L-Asp, and the heterogeneous rate of synthesis and or accumulation between the various tissues of the CNS (pleural versus cerebral ganglia versus sheath tissue), suggest that the D-Asp levels are regulated. Most D-amino acids within the organism are oxidized by the enzyme, D-amino acid oxidase; however, neither D-Asp nor D-Glu are oxidized using this enzyme (D’Aniello et al. 1993b). Their oxidation requires a separate enzyme, D-Asp oxidase. Both of these D-amino acid-degrading enzymes are yet to be found in A. californica, although this may change as the A. californica genome is finalized (Moroz et al. 2006a, Moroz et al. 2006b).
D-Asp release
Several groups described D-Asp release from brain tissue upon chemical or electrical stimulation in mammalian endocrine tissues using radioactive labeling (Palmer & Reiter 1994, Muzzolini et al. 1997, Savage et al. 2001) and HPLC (Wolosker et al. 2000, Nakatsuka et al. 2001). Both KCl and acetylcholine-induced D-Asp release were more pronounced than that of L-Asp in adrenal slices, and only D-Asp (but not L-Asp or L-Glu) was shown to be released from rat adrenal gland when stimulated with nicotine (Wolosker et al. 2000). In the rat brain, electrically evoked [3H]-D-Asp release from hippocampal slices mimics the release of L-Glu (Savage et al. 2001). Similarly, electrical field-induced D-Asp release has been demonstrated in cerebral cultures and is not only Ca2+-dependent but also sensitive to synaptic vesicle toxins (including the relatively specific V-type H+-ATPase inhibitor), suggesting that D-Asp can accumulate in synaptic vesicles (Cousin et al. 1997). Studies on different animal models have also shown that D-Asp is stored in secretary granules (Nakatsuka et al. 2001, Spinelli et al. 2006), actively transported in nerves in a colchicine-dependent manner (Miao et al. 2006), present in synaptosomes (Spinelli et al. 2006) and released in a Ca2+-dependent manner, similar to that of dopamine (Nakatsuka et al. 2001). However, these experiments are not often at the level of a single cell, but rather, are made using large populations of cells containing multiple cell types (e.g., neurons, glia, etc.), thereby making it difficult to differentiate the neuronal contribution to D-Asp release.
Our findings show that F-cluster cells are capable of releasing a relatively high quantity of D-Asp at A. californica neurohemal areas. Released D-Asp may influence nearby neuronal terminals, sheath and vasculature cells at these locations, as well as distant targets via a hormonal role. It is particularly intriguing that different chemical stimuli induce Asp release where the ratio of D-Asp to L-Asp varies. For example, ionomycin stimulation results in the predominant release of D-Asp, and was also found to be a more potent stimulus compared to an elevated potassium concentration for D-Asp release from A. limacina synaptosomes (Spinelli et al. 2006). Thus, it appears that D-Asp and L-Asp release from the same physical location can be independently varied depending on a number of factors.
Uptake and accumulation of D-Asp
Our results show that the uptake and accumulation of D-Asp appears to be tissue-specific in the Aplysia californica CNS. This finding well corroborates with results showing a heterogeneous distribution of endogenous D-Asp levels within the CNS (Miao et al. 2006). Giving consideration to the total protein levels, the cerebral ganglion accumulates [14C]-D-Asp at a higher Asp-to-mg-protein ratio than that of the pleural-pedal ganglion. Taking into account the relatively low constitutive release of Asp enantiomers, the heterogeneous preference for D-Asp accumulation in those tissues that are known to contain a high abundance of D-Asp suggests a physiological uptake mechanism for D-Asp, and not that the uptake of D-Asp is due to a non-selective transporter that is taking up the L-Asp.
Using multiple forms of radiolabeled Asp under a variety of conditions, we have shown that the accumulation of D-Asp in cells and nervous tissue depends on temperature and has an active sodium-dependent mechanism. In our experiments with equal concentrations of the enantiomers, an accumulation preference for D-Asp was observed. This preference may be the result of higher affinity binding by a transporter that is preferentially expressed in neurons of the cerebral ganglion. This mirrors previous studies performed on the stereoselectivity of the RNA-transcribed, sodium-dependent, high-affinity Glu transporter isolated from rabbit (Kanai & Hediger 1992). While it has been reported that Asp is unable to activate Glu receptors in A. californica neurons, it has also been shown that Asp competes with Glu for Glu transporters (Carpenter et al. 1995). The surprisingly high percentage of radioactivity accumulated by cells incubated in [14C]-D-Asp-containing media suggests that D-Asp is taken up preferentially over L-Asp, although the amino acid release experiments demonstrated a higher constitutive release of L-Asp. Additional experiments are needed to determine the balance between uptake and release of these enantiomers by the neurons.
Conclusions
The advantage of combining Aplysia californica CNS studies with small volume analytical methods is the ability to study D-Asp dynamics at the neuronal cluster or even single neuron level. Information on the formation, release and uptake/accumulation of D-Asp in the A. californica nervous system indicates it plays a likely role in cell-cell signaling and justifies follow-up physiological studies on D-Asp function in neuronal systems. Additional work is now aimed at determining the specific enzymes involved in D-Asp synthesis.
Supplementary Material
Acknowledgments
The project described was supported by Award No. CHE-05-26692 from the National Science Foundation (NSF), Award No. P30 DA018310 from the National Institute On Drug Abuse (NIDA) and by Award No. 5RO1NS031609 from the National Institute of Neurological Disorders and Stroke (NINDS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NSF, NIDA, NINDS, or National Institutes of Health.
References
- Arnow LE, Opsahl JC. The configuration of the glutamic acid of adenocarcinoma protein. Science. 1939;90:257–258. doi: 10.1126/science.90.2333.257. [DOI] [PubMed] [Google Scholar]
- Assisi L, Botte V, D’Aniello A, Di Fiore MM. Enhancement of aromatase activity by D-aspartic acid in the ovary of the lizard Podarcis s. sicula. Reproduction. 2001;121:803–808. doi: 10.1530/rep.0.1210803. [DOI] [PubMed] [Google Scholar]
- Berg CP. Physiology of the D-amino acids. Physiol Rev. 1953;33:145–189. doi: 10.1152/physrev.1953.33.2.145. [DOI] [PubMed] [Google Scholar]
- Carpenter DO, King WM, McCreery MJ. The role of glutamate reuptake in regulation of glutamate responses in Aplysia neurons. Acta Biol Hung. 1995;46:363–373. [PubMed] [Google Scholar]
- Cousin MA, Hurst H, Nicholls DG. Presynaptic calcium channels and field-evoked transmitter exocytosis from cultured cerebellar granule cells. Neuroscience. 1997;81:151–161. doi: 10.1016/s0306-4522(97)00047-x. [DOI] [PubMed] [Google Scholar]
- D’Aniello A. D-aspartic acid: an endogenous amino acid with an important neuroendocrine role. Brain Res Rev. 2007;53:215–234. doi: 10.1016/j.brainresrev.2006.08.005. [DOI] [PubMed] [Google Scholar]
- D’Aniello A, Di Fiore MM, Fisher GH, Milone A, Seleni A, D’Aniello S, Perna AF, Ingrosso D. Occurrence of D-aspartic acid and N-methyl-D-aspartic acid in rat neuroendocrine tissues and their role in the modulation of luteinizing hormone and growth hormone release. Faseb J. 2000;14:699–714. doi: 10.1096/fasebj.14.5.699. [DOI] [PubMed] [Google Scholar]
- D’Aniello A, Dicosmo A, Dicristo C, Fisher G. D-Aspartate in the male and female reproductive-system of Octopus vulgaris lam. Gen Comp Endocr. 1995a;100:69–72. doi: 10.1006/gcen.1995.1134. [DOI] [PubMed] [Google Scholar]
- D’Aniello A, Nardi G, DeSantis A, Vetere A, diCosmo A, Marchelli R, Dossena A, Fisher G. Free L-amino acids and D-aspartate content in the nervous system of Cephalopoda. A comparative study. Comp Biochem Phys B. 1995b;112:661–666. [Google Scholar]
- D’Aniello A, Nardi G, Vetere A, Ferguson GP. Occurrence of free D-aspartic acid in the circumsoesophageal ganglia of Aplysia fasciata. Life Sci. 1993a;52:733–736. doi: 10.1016/0024-3205(93)90235-u. [DOI] [PubMed] [Google Scholar]
- D’Aniello A, Spinelli P, De Simone A, D’Aniello S, Branno M, Aniello F, Fisher GH, Di Fiore MM, Rastogi RK. Occurrence and neuroendocrine role of D-aspartic acid and N-methyl-D-aspartic acid in Ciona intestinalis. Febs Lett. 2003;552:193–198. doi: 10.1016/s0014-5793(03)00921-9. [DOI] [PubMed] [Google Scholar]
- D’Aniello A, Vetere A, Petrucelli L. Further study on the specificity of D-amino-acid oxidase and of D-aspartate oxidase and time-course for complete oxidation of D-amino acids. Comp Biochem Phys B. 1993b;105:731–734. doi: 10.1016/0305-0491(93)90113-j. [DOI] [PubMed] [Google Scholar]
- Di Fiore MM, Assisi L, Botte V, D’Aniello A. D-aspartic acid is implicated in the control of testosterone production by the vertebrate gonad. Studies on the female green frog, Rana esculenta. J Endocrinol. 1998;157:199–207. doi: 10.1677/joe.0.1570199. [DOI] [PubMed] [Google Scholar]
- Fisher GH, Petrucelli L, Gardner C, et al. Free D-amino acids in human cerebrospinal fluid of Alzheimer disease, multiple sclerosis, and healthy control subjects. Mol Chem Neuropathol. 1994;23:115–124. doi: 10.1007/BF02815405. [DOI] [PubMed] [Google Scholar]
- Floyd PD, Li L, Rubakhin SS, et al. Insulin prohormone processing, distribution, and relation to metabolism in Aplysia californica. J Neurosci. 1999;19:7732–7741. doi: 10.1523/JNEUROSCI.19-18-07732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii N, Tajima S, Tanaka N, Fujimoto N, Takata T, Shimo-Oka T. The presence of D-beta-aspartic acid-containing peptides in elastic fibers of sun-damaged skin: a potent marker for ultraviolet-induced skin aging. Biochem Bioph Res Co. 2002;294:1047–1051. doi: 10.1016/S0006-291X(02)00597-1. [DOI] [PubMed] [Google Scholar]
- Hamase K, Homma H, Takigawa Y, Fukushima T, Santa T, Imai K. Regional distribution and postnatal changes of D-amino acids in rat brain. Biochim Biophys Acta. 1997;1334:214–222. doi: 10.1016/s0304-4165(96)00095-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Kumashiro S, Nishikawa T, et al. Embryonic development and postnatal changes in free D-aspartate and D-serine in the human prefrontal cortex. J Neurochem. 1993a;61:348–351. doi: 10.1111/j.1471-4159.1993.tb03575.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Nishikawa T, Kumashiro S, Oka T, Takahashi K, Mito T, Takashima S. Embryonic and postnatal changes in free D-aspartate and D-serine in the human prefrontal cortex. J Neurochem. 1993b;61:S266–S266. doi: 10.1111/j.1471-4159.1993.tb03575.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Nishikawa T, Oka T, Takahashi K, Hayashi T. Determination of free amino acid enantiomers in rat brain and serum by high-performance liquid chromatography after derivatization with N-tert.-butyloxycarbonyl-L-cysteine and o-phthaldialdehyde. J Chromatogr. 1992;582:41–48. doi: 10.1016/0378-4347(92)80300-f. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Oka T. Free D-aspartate and D-serine in the mammalian brain and periphery. Prog Neurobiol. 1997;52:325–353. doi: 10.1016/s0301-0082(97)00019-1. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Oka T, Nishikawa T. Anatomical distribution and postnatal changes in endogenous free D-aspartate and D-serine in rat brain and periphery. Eur J Neurosc. 1995;7:1657–1663. doi: 10.1111/j.1460-9568.1995.tb00687.x. [DOI] [PubMed] [Google Scholar]
- Hatcher NG, Richmond TA, Rubakhin SS, Sweedler JV. Monitoring activity-dependent peptide release from the CNS using single-bead solid-phase extraction and MALDI TOF MS detection. Anal Chem. 2005;77:1580–1587. doi: 10.1021/ac0487909. [DOI] [PubMed] [Google Scholar]
- Hatcher NG, Sweedler JV. Aplysia bag cells function as a distributed neurosecretory network. J Neurophysiol. 2008;99:333–343. doi: 10.1152/jn.00968.2007. [DOI] [PubMed] [Google Scholar]
- Huang Y, Shi M, Zhao S. Quantification of D-Asp and D-Glu in rat brain and human cerebrospinal fluid by microchip electrophoresis. J Sep Sci. 2009;32:3001–3006. doi: 10.1002/jssc.200900026. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360:467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. McGraw-Hill; New York: 2000. [Google Scholar]
- Kim PM, Duan X, Huang AS, Liu CY, Ming GL, Song H, Snyder SH. Aspartate racemase, generating neuronal D-aspartate, regulates adult neurogenesis. Proc Natl Acad Sci U S A. 2010;107:3175–3179. doi: 10.1073/pnas.0914706107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Li L, Floyd PD, Rubakhin SS, et al. Cerebrin prohormone processing, distribution and action in Aplysia californica. J Neurochem. 2001;77:1569–1580. doi: 10.1046/j.1471-4159.2001.00360.x. [DOI] [PubMed] [Google Scholar]
- Lipmann F, Behrens OK, Kabit EA, Burk D. The determination of the total D-amino acid content of human tumors and normal tissues by means of D-amino acid oxidase. Science. 1940;91:21–23. doi: 10.1126/science.91.2349.21. [DOI] [PubMed] [Google Scholar]
- Long ZQ, Homma H, Lee JA, Fukushima T, Santa T, Iwatsubo T, Yamada RH, Imai K. Biosynthesis of D-aspartate in mammalian cells. Febs Lett. 1998;434:231–235. doi: 10.1016/s0014-5793(98)00986-7. [DOI] [PubMed] [Google Scholar]
- Mangas A, Covenas R, Bodet D, Geffard M, Aguilar LA, Yajeya J. Immunocytochemical visualization of D-glutamate in the rat brain. Neuroscience. 2007;144:654–664. doi: 10.1016/j.neuroscience.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Miao H, Rubakhin SS, Scanlan CR, Wang L, Sweedler JV. D-Aspartate as a putative cell-cell signaling molecule in the Aplysia californica central nervous system. J Neurochem. 2006;97:595–606. doi: 10.1111/j.1471-4159.2006.03791.x. [DOI] [PubMed] [Google Scholar]
- Miao H, Rubakhin SS, Sweedler JV. Subcellular analysis of D-aspartate. Anal Chem. 2005;77:7190–7194. doi: 10.1021/ac0511694. [DOI] [PubMed] [Google Scholar]
- Morikawa A, Hamase K, Inoue T, Konno R, Zaitsu K. Alterations in D-amino acid levels in the brains of mice and rats after the administration of D-amino acids. Amino Acids. 2007;32:13–20. doi: 10.1007/s00726-005-0357-8. [DOI] [PubMed] [Google Scholar]
- Morikawa A, Hamase K, Miyoshi Y, Koyanagi S, Ohdo S, Zaitsu K. Circadian changes of D-alanine and related compounds in rats and the effect of restricted feeding on their amounts. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;875:168–173. doi: 10.1016/j.jchromb.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Moroz LL, Edwards JR, Puthanveettil SV, et al. Neuronal transcriptome of Aplysia: neuronal compartments and circuitry. Cell. 2006a;127:1453–1467. doi: 10.1016/j.cell.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz LL, Ju J, Russo JJ, et al. Proposal to sequence the genome of Aplysia californica. 2006b http://www.genome.gov/Pages/Research/Sequencing/SeqProposals/AplysiaSeq.pdf.
- Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzolini A, Bregola G, Bianchi C, Beani L, Simonato M. Characterization of glutamate and [3H]D-aspartate outflow from various in vitro preparations of the rat hippocampus. Neurochem Int. 1997;31:113–124. doi: 10.1016/s0197-0186(96)00129-5. [DOI] [PubMed] [Google Scholar]
- Nakatsuka S, Hayashi M, Muroyama A, Otsuka M, Kozaki S, Yamada H, Moriyama Y. D-Aspartate is stored in secretory granules and released through a Ca(2+)-dependent pathway in a subset of rat pheochromocytoma PC12 cells. J Biol Chem. 2001;276:26589–26596. doi: 10.1074/jbc.M011754200. [DOI] [PubMed] [Google Scholar]
- Neidle A, Dunlop DS. Developmental changes in free D-aspartic acid in the chicken embryo and in the neonatal rat. Life Sci. 1990;46:1517–1522. doi: 10.1016/0024-3205(90)90424-p. [DOI] [PubMed] [Google Scholar]
- Nicholls JG, Martin AR, Wallace BG, Fuchs PA. From Neuron to Brain: A Cellular and Molecular Approach to the Function of the Nervous System. Sinauer Associates, Inc.; Sunderland, MA: 2001. [Google Scholar]
- Palmer AM, Reiter CT. Comparison of the superfused efflux of preaccumulated D-[3H]aspartate and endogenous L-aspartate and L-glutamate from rat cerebrocortical minislices. Neurochem Int. 1994;25:441–450. doi: 10.1016/0197-0186(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Sakai K, Homma H, Lee JA, Fukushima T, Santa T, Tashiro K, Iwatsubo T, Imai K. Localization of D-aspartic acid in elongate spermatids in rat testis. Arch Biochem Biophys. 1998;351:96–105. doi: 10.1006/abbi.1997.0539. [DOI] [PubMed] [Google Scholar]
- Savage DD, Galindo R, Queen SA, Paxton LL, Allan AM. Characterization of electrically evoked [3H]-D-aspartate release from hippocampal slices. Neurochem Int. 2001;38:255–267. doi: 10.1016/s0197-0186(00)00077-2. [DOI] [PubMed] [Google Scholar]
- Schell MJ, Cooper OB, Snyder SH. D-aspartate localizations imply neuronal and neuroendocrine roles. Proc Natl Acad Sci U S A. 1997;94:2013–2018. doi: 10.1073/pnas.94.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K, Watanabe T, Yoshikawa H, Abe K, Takahashi S, Kera Y, Yamada RH. Purification and characterization of aspartate racemase from the bivalve mollusk Scapharca broughtonii. Comp Biochem Phys B. 2003;134:307–314. doi: 10.1016/s1096-4959(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Spinelli P, Brown ER, Ferrandino G, et al. D-aspartic acid in the nervous system of Aplysia limacina: Possible role in neurotransmission. J Cell Physiol. 2006;206:672–681. doi: 10.1002/jcp.20513. [DOI] [PubMed] [Google Scholar]
- Spinelli P, D’Aniello E, Ferrandino G, D’Aniello A. D-Aspartic acid: a putative neurotransmiter in the marine mollusk Aplysia limacina. Febs J. 2005;272:343–344. [Google Scholar]
- Wang L, Ota N, Sweedler JV. Society for Neuroscience Meeting. Society for Neuroscience; Chicago: 2009. D-amino acids as a signaling molecule: from localization and release to synthesis. A novel amino acid racemase from the central nervous system of Aplysia californica; p. 889.818/GG843. [Google Scholar]
- Watanabe T, Shibata K, Kera Y, Yamada R. Occurrence of free D-aspartate and aspartate racemase in the blood shell Scapharca broughtonii. Amino Acids. 1998;14:353–360. doi: 10.1007/BF01318854. [DOI] [PubMed] [Google Scholar]
- Wolosker H, D’Aniello A, Snyder SH. D-aspartate disposition in neuronal and endocrine tissues: ontogeny, biosynthesis and release. Neuroscience. 2000;100:183–189. doi: 10.1016/s0306-4522(00)00321-3. [DOI] [PubMed] [Google Scholar]
- Zhao SL, Liu YM. Quantification of D/L-aspartic acids in Aplysia californica central nervous system by beta-cyclodextrin modified micellar electrokinetic chromatography. Biomed Chromatogr. 2001;15:274–279. doi: 10.1002/bmc.72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


