Abstract
A reproducible method, adapted from renal micropuncture techniques, was developed for sampling 10-40 mμl of a clear fluid from epiphyseal cartilage of normal or rachitic rats in vivo, either from the hypertrophic cell zone (Cf1) or surface resting cell cartilage (Lf1).
Characterization of this fluid depended upon quantitation of protein, total inorganic phosphate (Pit), total calcium (Cat), nucleotide, and hemoglobin in volumes of 20 mμl. Established methods for macroscale measurements of each of these parameters have been modified to permit direct spectrophotometric readings on samples of 10-10-10-11 g.
The fluid from hypertrophic and peripheral resting cell cartilage was of an extracellular nature as evidenced by a high chloride and sodium, as well as low potassium, protein, and nucleotide content.
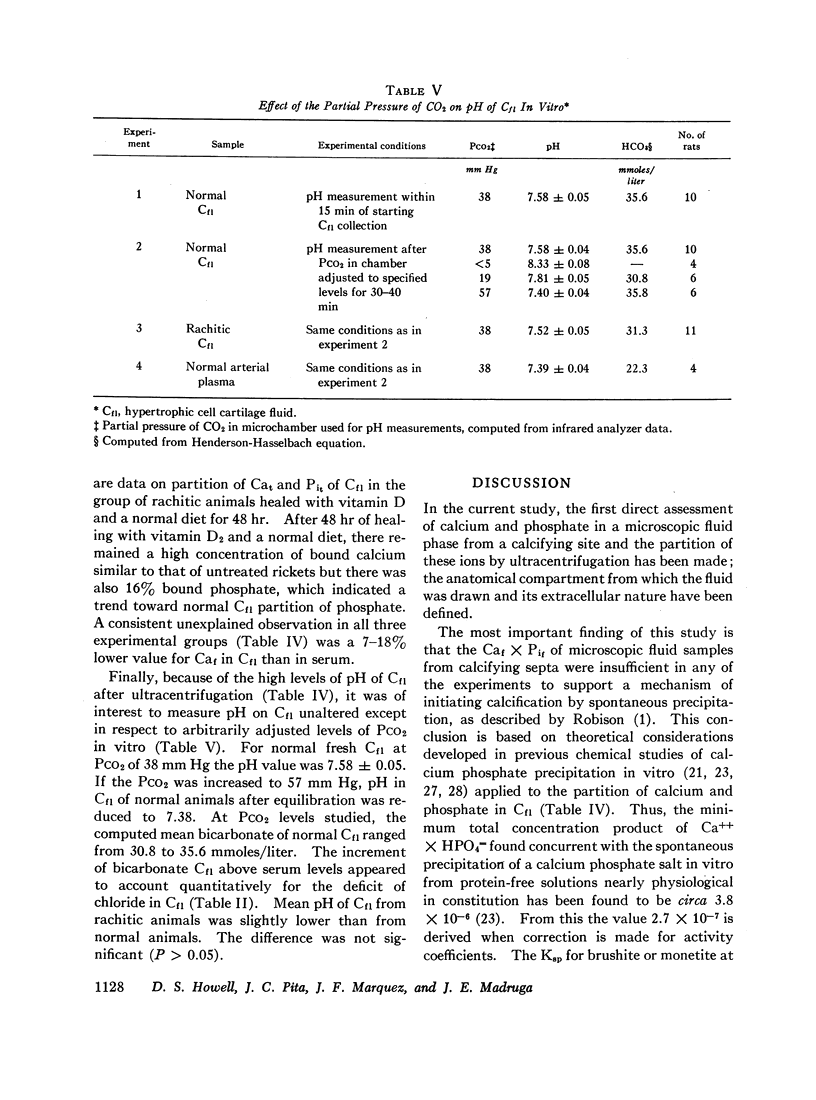
The pH of fluid isolated from endochrondral plates in vivo was measured under oil as a function of PCO2 and the computed bicarbonate was elevated above concurrent serum levels.
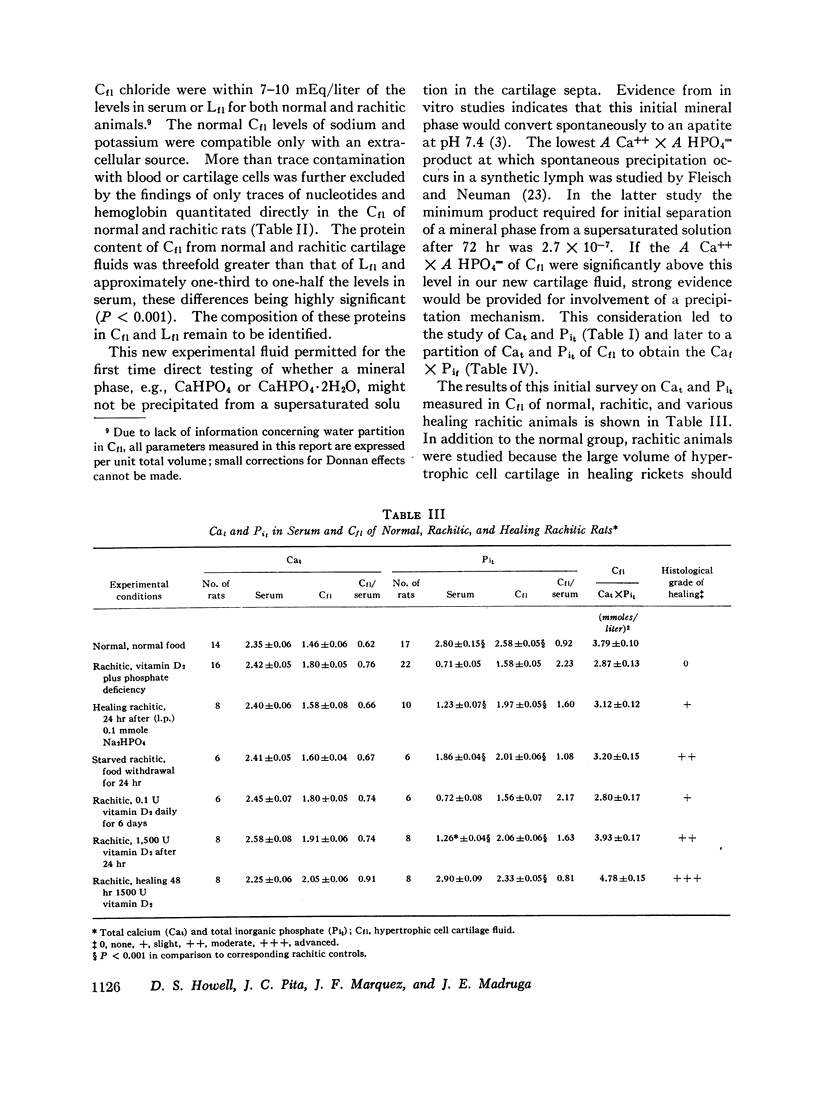
After ultracentrifugation of Cf1 of normal, rachitic, and healing rachitic animals, nonprotein-bound calcium (Caf) and phosphate (Pif) were determined on supernatant fluids. The hypertrophic cell cartilage fluid of rachitic rats was distinguished by a high ratio Cf1/serum of Pif. This ratio returned to normal during treatment of rickets. The upper limit for ionic activity A1 Ca++ × A HPO4= was too low to initiate precipitation of brushite or dicalcium phosphate but was in a range of supersaturation in respect to crystalline apatites. Thus these data are consistent with initiation of calcification by heterogeneous nucleation of mineral in the septal matrix but can be reconciled alternately with a precipitation mechanism only if the site of initial mineral phase separation is outside the septal matrix.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachra B. N. Some molecular aspects of tissue calcification. Clin Orthop Relat Res. 1967 Mar-Apr;51:199–222. [PubMed] [Google Scholar]
- Ellison A. C. Determination of carbonic anhydrase in the epiphysis of endochondral bone. Proc Soc Exp Biol Med. 1965 Nov;120(2):415–418. doi: 10.3181/00379727-120-30551. [DOI] [PubMed] [Google Scholar]
- FLEISCH H., BISAZ S. Isolation from urine of pyrophosphate, a calcification inhibitor. Am J Physiol. 1962 Oct;203:671–675. doi: 10.1152/ajplegacy.1962.203.4.671. [DOI] [PubMed] [Google Scholar]
- HOWARD J. E. Calcium metabolism, bones and calcium homeostasis; a review of certain current concepts. J Clin Endocrinol Metab. 1957 Sep;17(9):1105–1123. doi: 10.1210/jcem-17-9-1105. [DOI] [PubMed] [Google Scholar]
- HOWELL D. S., DELCHAMPS E., RIEMER W., KIEM I. A profile of electrolytes in the cartilaginous plate of growing ribs. J Clin Invest. 1960 Jun;39:919–929. doi: 10.1172/JCI104112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell D. S., Pita J. C., Marquez J. F. Ultramicro spectrophotometric determination of calcium in biologic fluids. Anal Chem. 1966 Mar;38(3):434–438. doi: 10.1021/ac60235a015. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOKEN H. F., HAVEL R. J., GORDAN G. S., WHITTINGTON S. L. Ultracentrifugal analysis of protein-bound and free calcium in human serum. J Biol Chem. 1960 Dec;235:3654–3658. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAREN T. H., MAYER E., WADSWORTH B. C. Carbonic anhydrase inhibition. I. The pharmacology of diamox 2-acetylamino-1,3,4-thiadiazole-5-sulfonamide. Bull Johns Hopkins Hosp. 1954 Nov;95(5):199–243. [PubMed] [Google Scholar]
- RECTOR F. C., Jr, CARTER N. W., SELDIN D. W. THE MECHANISM OF BICARBONATE REABSORPTION IN THE PROXIMAL AND DISTAL TUBULES OF THE KIDNEY. J Clin Invest. 1965 Feb;44:278–290. doi: 10.1172/JCI105142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REID E. L., HILLS A. G. DIFFUSION OF CARBON DIOXIDE OUT OF THE DISTAL NEPHRON IN MAN DURING ANTIDIURESIS. Clin Sci. 1965 Feb;28:15–28. [PubMed] [Google Scholar]
- Robison R. The Possible Significance of Hexosephosphoric Esters in Ossification. Biochem J. 1923;17(2):286–293. doi: 10.1042/bj0170286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman B. S., Sobel A. E. Differentiating between nucleation and crystal growth in mineralizing tissues and macromolecules. Arch Oral Biol. 1965 May-Jun;10(3):323–342. doi: 10.1016/0003-9969(65)90100-7. [DOI] [PubMed] [Google Scholar]
- Termine J. D., Wuthier R. E., Posner A. S. Amorphous-crystalline mineral changes during endochondral and periosteal bone formation. Proc Soc Exp Biol Med. 1967 May;125(1):4–9. doi: 10.3181/00379727-125-31999. [DOI] [PubMed] [Google Scholar]
- Vurek G. G., Bowman R. L. Helium-Glow Photometer for Picomole Analysis of Alkali Metals. Science. 1965 Jul 23;149(3682):448–450. doi: 10.1126/science.149.3682.448. [DOI] [PubMed] [Google Scholar]
- WALSER M. The separate effects of hyperparathyroidism, hypercalcemia of malignancy, renal failure, and acidosis on the state of calcium, phosphate, and other ions in plasma. J Clin Invest. 1962 Jul;41:1454–1471. doi: 10.1172/JCI104601. [DOI] [PMC free article] [PubMed] [Google Scholar]



