Abstract
Despite the ubiquity of invasive organisms and their often deleterious effects on native flora and fauna, the consequences of biological invasions for human health and the ecological mechanisms through which they occur are rarely considered. Here we demonstrate that a widespread invasive shrub in North America, Amur honeysuckle (Lonicera maackii), increases human risk of exposure to ehrlichiosis, an emerging infectious disease caused by bacterial pathogens transmitted by the lone star tick (Amblyomma americanum). Using large-scale observational surveys in natural areas across the St. Louis, Missouri region, we found that white-tailed deer (Odocoileus virginianus), a preeminent tick host and pathogen reservoir, more frequently used areas invaded by honeysuckle. This habitat preference translated into considerably greater numbers of ticks infected with pathogens in honeysuckle-invaded areas relative to adjacent honeysuckle-uninvaded areas. We confirmed this biotic mechanism using an experimental removal of honeysuckle, which caused a decrease in deer activity and infected tick numbers, as well as a proportional shift in the blood meals of ticks away from deer. We conclude that disease risk is likely to be reduced when honeysuckle is eradicated, and suggest that management of biological invasions may help ameliorate the burden of vector-borne diseases on human health.
Keywords: Amur honeysuckle, disease ecology, ehrlichiosis, lone star tick, white-tailed deer
Invasive species, defined here as nonnative species that spread rapidly and often become dominant members of local assemblages, constitute a significant threat to native biological diversity (1). Invasives often directly degrade various important ecosystem-level properties, including disturbance regimes, nutrient cycling, microbial processes, and hydrology (2–6). Additional indirect effects of biological invasions frequently manifest via ecological interactions within wildlife communities (7). These indirect effects may include changes in the distribution and abundance of parasites and pathogens, which are often deeply embedded in the complex, interactive webs of wildlife communities (8). However, the field of ecology has only recently begun to address the potential consequences of biological invasions for the transmission of parasites and pathogens that cause disease in humans (9).
Among the most ecologically complex disease dynamics are those involving pathogens that are transmitted among a community of hosts via arthropod vectors (10). The possible influences of invasive species on community interactions that govern host–pathogen dynamics are manifold, but can be divided into two broad mechanistic pathways. First, invasive species can alter the distribution, abundance, and/or diversity of hosts for infectious agents or their arthropod vectors (i.e., biotic pathways) (10). Second, biological invasions can alter abiotic features of the local environment (e.g., temperature, humidity), which can potentially alter vector survival rates and ultimately their transmission rates of pathogens to hosts (11, 12). In this study, we used surveys of communities invaded and uninvaded by an exotic shrub, Amur honeysuckle (Lonicera maackii), coupled with a shrub removal experiment, to distinguish between these pathways. In so doing, we provide a comprehensive examination of how an invasive plant affects vertebrate host communities, tick vectors, and the pathogens they carry to ultimately influence human disease risk.
L. maackii (“honeysuckle” hereafter) is a woody shrub native to Asia that has become a noxious understory invader in many deciduous forests of eastern North America (13), with myriad biotic and abiotic consequences (14). By reducing light levels (15) and through allelopathy (16), honeysuckle has wreaked havoc on native plant diversity and abundance (17–19). The implications of these changes for the composition and diversity of vertebrate species that occupy these habitats remain largely unknown, although some animals appear to take advantage of the thick cover provided by honeysuckle to evade predators (20).
The invasion of eastern North American forests by honeysuckle has occurred throughout much of the range of the lone star tick (Amblyomma americanum). Once considered a nuisance but a nonvector species, the lone star tick is now known to be an important vector of infectious diseases from wildlife to humans (i.e., zoonoses) in the United States (21), including Ehrlichia chaffeensis and E. ewingii (agents of human ehrlichiosis). Because E. chaffeensis and E. ewingii are not transovarially transmitted (i.e., from mother to offspring), it is the acquisition of a blood meal from a reservoir-competent and infective host in a juvenile life stage (i.e., larvae and nymphs) that results in an infected vector life stage tick (i.e., nymphs and adults) capable of transmitting pathogens to humans (21). Recent insights into the ecology of lone star tick-associated zoonoses suggest that white-tailed deer (Odocoileus virginianus) may serve as both the primary host for the lone star tick and a wildlife reservoir for multiple emerging bacterial pathogens, including E. chaffeensis and E. ewingii (22, 23).
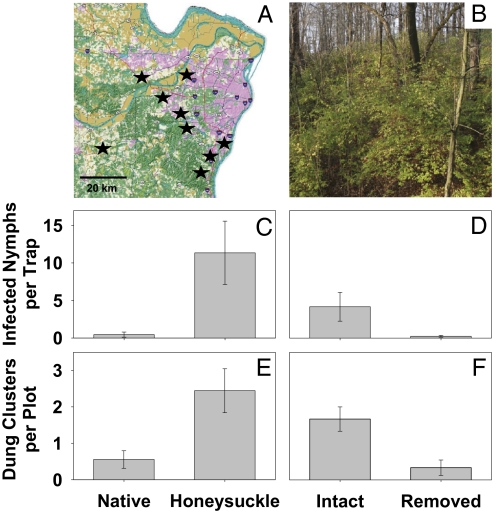
To quantify the impact of honeysuckle invasion on ticks and their associated pathogens, as well as on tick hosts, we conducted field surveys of paired honeysuckle-invaded and honeysuckle-uninvaded plots (measuring at least 30 × 30 m) in nine natural areas throughout the St. Louis, Missouri region (Fig. 1A). Furthermore, to provide a strong experimental test of the underlying mechanisms through which honeysuckle invasion alters community interactions and to examine whether eradication of the invasive plant can reduce tick-borne disease risk, we conducted an experimental removal of honeysuckle and measured tick survival rates in invaded and restored habitats at one of our most heavily invaded and high-disease risk study sites (Fig. 1B).
Fig. 1.
Effects of honeysuckle invasion and eradication on tick-borne disease dynamics. (A) Land-use map of the St. Louis, Missouri metropolitan area indicating the nine natural areas used in the regional survey of the effects of Amur honeysuckle invasion on tick-borne disease risk. (Map produced using the Geographic Resources Center, copyright 2002.) (B) Honeysuckle in the understory of an oak-hickory forest before eradication. (C and D) Density of A. americanum nymphs infected with E. chaffeensis in native vegetation vs. honeysuckle plots distributed across nine natural areas (C) and E. ewingii in honeysuckle-intact versus -eradicated experimental plots at the Busch Conservation Area (D). (E and F) Density of white-tailed deer dung clusters in native vegetation vs. honeysuckle plots distributed across nine natural areas (E) and honeysuckle-intact vs. -eradicated experimental plots at the Busch Conservation Area (F). Error bars reflect 1 SE.
Results and Discussion
Regional Survey: Ticks, Deer, and Disease Risk in Native Vegetation Versus Honeysuckle-Invaded Plots.
We found significantly higher abundances of both nymph (t = −4.011; P = 0.004) and adult (t = −3.117; P = 0.014) life stage ticks in honeysuckle-invaded plots relative to neighboring uninvaded native vegetation plots in the nine surveyed natural areas (Fig. S1A). We found no significant differences in the proportion of ticks infected with pathogens across the sites (range, 0.011–0.078 nymph infection prevalence); however, the density of nymphs infected with E. chaffeensis was ∼10-fold higher in the honeysuckle-invaded plots compared with the native vegetation plots (t = −3.766; P = 0.020; Fig. 1C), indicating that the presence of honeysuckle is associated with a substantial increase in disease risk.
Because white-tailed deer represent the primary host for lone star ticks and several of their associated pathogens (22, 23), we conducted field surveys to estimate their abundance based on scats found in the same plots surveyed for ticks. These surveys indicated a nearly fivefold greater density of deer in honeysuckle-invaded areas relative to uninvaded areas (t = −3.420; P = 0.009; Fig. 1E). One possible mechanism through which deer might use honeysuckle-invaded areas more frequently would be if the invaded areas had a higher overall vegetation density, providing possible food or security to resting deer. Indeed, we found a positive relationship between the density of honeysuckle in a given area and the overall vegetation density (R2 = 0.74; P < 0.0001), as well as an 18-fold increase in the overall density of plants in honeysuckle-invaded areas relative to uninvaded areas (1 ± 1.7 contacts with vegetation per 20 m in plots of native vegetation vs. 18.1 ± 13.8 contacts per 20 m in honeysuckle-invaded plots). Overall, these results suggest that increased use by deer of the densely vegetated habitat created by invasive honeysuckle might trigger a chain of ecological events that increases the local densities of ticks and their associated pathogens.
Removal Experiment: Ticks, Deer, and Disease Risk in Honeysuckle-Intact Versus Honeysuckle-Eradicated Plots.
The results of our honeysuckle eradication experiment mirrored those from our survey of naturally invaded and uninvaded areas. Specifically, we found significantly reduced densities of nymphs (F = 7.18; P = 0.043), but not adults (F = 3.04; P = 0.104), in plots in which honeysuckle was removed relative to plots in which it was left intact (Fig. S1B). The density of nymphs infected with E. ewingii was significantly reduced in honeysuckle-eradicated plots (F = 5.99; P = 0.028; Fig. 1D), although the percentage of infected nymphs did not differ significantly between the two types of plots (F = 0.24, P = 0.672). We found higher densities of deer scat in the honeysuckle-intact plots (F = 11.29; P = 0.02; Fig. 1F), and also found that vegetation density corresponded strongly with honeysuckle density (R2 = 0.82; P < 0.0001), with 22.7 ± 11.3 contacts per 20 m in the honeysuckle-intact plots versus 5.0 ± 6.9 contacts per 20 m in the honeysuckle-eradicated plots. Overall, these experimental results confirm the hypothesis that deer preferentially use areas invaded by honeysuckle, increasing the abundance of ticks in those areas and increasing the resulting disease risk.
To tease apart possible mechanisms through which tick abundances and their associated pathogen prevalences were influenced by the honeysuckle removal treatment, we performed two more detailed analyses. First, to discern whether differences in tick abundances might have been due to differences in the survivorship of ticks in honeysuckle-intact versus honeysuckle-eradicated areas as a result of abiotic differences between the habitat types (e.g., temperature, humidity), we performed a tick-survival experiment in each of the plots (24). We found no differences in survival of nymphs (z = −0.716; P = 0.470; Fig. S2A) and adults (z = 0.728, P = 0.47; Fig. S2B), suggesting that the observed differences were more likely due to honeysuckle-mediated changes in deer activity and not to abiotic changes imposed by honeysuckle removal.
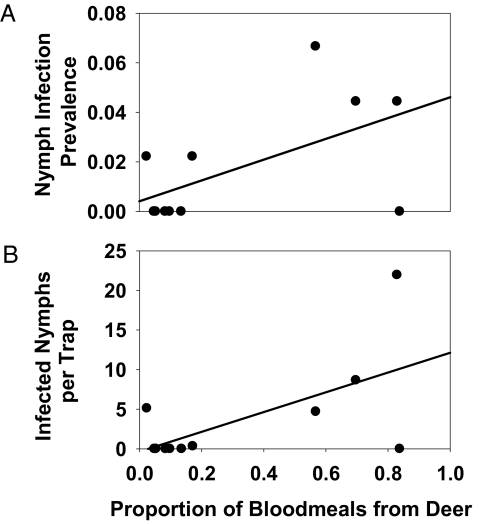
Second, to determine whether the distribution of tick blood meals among hosts, and thus the potential to acquire pathogens from different hosts, changes as host abundance changes, we used molecular techniques to identify the sources of the previous instar's blood meal from field-collected nymphs (23, 25). We found a trend toward an increased proportion of tick blood meals taken from deer in honeysuckle-intact plots relative to honeysuckle-eradicated plots (F = 4.79; P = 0.079) (Fig. S3). We also found a significant correlation between the proportion of blood meals derived from deer within a plot and both the infection prevalence for E. ewingii (R2 = 0.346; P = 0.044; Fig. 2A) and the density of nymphs infected with E. ewingii (R2 = 0.397; P = 0.028; Fig. 2B).
Fig. 2.
Proportion of blood meals from white-tailed deer vs. the proportion of A. americanum nymphs infected with E. ewingii (A) and the density of A. americanum nymphs infected with E. ewingii (B) in 12 experimental plots sampled at the Busch Conservation Area in May 2008.
Conclusions
Overall, our results show a mechanistic link between an invasive shrub and human risk of exposure to tick-borne diseases through a cascade of ecological interactions. First and foremost, honeysuckle alters the habitat use of white-tailed deer, which in turn alters the abundance of lone star ticks and human risk of exposure to the bacterial pathogens that they vector. Possible factors that cause white-tailed deer to select honeysuckle-invaded habitats are diverse but are likely related to deer foraging on some component of honeysuckle vegetative structure (e.g., leaves, bark) and/or using dense honeysuckle stands for shelter. In the St. Louis, Missouri region, honeysuckle invasion alters the nature of understory vegetation, forming a monoculture in the understory that is ≈18 times denser than uninvaded areas. Further, relative to native plants, honeysuckle produces leaves earlier and retains them longer during the growing season (26). The combined effect of increased stem density and altered leaf phenology is increased understory complexity and density. Wildlife may seek out such refuges for several reasons, including favorable microclimate and protection from predators (27). Viewed in light of evidence that deer prefer to select bed sites in more densely vegetated woody habitats (28), and that honeysuckle cover alters the behavior of other native vertebrates (20), our patterns of deer habitat use are consistent with the hypothesis that honeysuckle may provide a refuge (e.g., ref. 29) in which deer preferentially bed when not feeding.
The generalizability of our results to other plant invasions and infectious agents is an area in critical need of further study. The results of our tick survival study suggest that abiotic pathways were not responsible for the increase in lone star tick abundance observed in the honeysuckle-invaded plots. However, a recent study demonstrated that survival of lone star and American dog ticks was reduced by Japanese stiltgrass (Microstegium vimineum), an exotic annual grass invasive to eastern North America (12). Thus, the abiotic effects of plant invasions on tick-borne disease risk may vary depending on the species of tick and invasive plant. There is widespread evidence for biotic effects of environmental change on human risk of exposure to zoonotic diseases due to changes in the composition of wildlife communities (30). However, although there is phenomenological support for potential biotic effects of plant invasions on tick-borne disease risk mediated via tick hosts, mechanistic understanding has remained elusive. Studies conducted in the northeastern United States (31, 32) have demonstrated that human risk of exposure to Lyme disease, which is caused by the bacterium Borrelia burgdorferi and transmitted by the black-legged tick (Ixodes scapularis), is increased by several exotic shrubs, including honeysuckle and Japanese barberry (Berberis thunbergii). The ecological mechanisms through which these plant invasions influence Lyme disease risk remain unknown, however, and an enhanced understanding of the mechanisms that drive disease risk is critical to mitigation and control strategies.
Furthermore, determining the spatial scale over which invasive honeysuckle increases tick-borne disease risk is a crucial area of future research. Although our results clearly indicate an increase in disease risk at the scale of a local honeysuckle patch, increased use of invaded areas by deer could cumulatively decrease the time spent in native vegetation, such that disease risk in native areas is decreased relative to preinvasion conditions. Alternatively, proximity to honeysuckle-invaded sites could increase the disease risk in native vegetation due to a spillover effect of high disease risk from invaded areas. A broad-scale survey that includes large areas of uninvaded and fully invaded sites is needed to determine whether honeysuckle invasion increases disease risk beyond the scale of the local honeysuckle patch.
Our findings contribute to a growing body of literature that illustrates how extensively invasive species can alter interactions in native communities (29, 33). An accumulation of evidence indicates that the loss of biological diversity and the homogenization of wildlife communities have the potential to increase the prevalence of and risk of exposure to zoonotic diseases (10, 34). Our results illustrate an underappreciated consequence of anthropogenic global change: that biological invasions may indirectly contribute to human risk of exposure to infectious diseases, mediated by how invasive species alter ecological interactions in the communities that they invade. Further, our finding that removal of the invader mitigates disease risk, coupled with the benefits of invasive plant removal to wildlife communities, suggests a potential “win-win” scenario (35) for biodiversity conservation and human health.
Methods
Regional Survey.
Our survey in the St. Louis, Missouri region was conducted in nine natural areas that are naturally dominated by oak-hickory forests with an herbaceous understory (36) but are undergoing extensive invasion by honeysuckle. In each of these natural areas, we selected three plots of primarily native vegetation and three plots dominated by honeysuckle in which to conduct our surveys. Plots were intentionally selected to consist primarily of native or honeysuckle vegetation, and thus were not selected randomly within sites. All plots were at least 30 × 30 m in area, although many plots were much larger. To assess the impact of honeysuckle invasion on the complexity of understory vegetation, we measured vegetation density along one 20-m transect in one native plot and one invaded plot at each of the nine natural areas. Across this transect, we counted the number of times that any plant material touched a polyester line (i.e., encounters) held 2 m above the ground and counted the number of L. maackii individuals within 2 m of the line.
Human risk of exposure to tick-borne diseases is often quantified by measuring the density of vector life stages, their pathogen infection rates, and the product of these two variables, the density of infected ticks. This latter metric is widely considered the best estimate of human risk of encountering an infected tick (37). We sampled three native vegetation plots and three honeysuckle-invaded plots in each of the nine natural areas for the density of host-seeking ticks using CO2 traps baited with dry ice, a highly effective method for sampling lone star ticks (38). Nymph and adult life stage ticks were sampled by placing two CO2 traps ∼10 m apart near the center of each of the six plots at each study site. Traps were baited with 1 kg of dry ice and set out for 24 h. Sites were sampled once each in random order under constant meteorological conditions between June 12 and July 11, 2008, for a total of 108 trap-nights. Sampling coincided with the peak in abundance of the nymph and adult life stages of lone star ticks in Missouri (39).
Dung surveys were conducted on October 22–31, 2008, coinciding with the peak in abundance for larval life stage lone star ticks in Missouri (39) and thus indicative of the availability of deer for larval blood meals at our study sites. We randomly selected one plot of native vegetation and one plot of honeysuckle at each study site and delineated a central 20 × 20-m area using stake-wire flags. The entire grid was surveyed by a single observer (B.F.A.), who walked a transect every 2.5 m up and down each row of the survey area and scanned side-to-side for dung clusters. All dung clusters observed were marked with an additional flag to prevent recounting.
For pathogen analyses, we focused on nymph life stage ticks, because previous studies of tick-borne diseases have shown that this is often the primary vector life stage (37). Five of the nine natural areas yielded sufficient quantities of nymphs for pathogen analyses. We selected 90 nymphs from native vegetation plots and 90 nymphs from honeysuckle plots from each of these five areas. In brief, we screened ticks for pathogens using a combination of PCR with general primers to amplify any bacterial DNA that might be present and reverse line blot (RLB) hybridization with a series of pathogen-specific oligonucleotide probes to identify amplified bacterial DNA from the tick samples (25, 40). We used established RLB methods and probes (23) to screen for E. chaffeensis and E. ewingii.
Removal Experiment.
Our honeysuckle removal experiment was conducted at one study site (August A. Busch Memorial Conservation Area, Missouri Department of Conservation) that was heavily invaded by honeysuckle. We implemented a second study treatment that entailed the removal of honeysuckle fruits to tease apart the importance of honeysuckle vegetation versus honeysuckle fruits. We implemented this experimental removal of honeysuckle vegetation and fruits using a random-block study design, with one of four study treatments (honeysuckle vegetation intact or removed combined with honeysuckle fruits left intact or removed) in each of three experimental blocks.
The oak-hickory overstory was consistent among the experimental blocks, and treatments were randomly assigned to each 30 × 30 m-quadrant of each block. Honeysuckle individuals were physically removed by cutting the stem at the base in the fall of 2006 and pruning continuously until the end of the study period. Honeysuckle berries were individually removed by hand starting in the fall of 2006 and were continually removed each fall thereafter. In sites where vegetation was removed but honeysuckle fruits left intact, fruits were removed by hand and dropped on the ground before the vegetation was removed in the first year of the study. In subsequent years, fruits from “vegetation-intact fruits-removed” plots were added to the “vegetation-removed fruits-intact” plots on a weekly basis. Few significant effects were detected from the fruit removal treatment (Table S1), suggesting that the treatment had a minimal biologically relevant effect on tick-borne disease risk. Furthermore, no significant interactions were detected between the fruit and vegetation removal treatments in any of our analyses (Table S1), indicating that the effect of honeysuckle vegetation removal was not contingent on the influence of fruit removal. Therefore, we focused solely on the results from the vegetation removal treatment and conducted vegetation surveys using the same methods as described above for all 12 study plots, including removal areas.
The abundance of nymph and adult life stage ticks were sampled by CO2 traps as described above, with two surveys conducted in May and July of both 2007 and 2008, for a total of four surveys in each of the 12 plots. All 12 plots were sampled simultaneously with two CO2 traps each under relatively constant meteorological conditions. To avoid any potential edge effects, white-tailed deer dung cluster surveys were performed in the inner 20 × 20-m area of each plot on October 20–21, 2008, as described above for the regional survey.
We determined the prevalence of pathogens in nymphs for all 12 plots from the May 2007 and 2008 tick surveys. We tested at least 45 nymphs from each plot from the May 2007 survey (except for four plots that yielded <45 ticks; mean number tested, 41.3 ± 23.2) and exactly 45 nymphs from all 12 plots for May 2008.
For the removal experiment only, we also used a combination of PCR and RLB to identify blood meals derived from white-tailed deer for the aforementioned nymphs collected in May 2007 and 2008. As in our method for pathogen detection, we used universal primers to amplify a region of vertebrate 18S rDNA that is highly conserved across vertebrate taxa. We then identified this amplified vertebrate DNA using host-specific oligonucleotide probes in a RLB panel (23).
Finally, to explore the effects of abiotic conditions in our experimental plots on tick survival, we conducted a tick survival study (24) in all 12 study plots in 2008. In this study, 20 nymph and 10 adult life stage ticks were placed in each of 12 mesh bags, one of which was then partially buried in the leaf litter at the center of each study plot, protected by a chicken wire cage. The bags were examined weekly to determine the number of surviving nymphs and adults, until all ticks in all bags had succumbed to desiccation. The survival experiment was established on May 30, 2008, and continued for 22 wk until November 14, 2008, when all ticks in all plots were observed to have desiccated.
Statistical Analyses.
For the regional study, all samples from the three control plots of native vegetation and the three honeysuckle-invaded plots were averaged for each site, allowing for paired comparisons, with study sites serving as the level of replication. We used paired t tests to identify any significant difference between native vegetation versus honeysuckle for all response variables sampled (i.e., density of nymphs and adults, proportion of nymphs infected with zoonotic pathogens, density of infected nymphs, and density of deer dung clusters). All analyses were conducted in Systat.
For the experimental study, we used permutational ANOVA, using the PERMANOVA program (41, 42), to explore the effects of honeysuckle vegetation removal on the abundance of ticks, nymph infection rates with pathogens, density of infected nymphs, density of dung clusters, and proportion of blood meals from deer. We also explored for the effects of block, month (May vs. July), year (2007 vs. 2008), and all possible interactions among variables. PERMANOVA makes no particular assumptions regarding the distributions of original variables, because all P values are obtained by permutation. Analyses were performed with type III sums of squares and 9,999 unrestricted permutations of the raw data, using correct permutable units for the permutational ANOVA.
For the tick survival study, we conducted a Cox proportional hazards survival analysis for both nymphs and adults in the R programming environment using the “survival” library. The Cox proportional hazards approach has the benefit of being semiparametric, in that the baseline hazard function is left unspecified, whereas the covariates enter the model linearly. Again, we tested for the effects of vegetation removal, block effects, and all possible combinations of interactions on survival of nymphs and adults.
Supplementary Material
Acknowledgments
We thank the land managers of numerous private and public natural areas, including the Tyson Research Center, Missouri Department of Conservation, Missouri Department of Natural Resources, and St. Louis County Parks Department, for providing logistical support for this study. We thank the numerous volunteers who provided invaluable assistance in the field. Helpful comments on this research were provided by K. Clay, E. Damschen, F. Keesing, T. Knight, R. Ostfeld, A. Templeton, the Chase and Marquis Laboratory groups, and two anonymous reviewers. This research was funded by National Science Foundation Doctoral Dissertation Improvement Grants (to B.F.A. and H.P.D.), an Environmental Protection Agency Science to Achieve Results Fellowship and an American Philosophical Society Lewis and Clark Grant (to B.F.A.), Whitney R. Harris World Ecology Center and Webster Groves Nature Society Scholarships (to H.P.D.), and a Washington University Research Support for Senior Administrators grant (to R.E.T.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008362107/-/DCSupplemental.
References
- 1.Wilcove DS, Rothstein D, Dubow J, Phillips A, Losos E. Quantifying threats to imperiled species in the United States. Bioscience. 1998;48:607–615. [Google Scholar]
- 2.Mack MC, D'Antonio CM. Impacts of biological invasions on disturbance regimes. Trends Ecol Evol. 1998;13:195–198. doi: 10.1016/S0169-5347(97)01286-X. [DOI] [PubMed] [Google Scholar]
- 3.Brooks ML, et al. Effects of invasive alien plants on fire regimes. Bioscience. 2004;54:677–688. [Google Scholar]
- 4.Ehrenfeld JG. Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems (N Y, Print) 2003;6:503–523. [Google Scholar]
- 5.Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol. 2006;57:233–266. doi: 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- 6.Zavaleta ES, Hobbs RJ, Mooney HA. Viewing invasive species removal in a whole-ecosystem context. Trends Ecol Evol. 2001;16:454–459. [Google Scholar]
- 7.Levine JM, et al. Mechanisms underlying the impacts of exotic plant invasions. Proc Biol Sci. 2003;270:775–781. doi: 10.1098/rspb.2003.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostfeld RS, Keesing F, Eviner V. Infectious Disease Ecology: Effects of Ecosystems on Disease and of Disease on Ecosystems. Princeton: Princeton Univ Press; 2008. [Google Scholar]
- 9.Pearson DE, Callaway RM. Biological control agents elevate hantavirus by subsidizing deer mouse populations. Ecol Lett. 2006;9:443–450. doi: 10.1111/j.1461-0248.2006.00896.x. [DOI] [PubMed] [Google Scholar]
- 10.Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecol Lett. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- 11.Needham GR, Teel PD. Off-host physiological ecology of ixodid ticks. Annu Rev Entomol. 1991;36:659–681. doi: 10.1146/annurev.en.36.010191.003303. [DOI] [PubMed] [Google Scholar]
- 12.Civitello DJ, Flory SL, Clay K. Exotic grass invasion reduces survival of Amblyomma americanum and Dermacentor variabilis ticks (Acari: Ixodidae) J Med Entomol. 2008;45:867–872. doi: 10.1603/0022-2585(2008)45[867:egirso]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Luken JO, Thieret JW. Amur honeysuckle, its fall from grace. Bioscience. 1996;46:18–24. [Google Scholar]
- 14.Webster CR, Jenkins MA, Jose S. Woody invaders and the challenges they pose to forest ecosystems in the eastern United States. J For. 2006;104:366–374. [Google Scholar]
- 15.Gorchov DL, Trisel DE. Competitive effects of the invasive shrub, Lonicera maackii (Rupr.) Herder (Caprifoliaceae), on the growth and survival of native tree seedlings. Plant Ecol. 2003;166:13–24. [Google Scholar]
- 16.Dorning M, Cipollini D. Leaf and root extracts of the invasive shrub, Lonicera maackii, inhibit seed germination of three herbs with no autotoxic effects. Plant Ecol. 2006;184:287–296. [Google Scholar]
- 17.Gould AMA, Gorchov DL. Effects of the exotic invasive shrub Lonicera maackii on the survival and fecundity of three species of native annuals. Am Midl Nat. 2000;144:36–50. [Google Scholar]
- 18.Collier MH, Vankat JL, Hughes MR. Diminished plant richness and abundance below Lonicera maackii, an invasive shrub. Am Midl Nat. 2002;147:60–71. [Google Scholar]
- 19.Miller KE, Gorchov DL. The invasive shrub, Lonicera maackii, reduces growth and fecundity of perennial forest herbs. Oecologia. 2004;139:359–375. doi: 10.1007/s00442-004-1518-2. [DOI] [PubMed] [Google Scholar]
- 20.Mattos KJ, Orrock JL. Behavioral consequence of plant invasion: An invasive plant alters rodent antipredator behavior. Behav Ecol. 2010;21:556–561. [Google Scholar]
- 21.Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- 22.Paddock CD, Yabsley MJ. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum–associated zoonoses in the United States. Curr Top Microbiol Immunol. 2007;315:289–324. doi: 10.1007/978-3-540-70962-6_12. [DOI] [PubMed] [Google Scholar]
- 23.Allan BF, Goessling LS, Storch GA, Thach RE. Identification of reservoir hosts for Amblyomma americanum–associated zoonoses using bloodmeal analysis. Emerg Infect Dis. 2010;16:433–440. doi: 10.3201/eid1603.090911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertrand MR, Wilson ML. Microhabitat-independent regional differences in survival of unfed Ixodes scapularis nymphs (Acari: Ixodidae) nymphs in Connecticut. J Med Entomol. 1997;34:167–172. doi: 10.1093/jmedent/34.2.167. [DOI] [PubMed] [Google Scholar]
- 25.Pichon B, Egan D, Rogers M, Gray J. Detection and identification of pathogens and host DNA in unfed host-seeking Ixodes ricinus L. (Acari: Ixodidae) J Med Entomol. 2003;40:723–731. doi: 10.1603/0022-2585-40.5.723. [DOI] [PubMed] [Google Scholar]
- 26.Trisel DE, Gorchov DL. Regional distribution, leaf phenology, and herbivory of the invasive shrub, Lonicera maackii. Bull Ecol Soc Am. 1994;75:231–232. [Google Scholar]
- 27.Caro TM. Antipredator Defenses in Birds and Mammals. Chicago: Univ of Chicago Press; 2005. [Google Scholar]
- 28.Huegel CN, Dahlgren RB, Gladfelter HL. Bedsite selection by white-tailed deer fawns in Iowa. J Wildl Manage. 1986;50:474–480. [Google Scholar]
- 29.Orrock JL, Holt RD, Baskett ML. Refuge-mediated apparent competition in plant–consumer interactions. Ecol Lett. 2010;13:11–20. doi: 10.1111/j.1461-0248.2009.01412.x. [DOI] [PubMed] [Google Scholar]
- 30.Ostfeld RS. Biodiversity loss and the rise of zoonotic pathogens. Clin Microbiol Infect. 2009;15(Suppl 1):40–43. doi: 10.1111/j.1469-0691.2008.02691.x. [DOI] [PubMed] [Google Scholar]
- 31.Elias SP, et al. Deer browse resistant exotic-invasive understory: An indicator of elevated human risk of exposure to Ixodes scapularis (Acari: Ixodidae) in southern coastal Maine woodlands. J Med Entomol. 2006;43:1142–1152. doi: 10.1603/0022-2585(2006)43[1142:dbreua]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Williams SC, Ward JS, Worthley TE, Stafford KC., 3rd Managing Japanese barberry (Ranunculales: Berberidaceae) infestations reduces blacklegged tick (Acari: Ixodidae) abundance and infection prevalence with Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) Environ Entomol. 2009;38:977–984. doi: 10.1603/022.038.0404. [DOI] [PubMed] [Google Scholar]
- 33.Didham RK, Tylianakis JM, Hutchison MA, Ewers RM, Gemmell NJ. Are invasive species the drivers of ecological change? Trends Ecol Evol. 2005;20:470–474. doi: 10.1016/j.tree.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Pongsiri MJ, et al. Biodiversity loss affects global disease ecology. Bioscience. 2009;59:945–954. [Google Scholar]
- 35.Rosenzweig ML. Reconciliation ecology and the future of species diversity. Oryx. 2003;37:194–205. [Google Scholar]
- 36.Yang J, He HS, Shifley SR. Spatial controls of occurrence and spread of wildfires in the Missouri Ozark Highlands. Ecol Appl. 2008;18:1212–1225. doi: 10.1890/07-0825.1. [DOI] [PubMed] [Google Scholar]
- 37.Barbour AG, Fish D. The biological and social phenomenon of Lyme disease. Science. 1993;260:1610–1616. doi: 10.1126/science.8503006. [DOI] [PubMed] [Google Scholar]
- 38.Schulze TL, Jordan RA, Hung RW. Biases associated with several sampling methods used to estimate abundance of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 1997;34:615–623. doi: 10.1093/jmedent/34.6.615. [DOI] [PubMed] [Google Scholar]
- 39.Kollars TM, Jr, Oliver JH, Jr, Durden LA, Kollars PG. Host association and seasonal activity of Amblyomma americanum (Acari: Ixodidae) in Missouri. J Parasitol. 2000;86:1156–1159. doi: 10.1645/0022-3395(2000)086[1156:HAASAO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 40.Rijpkema SGT, Molkenboer MJ, Schouls LM, Jongejan F, Schellekens JF. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus ticks by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J Clin Microbiol. 1995;33:3091–3095. doi: 10.1128/jcm.33.12.3091-3095.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26:32–46. [Google Scholar]
- 42.McArdle BH, Anderson MJ. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology. 2001;82:290–297. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.