Abstract
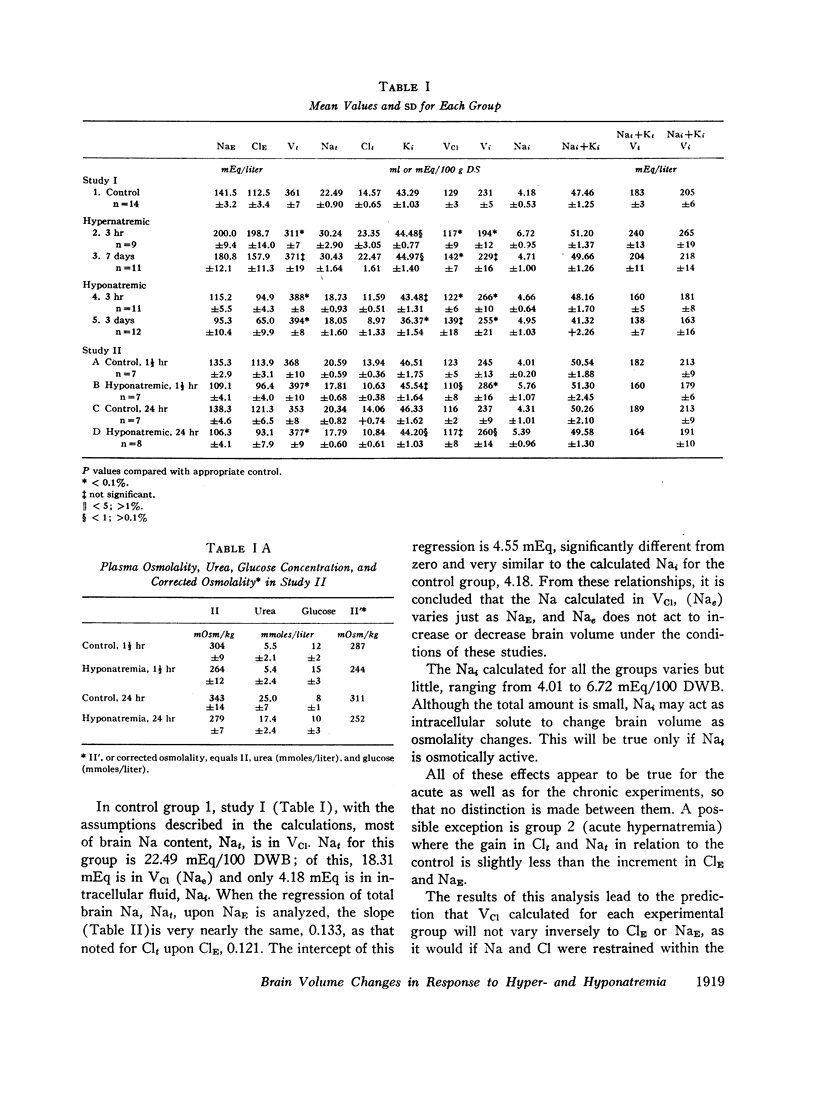
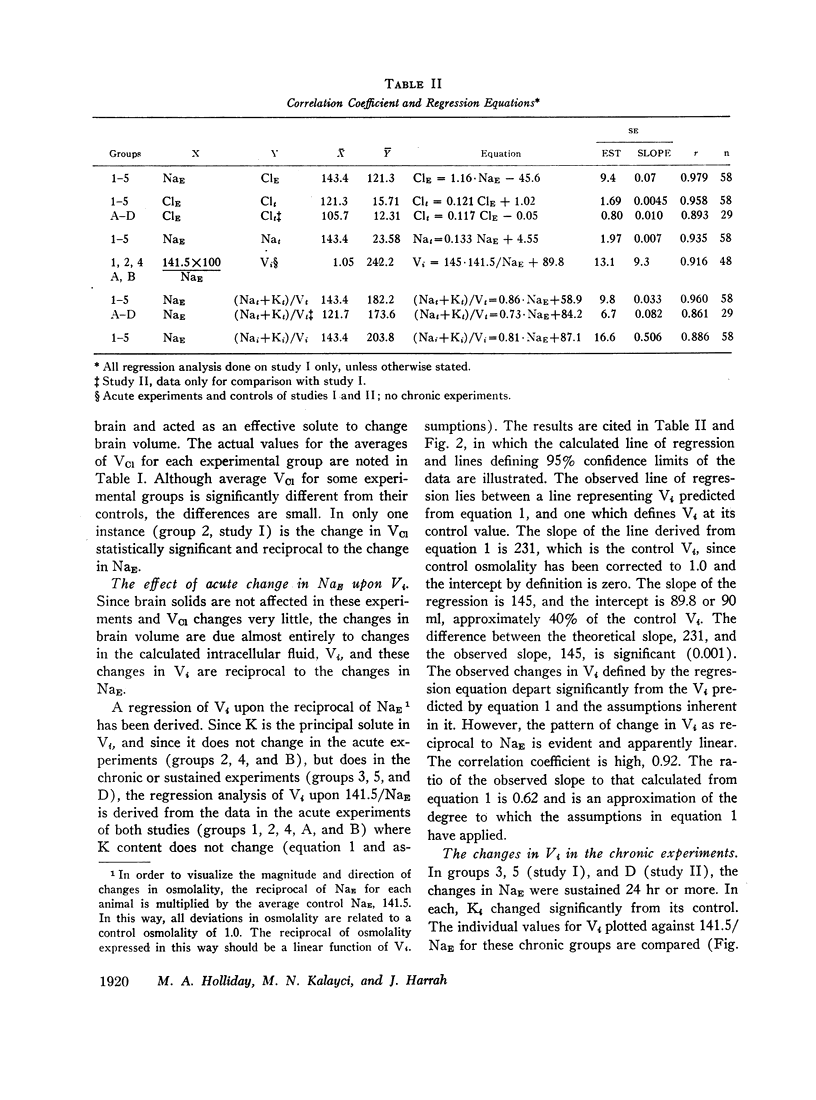
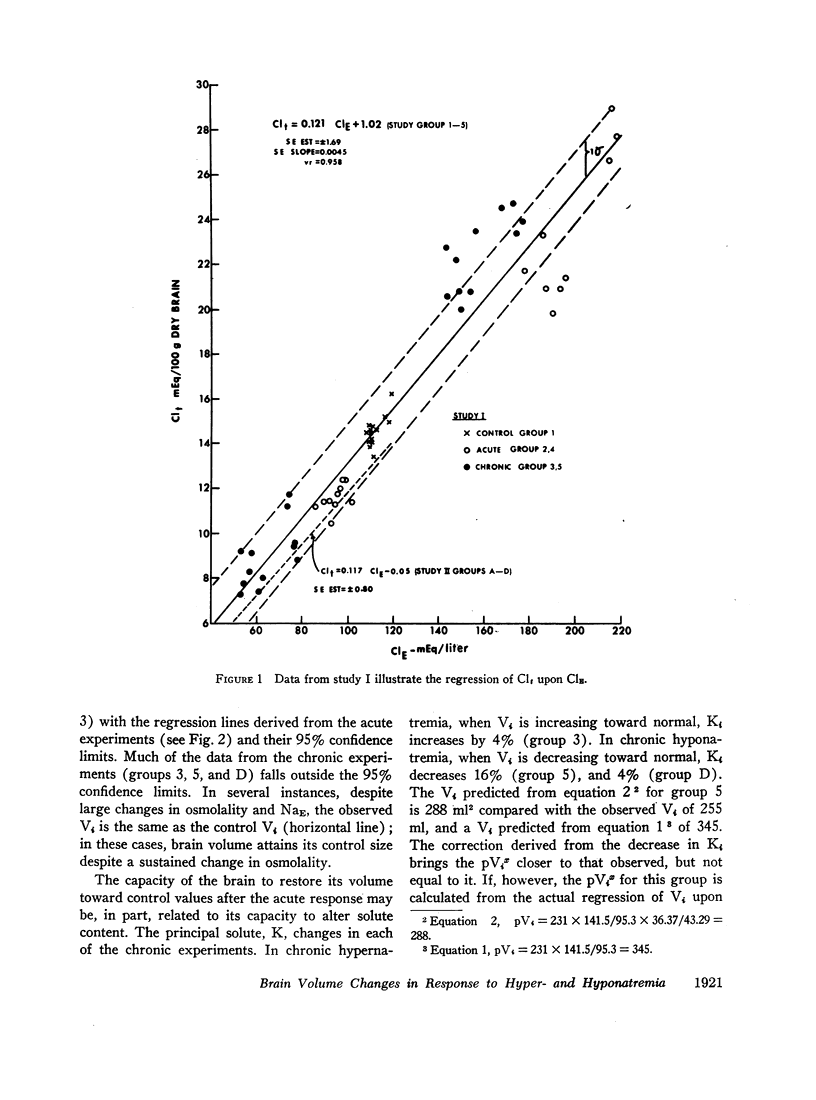
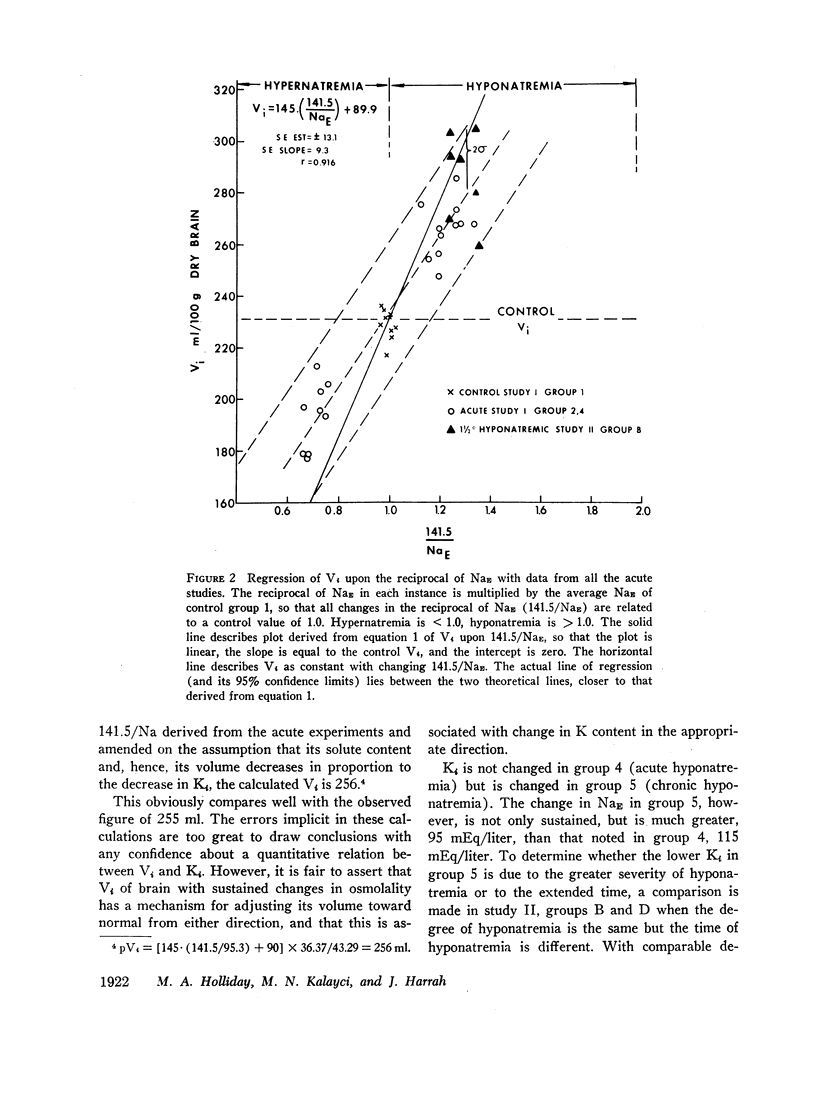
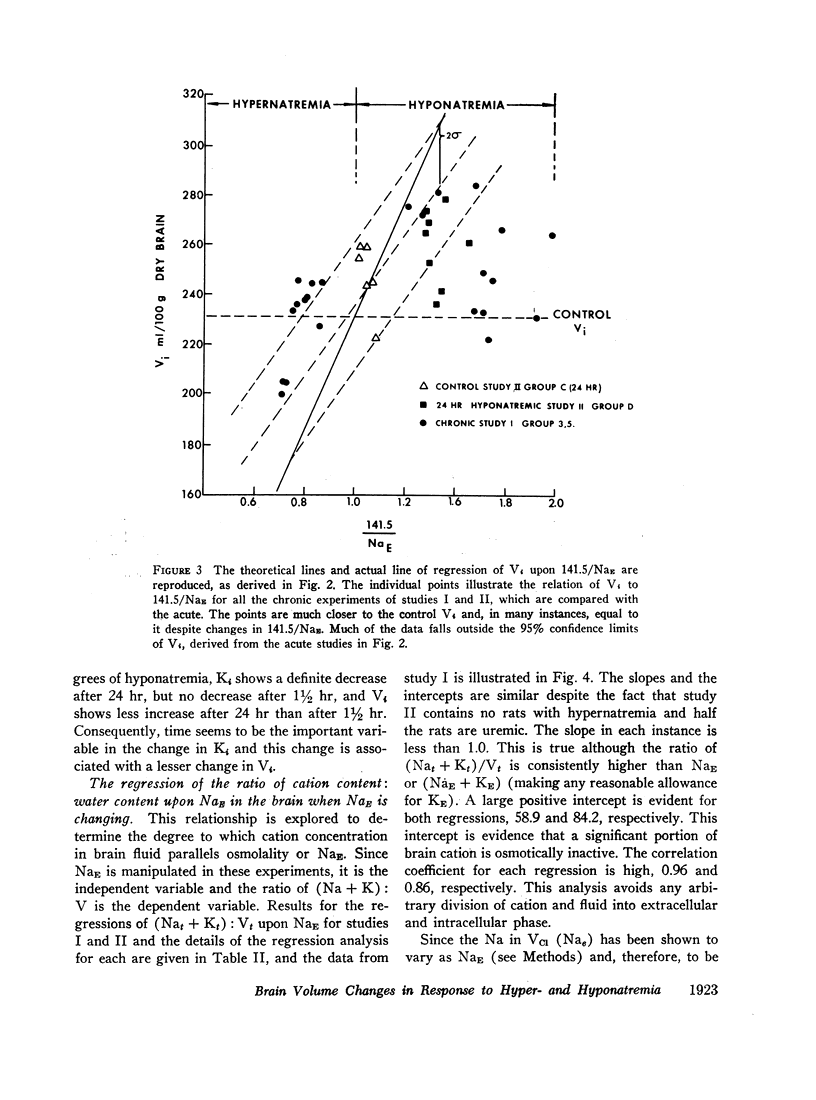
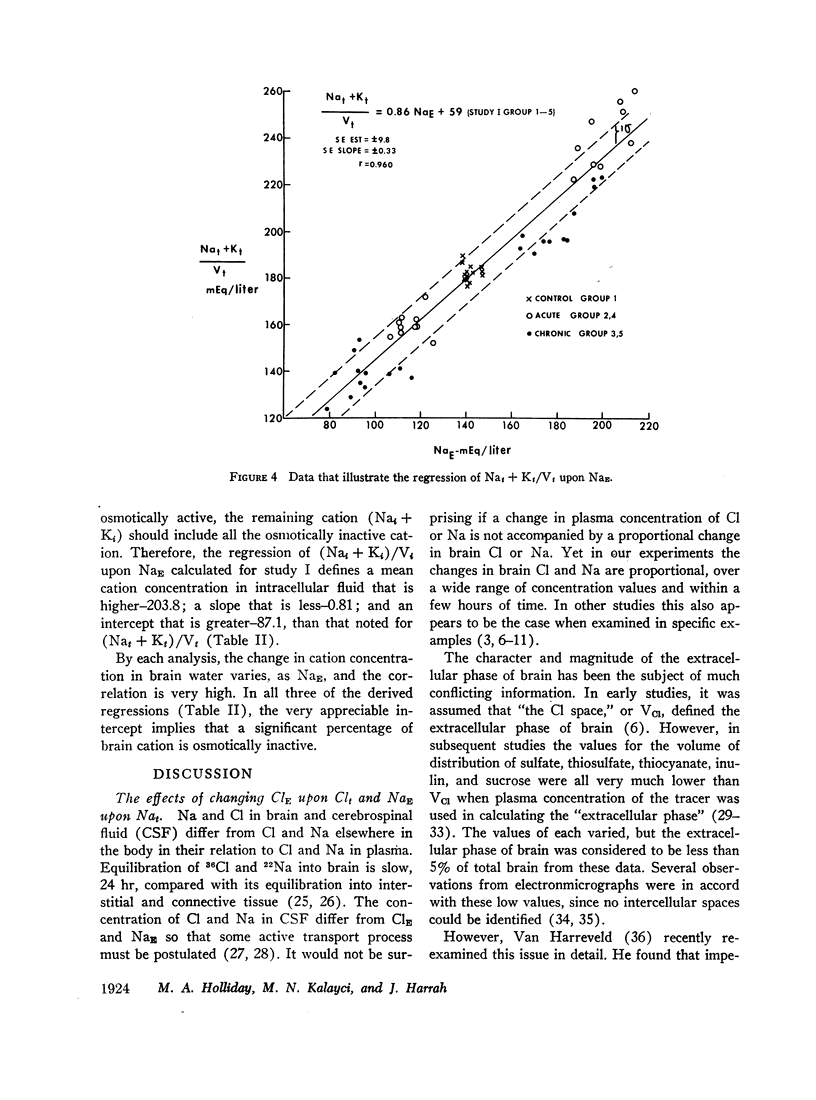
Rats were made acutely hyper- or hyponatremic by infusion of hypertonic saline or water, respectively. Other rats were maintained in these states from 1 to 7 days to observe the effects of time. Brain tissue water, Na, Cl, and K were compared with serum Na and Cl concentration (NaE and ClE). The following observations are noted: Brain Cl content varies directly with ClE and brain Na content in the Cl space (Nae) varies directly with NaE, indicating little or no restraint on the inward or outward movement of Na or Cl from the Cl space of brain. The intracellular volume of brain fluid (Vi) derived as the difference between total water and Cl space, decreases with hypernatremia and increases with hyponatremia. The changes in Vi in the acute studies are not accompanied by any change in brain K content, or calculated intracellular Na content, and are approximately 0.6 the changes predicted from osmotic behavior of cells, which apply four assumptions: (a) NaE is proportional to osmolality; (b) brain osmolality remains equal to plasma osmolality; (c) Vi is osmotically active; and (d) there is no net gain or loss of solute from Vi. The validity of these assumptions is considered. When changes in osmolality are sustained, Vi is much closer to control values than when in the acute phase. K content increases in hypernatremia and decreases in hyponatremia. The changes in K content can account for some of the adjustment in Vi observed over the extended period of hyper- or hyponatremia. The regression of (Na + K)/v upon NaE describes a slope less than 1.0 and an intercept of (Na + K)/v equal to 40% of the control (Na + K)/v. These characteristics are interpreted to mean that significant quantities of Na and K in brain are osmotically inactive. The brain protects itself from acute volume changes in response to change in NaE by the freedom for Na and Cl to move from the Cl space, by Vi not changing acutely to the degree predicted from osmotic properties of cells in general, and by significant quantities of Na + K in Vi being osmotically inactive. With sustained changes in osmolality, Vi approaches normal values and brain K changes to account for part of this later adjustment.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELBOOM J. W., BRODSKY W. A., DENNIS W. H., DIAMOND I., MILEY J. F., REHM W. S. The freezing point depression of mammalian tissues in relation to the question of osmotic activity of cell fluid. J Gen Physiol. 1956 Nov 20;40(2):183–199. doi: 10.1085/jgp.40.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APPELBOOM J. W., BRODSKY W. A., TUTTLE W. S., DIAMOND I. The freezing point depression of mammalian tissues after sudden heating in boiling distilled water. J Gen Physiol. 1958 Jul 20;41(6):1153–1169. doi: 10.1085/jgp.41.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW C. F., DOMEK N. S., GOLDBERG M. A., ROTH L. J. Extracellular brain space measured by S35 sulfate. Arch Neurol. 1961 Jul;5:102–110. doi: 10.1001/archneur.1961.00450130104012. [DOI] [PubMed] [Google Scholar]
- BOURKE R. S., GREENBERG E. S., TOWER D. B. VARIATION OF CEREBRAL CORTEX FLUID SPACES IN VIVO AS A FUNCTION OF SPECIES BRAIN SIZE. Am J Physiol. 1965 Apr;208:682–692. doi: 10.1152/ajplegacy.1965.208.4.682. [DOI] [PubMed] [Google Scholar]
- COTLOVE E., HOLLIDAY M. A., SCHWARTZ R., WALLACE W. M. Effects of electrolyte depletion and acid-base disturbance on muscle cations. Am J Physiol. 1951 Dec;167(3):665–675. doi: 10.1152/ajplegacy.1951.167.3.665. [DOI] [PubMed] [Google Scholar]
- COTLOVE E., TRANTHAM H. V., BOWMAN R. L. An instrument and method for automatic, rapid, accurate, and sensitive titration of chloride in biologic samples. J Lab Clin Med. 1958 Mar;51(3):461–468. [PubMed] [Google Scholar]
- DAVSON H., POLLAY M. The turnover of 24Na in the cerebrospinal fluid and its bearing on the blood-brain barrier. J Physiol. 1963 Jul;167:247–255. doi: 10.1113/jphysiol.1963.sp007145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVSON H., SPAZIANI E. The blood-brain barrier and the extracellular space of brain. J Physiol. 1959 Dec;149:135–143. doi: 10.1113/jphysiol.1959.sp006330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE P. R., CRAWFORD J. D., PROBST T. H. Studies in experimental water intoxication. Arch Neurol. 1960 Nov;3:513–529. doi: 10.1001/archneur.1960.00450050033005. [DOI] [PubMed] [Google Scholar]
- Darrow D. C., Yannet H. THE CHANGES IN THE DISTRIBUTION OF BODY WATER ACCOMPANYING INCREASE AND DECREASE IN EXTRACELLULAR ELECTROLYTE. J Clin Invest. 1935 Mar;14(2):266–275. doi: 10.1172/JCI100674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN I. S., LEIBMAN J., O'MEARA M. P., BIRKENFELD L. W. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 1958 Sep;37(9):1236–1256. doi: 10.1172/JCI103712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINBERG L., LUTTRELL C., REDD H. Pathogenesis of lesions in the nervous system in hypernatremic states. II. Experimental studies of gross anatomic changes and alterations of chemical composition of the tissues. Pediatrics. 1959 Jan;23(1 Pt 1):46–53. [PubMed] [Google Scholar]
- FINBERG L. Pathogenesis of lesions in the nervous system in hypernatremic states. I. Clinical ovservations of infants. Pediatrics. 1959 Jan;23(1 Pt 1):40–45. [PubMed] [Google Scholar]
- FUISZ R. E. Hyponatremia. Medicine (Baltimore) 1963 Mar;42:149–170. doi: 10.1097/00005792-196303000-00003. [DOI] [PubMed] [Google Scholar]
- KARLSSON U., SCHULTZ R. L. FIXATION OF THE CENTRAL NERVOUS SYSTEM FROM ELECTRON MICROSCOPY BY ALDEHYDE PERFUSION. I. PRESERVATION WITH ALDEHYDE PERFUSATES VERSUS DIRECT PERFUSION WITH OSMIUM TETROXIDE WITH SPECIAL REFERENCE TO MEMBRANES AND THE EXTRACELLULAR SPACE. J Ultrastruct Res. 1965 Feb;12:160–186. doi: 10.1016/s0022-5320(65)80014-4. [DOI] [PubMed] [Google Scholar]
- KLOTZ I. M. Protein molecules in solution. Circulation. 1960 May;21:828–844. doi: 10.1161/01.cir.21.5.828. [DOI] [PubMed] [Google Scholar]
- MAFFLY R. H., EDELMAN I. S. The role of sodium, potassium and water in the hypo-osmotic states of heart failure. Prog Cardiovasc Dis. 1961 Jul;4:88–104. doi: 10.1016/s0033-0620(61)80010-8. [DOI] [PubMed] [Google Scholar]
- MAFFLY R. H., LEAF A. The potential of water in mammalian tissues. J Gen Physiol. 1959 Jul 20;42(6):1257–1275. doi: 10.1085/jgp.42.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON A. B. The distribution of intravenously-injected inulin in the fluids of the nervous system of the dog and rat. J Clin Invest. 1959 Oct;38:1769–1777. doi: 10.1172/JCI103955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappius H. M., Oh J. H., Dossetor J. B. The effects of rapid hemodialysis on brain tissues and cerebrospinal fluid of dogs. Can J Physiol Pharmacol. 1967 Jan;45(1):129–147. doi: 10.1139/y67-014. [DOI] [PubMed] [Google Scholar]
- REED D. J., WOODBURY D. M. KINETICS OF MOVEMENT OF IODIDE, SUCROSE, INULIN AND RADIO-IODINATED SERUM ALBUMIN IN THE CENTRAL NERVOUS SYSTEM AND CEREBROSPINAL FLUID OF THE RAT. J Physiol. 1963 Dec;169:816–850. doi: 10.1113/jphysiol.1963.sp007298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND J. E., HASTINGS A. B. Distribution of sulfate in blood and between cerebrospinal fluid and plasma in vivo. Am J Physiol. 1960 Nov;199:814–820. doi: 10.1152/ajplegacy.1960.199.5.814. [DOI] [PubMed] [Google Scholar]
- SAVITZ D., SIDEL V. W., SOLOMON A. K. OSMOTIC PROPERTIES OF HUMAN RED CELLS. J Gen Physiol. 1964 Sep;48:79–94. doi: 10.1085/jgp.48.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELIGSON D., SELIGSON H. A microdiffusion method for the determination of nitrogen liberated as ammonia. J Lab Clin Med. 1951 Aug;38(2):324–330. [PubMed] [Google Scholar]
- SIMS E. A. H., WELT L. G., ORLOFF J., NEEDHAM J. W. Asymptomatic hyponatremia in pulmonary tuberculosis. J Clin Invest. 1950 Nov;29(11):1545–1557. doi: 10.1172/JCI102396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- STERN W. E., COXON R. V. OSMOLALITY OF BRAIN TISSUE AND ITS RELATION TO BRAIN BULK. Am J Physiol. 1964 Jan;206:1–7. doi: 10.1152/ajplegacy.1964.206.1.1. [DOI] [PubMed] [Google Scholar]
- STERN W. E. Studies in experimental brain swelling and brain compression. J Neurosurg. 1959 Nov;16:676–704. doi: 10.3171/jns.1959.16.6.0676. [DOI] [PubMed] [Google Scholar]
- VAN HARREVELD A., HOOPER N. K., CUSICK J. T. Brain electrolytes and cortical impedance. Am J Physiol. 1961 Jul;201:139–143. doi: 10.1152/ajplegacy.1961.201.1.139. [DOI] [PubMed] [Google Scholar]
- VANHARREVELD A., CROWELL J., MALHOTRA S. K. A STUDY OF EXTRACELLULAR SPACE IN CENTRAL NERVOUS TISSUE BY FREEZE-SUBSTITUTION. J Cell Biol. 1965 Apr;25:117–137. doi: 10.1083/jcb.25.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Harreveld A., Ahmed N., Tanner D. J. Sulfate concentrations in cerebrospinal fluid and serum of rabbits and cats. Am J Physiol. 1966 Apr;210(4):777–780. doi: 10.1152/ajplegacy.1966.210.4.777. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A., Collewijn H., Malhotra S. K. Water, electrolytes, and extracellular space in hydrated and dehydrated brains. Am J Physiol. 1966 Feb;210(2):251–256. doi: 10.1152/ajplegacy.1966.210.2.251. [DOI] [PubMed] [Google Scholar]
- WISE B. L. EFFECTS OF INFUSION OF HYPERTONIC MANNITOL ON ELECTROLYTE BALANCE AND ON OSMOLARITY OF SERUM AND CEREBROSPINAL FLUID. J Neurosurg. 1963 Nov;20:961–967. doi: 10.3171/jns.1963.20.11.0961. [DOI] [PubMed] [Google Scholar]


