Abstract
Disruption of the blood–brain barrier (BBB) underlies the development of experimental autoimmune encephalomyelitis (EAE) and multiple sclerosis. Environmental factors, such as Bordetella pertussis, are thought to sensitize central endothelium to biogenic amines like histamine, thereby leading to increased BBB permeability. B. pertussis-induced histamine sensitization (Bphs) is a monogenic intermediate phenotype of EAE controlled by histamine H1 receptor (Hrh1/H1R). Here, we transgenically overexpressed H1R in endothelial cells of Hrh1-KO (H1RKO) mice to test the role of endothelial H1R directly in Bphs and EAE. Unexpectedly, transgenic H1RKO mice expressing endothelial H1R under control of the von Willebrand factor promoter (H1RKO-vWFH1R Tg) were Bphs-resistant. Moreover, H1RKO-vWFH1R Tg mice exhibited decreased BBB permeability and enhanced protection from EAE compared with H1RKO mice. Thus, contrary to prevailing assumptions, our results show that endothelial H1R expression reduces BBB permeability, suggesting that endothelial H1R signaling may be important in the maintenance of cerebrovascular integrity.
Keywords: endothelium, experimental autoimmune encephalomyelitis, vasoactive amine sensitization, vascular permeability, multiple sclerosis
The blood–brain barrier (BBB) involves endothelial cells that line the blood vessels of the central nervous system (CNS) and the tight junction protein complexes between these endothelial cells. The BBB thereby acts as a physical and metabolic barrier by separating the vasculature from the parenchyma of the CNS (1). Breakdown of the BBB is associated with the onset and pathogenesis of multiple sclerosis (MS), a degenerative, demyelinating, inflammatory disease of the CNS (2). In MS and its autoimmune model, experimental autoimmune encephalomyelitis (EAE), activated T cells cross the BBB into the perivascular space of the CNS, causing damage to neurons (2). Surveillance of the CNS by T cells does occur (3), but the paucity of leukocytes found in the CNS under noninflammatory conditions suggests that extravasation of cells across the BBB is tightly regulated. Therefore, identifying factors that modulate BBB permeability may be of therapeutical value in treating inflammatory demyelinating diseases of the CNS.
Bordetella pertussis-induced hypersensitivity to histamine (Bphs/Bphs) is a genetically controlled intermediate phenotype associated with susceptibility to EAE (4). Bphs is a state wherein mice are rendered highly susceptible to histamine after a preceding injection of B. pertussis toxin (PTX) (5). Bphs-susceptible strains of mice die within 30 min after histamine challenge, presumably attributable to hypotensive and hypovolemic shock. The cells targeted during Bphs are not known. Histamine has also been implicated in the pathophysiology of MS and EAE. Increased tissue levels of histamine correlate with the onset of EAE (6–8). Mast cells, the major source of histamine (9), are present in MS lesions (10–12), and evidence of mast cell activation is found in the cerebrospinal fluid (CSF) of patients who have MS (13). In mice, mast cells (14) and their activation (15) are required for early EAE onset and maximal disease severity.
The effects of histamine are mediated by four surface histamine receptors: H1R, H2R, H3R, and H4R (16). H1R protein and mRNA are highly expressed in MS lesions (17), and H1 antihistamines reduce EAE in mice and rats (17, 18). In patients with MS, the use of sedating H1 antihistamines is correlated with decreased disease incidence and amelioration of symptoms (19, 20). Our laboratory has shown that susceptibility to Bphs and EAE requires expression of Hrh1, the gene encoding H1R (21).
Expression of H1R on T cells is required for their full encephalitogenic potential (22), but the contribution of H1R signaling in other cell types to the pathogenesis of EAE has not been formally addressed. In this study, we focused on endothelial cells because they express H1R, play a role in both Bphs and EAE, and are important in controlling vascular permeability. We generated mice overexpressing H1R only on endothelial cells to test the hypothesis that signaling via endothelial H1R promotes BBB permeability and susceptibility to both Bphs and EAE. Contrary to our hypothesis, endothelial-specific reexpression of H1R did not restore Bphs susceptibility in Hrh1-KO (H1RKO) mice. Moreover, we found that selective expression of H1R on endothelial cells decreased BBB permeability and protected mice from EAE.
Results
Bphs Susceptibility Maps to the Nonhematopoietic Compartment.
In this study, we tested the hypothesis that PTX acts as an ancillary adjuvant in EAE by increasing BBB permeability via hypersensitization of endothelial cells to H1R signaling. First, we determined whether bone marrow (BM)-derived or non–BM-derived cells were responsible for histamine hypersensitivity by assessing Bphs in reciprocal BM chimeras of Bphs-resistant (BphsR) and Bphs-susceptible (BphsS) strains of mice (C3H/HeJ and C3H.BphsSJL/J, respectively). BphsS BM-derived cells failed to rescue the phenotype of BphsR C3H/HeJ recipients (Table 1), indicating that the recipient Bphs genotype determined Bphs susceptibility. Both sedating (cross the BBB) and nonsedating (do not cross the BBB) H1 antihistamines blocked Bphs in PTX-sensitized BphsS C57BL/6J mice (Table S1), indicating that histamine was not directly neurotoxic to the CNS. Thus, the H1R-expressing target cells involved in Bphs are not located exclusively in the CNS or in the BM but, instead, reside in a nonhematopoietic compartment.
Table 1.
Non–BM-derived cells mediate Bphs
| Donor | Recipient | No PTX | PTX | P value |
| C3H/HeJ | C3H/HeJ | 0/2 | 0/4 | |
| BphsS | BphsS | 0/2 | 7/8 | 0.004 |
| BphsS | C3H/HeJ | 0/2 | 2/10 | |
| C3H/HeJ | BphsS | 0/2 | 9/10 | 0.002 |
| Het | Het | 0/2 | 6/7 | 0.006 |
| Het | C3H/HeJ | 0/2 | 1/6 | |
| C3H/HeJ | Het | 0/2 | 6/6 | 0.007 |
Reciprocal BM chimeras using C3H/HeJ, C3H.BphsSJL/J (BphsS), or heterozygous C3H/HeJ × C3H.BphsSJL/J F1 hybrid (Het) mice were generated as described in Materials and Methods. After reconstitution, mice were injected i.v. with 200 ng of PTX, and 3 d later, they were challenged i.v. with histamine (12.5, 6.25, 3.125, and 1.56 mg/kg of histamine). The data are the total number of animals that died within 30 min divided by the total number of animals studied at all doses. Control mice received carrier alone on day 0 and were challenged with 12.5 mg/kg of histamine 3 d later. A χ2 test was used to detect the significance of differences.
Endothelial H1R Overexpression Does Not Rescue Bphs in H1R-Deficient Mice.
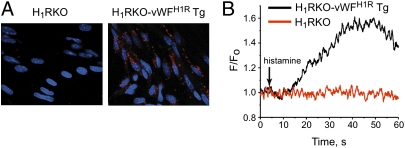
H1R-expressing endothelial cells are thought to control Bphs and BBB permeability, phenomena that are both important in susceptibility to EAE. In addition, endothelial cells regulate immune cell entry into the CNS during EAE (23). To directly test the hypothesis that endothelial H1R signaling is responsible for Bphs, increased BBB permeability, and EAE susceptibility, we generated transgenic H1RKO mice expressing endothelial H1R under control of the von Willebrand factor promoter (H1RKO-vWFH1R Tg). In these mice, endothelial-specific expression of H1R is driven by the vWF promoter (24) on an H1RKO background (Fig. S1A). We obtained two founder lines of H1RKO-vWFH1R Tg mice (Fig. S1B), but protein expression was only detectable in brain endothelial cells from the higher expressing line 4 (Fig. 1A), which we further studied. Vwf-HA-Hrh1 transgene expression was detectable in spleen, lymph node, and thymus that were depleted of lymphocytes (Fig. S1C). Activation of H1R increased Ca2+ levels in cells from H1RKO-vWFH1R Tg mice but not in cells from H1RKO mice (Fig. 1B). Taken together, these results demonstrate that H1RKO-vWFH1R Tg mice express H1R in endothelial cells of the CNS and lymphoid organs and that CNS-expressed H1Rs are functional.
Fig. 1.
Endothelial-specific H1R activation in vivo. (A) Brain endothelial cells from H1RKO and H1RKO-vWFH1R Tg mice were stained with anti-HA mAb, and HA-H1R expression (red) was visualized by confocal microscopy. Nuclei were stained with DAPI (blue). (B) Isolated brain endothelial cells were loaded with the Ca2+-sensing dye Fluo-4, and the change in Fluo-4 fractional fluorescence after stimulation with the H1R agonist 2-[(3-trifluoromethyl)phenyl] histamine dimaleate was measured by real-time confocal microscopy.
We then assessed whether H1RKO-vWFH1R Tg mice were susceptible to Bphs. As expected (21), H1RKO mice were resistant to Bphs, but, surprisingly, H1RKO-vWFH1R Tg mice were also completely resistant (Table 2). Sensitivity to histamine following PTX intoxication is prolonged (25), but mortality was not different between H1RKO and H1RKO-vWFH1R Tg mice up to 1 wk after initial histamine challenge (Table S2). These results show that endothelial-specific H1R activation does not influence acute or long-term histamine sensitivity following PTX intoxication.
Table 2.
H1RKO-vWFH1R Tg mice are resistant to Bphs
| Strain | Histamine (mg/kg) | No. affected/ Total |
| C57BL/6J | 100 | 4/4 |
| 50 | 4/4 | |
| 25 | 2/2 | |
| 12.5 | 2/2 | |
| H1RKO | 100 | 0/15 |
| H1RKO-vWFH1R Tg | 100 | 0/24 |
Mice were sensitized with 200 ng of purified PTX by i.v. injection on day 0. On day 3, animals were challenged with the indicated doses of histamine (mg/kg) by i.v. injection, and deaths were recorded at 30 min postchallenge. The results are expressed as the number of animals dead divided by the number of animals studied.
Endothelial-Specific H1R Activation Reduces BBB Permeability.
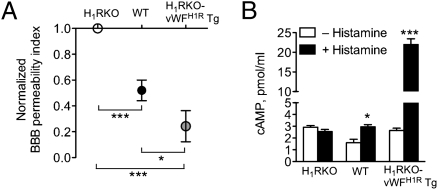
We assessed the BBB permeability index (SI Materials and Methods and ref. 26) in PTX-treated WT, H1RKO, and H1RKO-vWFH1R Tg mice. PTX-treated H1RKO mice exhibited the greatest concentration of FITC-BSA in the CSF after systemic administration, followed by WT mice, with the lowest concentration in H1RKO-vWFH1R Tg mice (Fig. 2A). Bphs/Hrh1 does not regulate sensitivity to PTX (27), indicating that differences observed were not attributable to ineffective PTX intoxication. Thus, contrary to the current dogma, the results obtained in our model system show that H1R overexpression in endothelial cells, in fact, negatively regulates BBB permeability, suggesting that H1R signaling in endothelial cells may be important in the maintenance of CNS barrier integrity.
Fig. 2.
Decreased BBB permeability mediated by endothelial H1R expression. (A) BBB permeability on day 10 after PTX injection of WT C57BL/6J (n = 9), H1RKO (n = 9), and H1RKO-vWFH1R Tg (n = 6) mice was determined as described in Materials and Methods. Values were normalized to H1RKO, and a one-sample t test was used to assess significance compared with H1RKO mice (***P < 0.0001, H1RKO vs. WT and H1RKO vs. H1RKO-vWFH1R Tg). WT and H1RKO-vWFH1R Tg mice were also significantly different from each other (*P < 0.05) as assessed by the Student's t test. (B) Brain endothelial cells from WT, H1RKO, and H1RKO-vWFH1R Tg mice (n = 4 per strain) were stimulated with PBS (no histamine) or with 10 μM histamine for 30 min, and intracellular cAMP was determined by enzyme immunoassay (*P < 0.05; ***P < 0.0001, as assessed by the Student's t test).
Activation of H1R through Gαq/11 proteins leads to an elevation of inositol 1,4,5-trisphosphate, which induces Ca2+ release from the endoplasmic reticulum of endothelial cells (28, 29). H1R can directly induce cAMP in cell lines (30) or indirectly increase cAMP levels by enhancing the effect of other cAMP-inducing stimuli (28, 31). With regard to BBB function, increased intracellular Ca2+ enhances tight junctions (32) and elevated cAMP increases BBB tightness (33). H1R triggering increased intracellular Ca2+ in H1RKO-vWFH1R Tg brain endothelial cells (Fig. 1B); thus, we next asked whether H1R signaling in endothelial cells could modulate cAMP levels. Endothelial cells from H1RKO mice did not increase cAMP in response to histamine, but cAMP was induced by WT endothelial cells and was strongly induced by histamine in endothelial cells from H1RKO-vWFH1R Tg mice (Fig. 2B). These results suggest that functional overexpression of H1R in endothelial cells directly contributes to maintenance of BBB integrity through a mechanism that may involve increased cAMP and intracellular Ca2+.
H1RKO-vWFH1R Tg Mice Are Highly Protected from Active EAE.
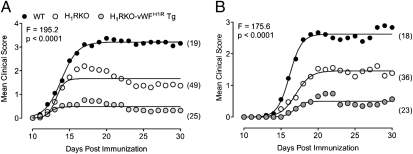
Given that breakdown of BBB integrity is implicated in the onset and pathogenesis of EAE/MS (34), we also determined whether H1RKO-vWFH1R Tg mice were susceptible to myelin oligodendrocyte glycoprotein peptides 35–55 (MOG35–55)-induced EAE. Age-matched cohorts of WT, H1RKO, and H1RKO-vWFH1R Tg mice were immunized with MOG35–55 + complete Freund’s adjuvant (CFA) + PTX, and clinical scores over a 30-d period were recorded. As expected (21, 22), H1RKO mice developed less severe disease than WT mice (Fig. 3A). Consistent with our findings on Bphs, H1RKO-vWFH1R Tg mice also did not develop severe EAE (Fig. 3A); surprisingly, however, EAE symptoms were even less severe in these mice than in H1RKO mice (Fig. 3A). Similar results were observed when using the 2× MOG35–55 + CFA protocol, which does not include PTX (Fig. 3B). EAE-associated clinical quantitative trait variables, including cumulative disease score, number of days affected, overall severity index, and peak score, were significantly different between WT, H1RKO, and H1RKO-vWFH1R Tg mice (Table S3); only day of onset was the same among genotypes. Compared with WT mice, the severity of disease parameters were decreased in H1RKO mice and further reduced in H1RKO-vWFH1R Tg mice. Similar disease trait results were obtained for cohorts immunized with the 2× MOG35–55 + CFA protocol for EAE (Table S4). At 30 d postimmunization with MOG35–55 + CFA + PTX, we also evaluated brain and spinal cord pathology, including demyelination, monocyte/lymphocyte infiltration, lesion score, and total disease score. Overall spinal cord pathology was significantly reduced in both H1RKO and H1RKO-vWFH1R Tg mice compared with WT mice, but the extent of this reduction was greater in H1RKO-vWFH1R Tg mice (Fig. S2). Thus, although global H1R expression is necessary for full development of EAE, selective overexpression of H1R on endothelial cells is protective.
Fig. 3.
Endothelial H1R activation is protective in EAE. EAE was elicited in WT, H1RKO, and H1RKO-vWFH1R Tg mice using a 1× MOG35–55 + CFA + PTX (A) or 2× MOG35–55 + CFA (B) immunization protocol. Number in parentheses indicates number of animals per group. Regression analysis revealed that the disease course in mouse strains fit a variable slope sigmoidal curve (shown as solid lines) that was significantly different (P < 0.0001) in all strains as assessed by the extra sum-of-squares F test.
Activation of Endothelial H1R Does Not Affect Encephalitogenic T-Cell Responses.
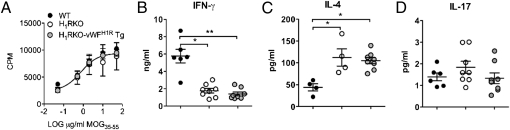
T cells and endothelial cells interact extensively at the BBB during EAE (35, 36). To ask whether endothelial H1R expression would have an impact on the encephalitogenic T-cell response, we examined the ex vivo MOG-specific response in spleen and draining lymph node cells from MOG35–55 + CFA + PTX-immunized WT, H1RKO, and H1RKO-vWFH1R Tg mice. MOG35–55-specific proliferative responses did not differ significantly in any of the strains (Fig. 4A). In agreement with our previous results (22), loss of H1R led to lower IFN-γ levels, higher IL-4 levels, and no change in IL-17 production by restimulated H1RKO cells compared with WT cells. However, cells from H1RKO-vWFH1R Tg mice responded like cells from H1RKO mice (Fig. 4 B–D). Similarly, none of the 20 other cytokines/chemokines we analyzed by multiplex assay were significantly affected by loss of H1R or by its overexpression in endothelial cells (Fig. S3). Furthermore, IFN-γ, IL-4, or IL-17 production by MOG35–55-restimulated cells from 2× MOG35–55 + CFA-immunized H1RKO mice was not different compared with cells from H1RKO-vWFH1R Tg mice (Fig. S4). In addition, expression of the endothelial activation marker intercellular adhesion molecule (ICAM)-1 (37) was not different in the CNS of 1× MOG35–55 + CFA + PTX-immunized WT, H1RKO, or H1RKO-vWFH1R Tg mice (Fig. S5). Likewise, endothelial degranulation, as assessed by serum vWF levels, in these mice was not different (Fig. S6). Thus, modulation of H1R expression did not affect the inflammatory capacity of endothelial cells.
Fig. 4.
Endothelial H1R expression did not alter the antigen-specific cytokine response in 1× MOG35–55 + CFA immunized mice. Spleen and draining lymph node cells were isolated from WT, H1RKO, and H1RKO-vWFH1R Tg (n = 15–17 per strain) mice that were immunized 10 d previously with the 1× MOG35–55 + CFA + PTX protocol. (A) Cells were restimulated ex vivo with the indicated doses of MOG35–55 for 72 h, and proliferation was determined by [H3]-thymidine incorporation. The mean cpm ± SD were calculated from triplicate wells, and the results shown are representative of two experiments. (B–D) Cells were isolated as in A and restimulated with 50 μg/mL MOG35–55 for 72 h. Supernatants were harvested and IFN-γ (B), IL-4 (C), and IL-17 (D) levels were determined by ELISA (*P < 0.05; **P < 0.01, as determined using one-way ANOVA with Kruskal–Wallis and Dunn's multiple comparison tests).
Discussion
Susceptibility to EAE and MS is determined, in part, through gene-by-environment interactions. PTX is an example of an environmental factor derived from an infectious agent that influences susceptibility to EAE; as such, it is widely used as an ancillary adjuvant in EAE. Exposure to PTX leads to increased BBB permeability that is thought to be controlled, in part, by sensitization of endothelial cells to vasoactive amines, such as histamine (38). In this study, we provide data demonstrating the opposite: that H1R signaling in endothelial cells decreases BBB permeability and strongly reduces susceptibility to EAE (Fig. S7).
Endothelial cells express H1R (28) and regulate vascular tone, BBB permeability, and migration and extravasation of leukocytes into tissues (39, 40). Because H1R is important for both Bphs and EAE, and because both Bphs and EAE involve some or all of these physiological processes, we asked whether endothelial H1R signaling could similarly affect EAE or Bphs susceptibility. BM chimera experiments suggested a nonhematopoietic compartment mediating Bphs. Endothelium-specific expression of H1R did not rescue the BphsR phenotype of H1RKO mice. Our results argue against a failure of H1R signaling in H1RKO-vWFH1R Tg endothelial cells because these cells responded to H1R stimulation with elevations in both intracellular Ca2+ and cAMP. It is also possible that concomitant H1R expression on another cell type, such as vascular smooth muscle cells or perivascular neurons, is required for Bphs. H1R signaling has been shown to modulate arterial tone via vascular endothelium and adrenergic and nonadrenergic/noncholinergic perivascular nerves (41). Our results do not support a role for endothelial H1R signaling as a physiological mechanism for Bphs. However, it is possible that H1R activation on perivascular nerves stimulates release of vasoactive neurotransmitters to elicit the characteristic hypovolemic and hypotensive shock associated with Bphs.
We have previously reported decreased EAE susceptibility in H1R-deficient mice (21) and that expression of H1R in T cells complements EAE in H1RKO mice (22). In contrast, in this study, we show that overexpression of H1R on endothelial cells further suppressed the residual disease symptoms present in H1RKO mice. Mechanistically, we propose that overexpression of H1R in endothelial cells enhanced histamine-induced Ca2+ flux and cAMP production to augment BBB function. The increased BBB function presumably impaired the entrance of encephalitogenic T cells into the CNS, resulting in nearly complete protection from EAE in H1RKO-vWFH1R Tg mice. Thus, although H1R activation in T cells is disease-promoting, its expression on endothelial cells impairs disease development, emphasizing cell-specific roles for susceptibility genes in complex traits, such as EAE.
In addition to their barrier function, endothelial cells store and release inflammatory mediators from Weibel–Palade bodies (WPBs) (42) and cytoplasmic granules (43). Although histamine is known to be a secretagogue for the release of WPB contents (44), our data do not support a role for H1R in triggering the inflammatory capacity of endothelial cells in the CNS. Modulation of H1R expression did not affect CNS endothelial activation status during EAE as assessed by ICAM-1 staining. Furthermore, endothelial H1R overexpression did not affect the MOG35–55-specific T-cell response compared with that of H1RKO mice. However, compared with WT mice, both H1RKO and H1RKO-vWFH1R Tg T cells produced less IFN-γ in response to MOG35–55 in vitro. We believe that the reduced encephalitogenicity of T cells in H1RKO and H1RKO-vWFH1R Tg mice likely reflects a T-cell–inherent defect, presumably attributable to the requirement for H1R for the full encephalitogenic capacity of T cells (22) rather than an effect on endothelial cells.
Histamine signaling appears to play differing and complex roles in the cerebral microvasculature during EAE. For leukocytes, H1R signaling promotes rolling in the cerebral microvasculature (45). Histamine also induces release of P-selectin from WPBs (44), which, within the CNS, is required for lymphocyte rolling but not adhesion (46). To our knowledge, it is not clear whether the secretagogue function of histamine for release of endothelial contents is H1R-dependent. Our data here do not support a role for H1R in the induction of ICAM-1 on CNS endothelial cells during disease. Nonetheless, regarding the barrier function of endothelial cells, our data indicate an important role for H1R in this process. H1RKO mice showed increased BBB permeability compared with WT mice, whereas overexpression of this receptor in the endothelium strongly reduced BBB permeability. We conclude that H1R activation primarily affects the barrier functions and not the inflammatory function of CNS endothelium during disease. Collectively, the enhanced barrier effect mediated by H1R in endothelial cells may increase the threshold for CNS immune surveillance by inhibiting activated T cells from entering the CNS without affecting their encephalitogenic potential.
Consistent with this notion, leukocyte infiltration into the spinal cord during EAE was lowest in H1RKO-vWFH1R Tg mice. However, compared with H1RKO mice, the Ag-specific immune response was not affected by restoration of endothelial H1R. However, if adhesion signals, such as the interaction between α4 integrin or lymphocyte function-associated antigen (LFA)-1 on activated T cells and vascular cell adhesion molecule (VCAM)-1 or intercellular adhesion molecule (ICAM)-1, respectively, on endothelial cells (37), are sufficiently high, T cells could still enter the CNS. Indeed, targeting α4 integrin is a viable but not comprehensive therapeutical approach for MS (47). Thus, we propose that H1R does not affect the elicitation of inflammatory cues produced by endothelial cells during the induction of disease but that an important role of endothelial H1R signaling is to increase the stringency for entry of immune cells into the CNS across the BBB. Finally, the results obtained here encourage the speculation that selectively activating H1R in endothelial cells may be an effective preventative or therapeutical strategy for EAE/MS.
Materials and Methods
Mice.
C57BL/6J (B6) mice and C3H/HeJ mice were purchased from the Jackson Laboratory. B6.129P-Hrh1tm1Wat (H1RKO) mice (48) and C3H.BphsSJL/J (BphsS) congenic mice (21) were maintained in-house at the University of Vermont. All animals were housed at the vivarium of the Given Medical Building at the University of Vermont. All animal studies were approved by the Institutional Animal Care and Use Committee of the University of Vermont.
H1RKO-vWFH1R Tg mice were generated by injection of a DNA fragment containing the murine Vwf promoter (24), the HA-tagged BphsS Hrh1 allele (22), and the human growth hormone (hGH) intron/polyadenylation signal directly into fertilized C57BL/6J eggs at the University of Vermont Transgenic/Knockout Mouse Facility. Transgene-positive founders were identified by DNA slot-blot using a BamHI-SacI 0.5-kb fragment from the hGH gene as a probe. Two founder lines were generated and were crossed to H1RKO mice to establish H1RKO-vWFH1R Tg mice.
Primary Mouse Brain Endothelial Cell Cultures.
Microvascular endothelial cells were isolated and cultured as previously described (49), except that dialyzed “histamine-free” FBS (22) was used in the culture medium.
Confocal Microscopy.
Brain endothelial cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% (vol/vol) Triton X-100 in PBS, and stained using an anti-HA mAb (Cell Signaling Technologies), followed by incubation with AlexaFluor 568-conjugated goat anti-mouse IgG Ab (Molecular Probes/Invitrogen). DAPI (Sigma–Aldrich) was used as a nuclear marker. Cells were examined by confocal microscopy using a Zeiss LSM 510 META Confocal Laser Scanning Imaging System (Carl Zeiss USA Microimaging, Inc.).
Ca2+ Imaging in Endothelial Cells.
Mouse brain endothelial cells were loaded with Fluo-4 (10 μM) for 7 min at 35 °C in the presence of pluronic acid (2.5 μg/mL). Ca2+ was imaged using a Revolution confocal system with an electron multiplying charge-coupled device camera (Andor Technology) mounted on an upright Nikon microscope with a 60× water-immersion objective (1.0 N.A.). After adding the H1R agonist 2-[(3-trifluoromethyl)phenyl] histamine dimaleate, images were acquired at 15 frames per second with Revolution TL acquisition software (Andor Technology). Fluo-4 fluorescence was excited by a krypton/argon laser (488 nm), and emitted fluorescence was collected at 495 nm. The images were processed using custom-designed software (supplied by A. Bonev, University of Vermont), and the fractional fluorescence was evaluated by dividing the fluorescence of a region of interest (ROI; 5 × 5 pixels per box) by the average fluorescence of 10 images from the same ROI.
Determination of cAMP Production.
Mouse brain endothelial cells were cultured in collagen-coated six-well plates and treated with PBS or 10 μM histamine for 30 min. Cells were then washed twice with ice-cold PBS and immediately lysed with 1 mL of 0.1 M HCl in PBS. The protein concentration in lysates was adjusted to 1.6 mg/mL with PBS, and cAMP was measured using an enzyme immunoassay kit (Cayman Chemical) according to the manufacturer's instructions.
PTX-Induced Sensitization to Histamine.
As described previously (21), mice were injected i.v. with 200 ng of purified PTX (List Biological Laboratories) dissolved in buffer containing 25 mM Tris, 0.5 M NaCl, and 0.017% (vol/vol) Triton X-100. Control mice received carrier. Three days later, sensitivity to histamine was determined by i.v. injection of indicated doses (mg/kg of dry base) of histamine dihydrochloride (Sigma–Aldrich) in PBS. H1 antihistamines were injected i.v. 20 min before histamine challenge. Deaths were recorded 30 min after histamine challenge or as indicated. Control mice received PBS. The results are expressed as the number of deaths divided by the number of animals studied.
BM Chimeras.
Femurs from 6- to 8-wk-old C3H/HeJ, C3H.BphsSJL/J (BphsS), or heterozygous C3H/HeJ × C3H.BphsSJL/J F1 hybrid (Het) mice were aspirated with RPMI through an 18-gauge needle to obtain BM cells. BM cells were washed and resuspended in RPMI and kept on ice until injection. Recipient mice (also 6–8 wk old) were irradiated with 700 rad divided over two doses given 4 h apart. Immediately following the second irradiation, mice received 2 × 106 BM cells of the indicated donor genotype i.v. and were allowed to reconstitute for 8 wk. Mice were then injected with 200 ng of PTX by i.v. injection, and 3 d later, they were challenged i.v. with histamine (12.5, 6.25, 3.125, and 1.56 mg/kg). Control mice received carrier alone on day 0 and were challenged with 12.5 mg/kg of histamine 3 d later.
Determination of BBB Permeability.
Determination of BBB permeability was performed essentially as described (26). Briefly, mice were injected i.v. with 200 ng of PTX, and 10 d later, they were given 50 μg/g of FITC-labeled BSA (Sigma–Aldrich) by i.v. injection. Four hours later, CSF and plasma were isolated (SI Materials and Methods), diluted in PBS, and centrifuged at 835 × g for 15 min. The fluorescence intensity (FI; excitation wavelength of 485 nm, emission wavelength of 528 nm) of FITC in the CSF and serum samples was determined with a microplate fluorescence reader (Flx-800-I; Bio-Tek Instruments, Inc.). The BBB permeability index is expressed as the ratio of the CSF FI divided by the plasma FI.
Induction and Evaluation of EAE.
Mice were immunized for MOG35–55-induced EAE and scored for clinical quantitative trait variables and quantitative histopathology as described previously (22). Non-linear regression analyses (50) were performed to examine the effect of strain on measures of disease severity (SI Materials and Methods).
Ex Vivo MOG35–55 Responses.
Mice were immunized for the induction of EAE, and 10 d later, spleens and draining lymph nodes were isolated. For proliferation assays, 5 × 105 cells per well were cultured in 96-well plates for 72 h at 37 °C in the presence of 0.05, 0.5, 2, 10, or 20 μg/mL MOG35–55. Cells were pulsed for the last 18 h of culture with 1.0 μCi of 3H-thymidine (PerkinElmer) and harvested onto glass fiber filters, and thymidine uptake was determined by liquid scintillation (Tomtec, Inc.). Counts per minute were measured, and data are presented as the mean ± SEM of triplicate wells. For cytokine assays, 1 × 106 cells per well were cultured in 24-well plates for 72 h in the presence of 50 μg/mL MOG35–55. Supernatants were harvested, and cytokines were analyzed by ELISA as described previously (22).
Statistical Analysis.
Statistical analyses, as indicated in the figure legends, were performed using GraphPad Prism 4 software (GraphPad Software, Inc.).
Supplementary Material
Acknowledgments
We thank John Dodge and Dr. Mercedes Rincón of the University of Vermont Transgenic Core Facility for generation of vWFH1R Tg mice. We thank Drs. David Hill-Eubanks, Diane Jaworski, Laure Case, and Emma Wall for critical reading of the manuscript. This work was supported by National Institutes of Health Grants AI045666, AI041747, AI058052, NS061014, NS060901, NS036526, and NS069628 (to C.T.) and HL44455 and HL089243 (to M.T.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008816107/-/DCSupplemental.
References
- 1.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–124. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- 4.Linthicum DS, Frelinger JA. Acute autoimmune encephalomyelitis in mice. II. Susceptibility is controlled by the combination of H-2 and histamine sensitization genes. J Exp Med. 1982;156:31–40. doi: 10.1084/jem.156.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudweeks JD, et al. Locus controlling Bordetella pertussis-induced histamine sensitization (Bphs), an autoimmune disease-susceptibility gene, maps distal to T-cell receptor beta-chain gene on mouse chromosome 6. Proc Natl Acad Sci USA. 1993;90:3700–3704. doi: 10.1073/pnas.90.8.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orr EL, Stanley NC. Brain and spinal cord levels of histamine in Lewis rats with acute experimental autoimmune encephalomyelitis. J Neurochem. 1989;53:111–118. doi: 10.1111/j.1471-4159.1989.tb07301.x. [DOI] [PubMed] [Google Scholar]
- 7.Stanley NC, Jackson FL, Orr EL. Attenuation of experimental autoimmune encephalomyelitis by Compound 48/80 in Lewis rats. J Neuroimmunol. 1990;29:223–228. doi: 10.1016/0165-5728(90)90165-j. [DOI] [PubMed] [Google Scholar]
- 8.Ichigi J. Histamine release from mast cells of EAE rats by Gi protein-dependent and IgE-dependent pathways. J Mol Neurosci. 1999;13:93–99. doi: 10.1385/JMN:13:1-2:93. [DOI] [PubMed] [Google Scholar]
- 9.Christy AL, Brown MA. The multitasking mast cell: Positive and negative roles in the progression of autoimmunity. J Immunol. 2007;179:2673–2679. doi: 10.4049/jimmunol.179.5.2673. [DOI] [PubMed] [Google Scholar]
- 10.Neuman J. Ueber das Vorkommen der sogneannten “Mastzellen” bei pathologischen Veraenderungen der Gehirns. Virchows Archiv. 1890;122:378–381. [Google Scholar]
- 11.Toms R, Weiner HL, Johnson D. Identification of IgE-positive cells and mast cells in frozen sections of multiple sclerosis brains. J Neuroimmunol. 1990;30:169–177. doi: 10.1016/0165-5728(90)90101-r. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim MZ, Reder AT, Lawand R, Takash W, Sallouh-Khatib S. The mast cells of the multiple sclerosis brain. J Neuroimmunol. 1996;70:131–138. doi: 10.1016/s0165-5728(96)00102-6. [DOI] [PubMed] [Google Scholar]
- 13.Rozniecki JJ, Hauser SL, Stein M, Lincoln R, Theoharides TC. Elevated mast cell tryptase in cerebrospinal fluid of multiple sclerosis patients. Ann Neurol. 1995;37:63–66. doi: 10.1002/ana.410370112. [DOI] [PubMed] [Google Scholar]
- 14.Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000;191:813–822. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietsch GN, Hinrichs DJ. The role of mast cells in the elicitation of experimental allergic encephalomyelitis. J Immunol. 1989;142:1476–1481. [PubMed] [Google Scholar]
- 16.Akdis CA, Simons FE. Histamine receptors are hot in immunopharmacology. Eur J Pharmacol. 2006;533:69–76. doi: 10.1016/j.ejphar.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 17.Pedotti R, et al. Multiple elements of the allergic arm of the immune response modulate autoimmune demyelination. Proc Natl Acad Sci USA. 2003;100:1867–1872. doi: 10.1073/pnas.252777399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimitriadou V, Pang X, Theoharides TC. Hydroxyzine inhibits experimental allergic encephalomyelitis (EAE) and associated brain mast cell activation. Int J Immunopharmacol. 2000;22:673–684. doi: 10.1016/s0192-0561(00)00029-1. [DOI] [PubMed] [Google Scholar]
- 19.Alonso A, Jick SS, Hernán MA. Allergy, histamine 1 receptor blockers, and the risk of multiple sclerosis. Neurology. 2006;66:572–575. doi: 10.1212/01.wnl.0000198507.13597.45. [DOI] [PubMed] [Google Scholar]
- 20.Logothetis L, et al. A pilot, open label, clinical trial using hydroxyzine in multiple sclerosis. Int J Immunopathol Pharmacol. 2005;18:771–778. doi: 10.1177/039463200501800421. [DOI] [PubMed] [Google Scholar]
- 21.Ma RZ, et al. Identification of Bphs, an autoimmune disease locus, as histamine receptor H1. Science. 2002;297:620–623. doi: 10.1126/science.1072810. [DOI] [PubMed] [Google Scholar]
- 22.Noubade R, et al. Histamine receptor H1 is required for TCR-mediated p38 MAPK activation and optimal IFN-gamma production in mice. J Clin Invest. 2007;117:3507–3518. doi: 10.1172/JCI32792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccio L, et al. Molecular mechanisms involved in lymphocyte recruitment in inflamed brain microvessels: Critical roles for P-selectin glycoprotein ligand-1 and heterotrimeric G(i)-linked receptors. J Immunol. 2002;168:1940–1949. doi: 10.4049/jimmunol.168.4.1940. [DOI] [PubMed] [Google Scholar]
- 24.Guan J, Guillot PV, Aird WC. Characterization of the mouse von Willebrand factor promoter. Blood. 1999;94:3405–3412. [PubMed] [Google Scholar]
- 25.Munoz JJ, Arai H, Bergman RK, Sadowski PL. Biological activities of crystalline pertussigen from Bordetella pertussis. Infect Immun. 1981;33:820–826. doi: 10.1128/iai.33.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noubade R, et al. von-Willebrand factor influences blood brain barrier permeability and brain inflammation in experimental allergic encephalomyelitis. Am J Pathol. 2008;173:892–900. doi: 10.2353/ajpath.2008.080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao JF, et al. Analysis of the role of Bphs/Hrh1 in the genetic control of responsiveness to pertussis toxin. Infect Immun. 2003;71:1281–1287. doi: 10.1128/IAI.71.3.1281-1287.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill SJ, et al. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol Rev. 1997;49:253–278. [PubMed] [Google Scholar]
- 29.Ledoux J, et al. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA. 2008;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maruko T, et al. Involvement of the betagamma subunits of G proteins in the cAMP response induced by stimulation of the histamine H1 receptor. Naunyn Schmiedebergs Arch Pharmacol. 2005;372:153–159. doi: 10.1007/s00210-005-0001-x. [DOI] [PubMed] [Google Scholar]
- 31.Leurs R, et al. Guinea pig histamine H1 receptor. II. Stable expression in Chinese hamster ovary cells reveals the interaction with three major signal transduction pathways. J Neurochem. 1994;62:519–527. doi: 10.1046/j.1471-4159.1994.62020519.x. [DOI] [PubMed] [Google Scholar]
- 32.Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24:719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- 33.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 34.Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: Anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Bartholomäus I, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 36.Reboldi A, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 37.Steffen BJ, Butcher EC, Engelhardt B. Evidence for involvement of ICAM-1 and VCAM-1 in lymphocyte interaction with endothelium in experimental autoimmune encephalomyelitis in the central nervous system in the SJL/J mouse. Am J Pathol. 1994;145:189–201. [PMC free article] [PubMed] [Google Scholar]
- 38.Linthicum DS, Munoz JJ, Blaskett A. Acute experimental autoimmune encephalomyelitis in mice. I. Adjuvant action of Bordetella pertussis is due to vasoactive amine sensitization and increased vascular permeability of the central nervous system. Cell Immunol. 1982;73:299–310. doi: 10.1016/0008-8749(82)90457-9. [DOI] [PubMed] [Google Scholar]
- 39.Choi J, Enis DR, Koh KP, Shiao SL, Pober JS. T lymphocyte-endothelial cell interactions. Annu Rev Immunol. 2004;22:683–709. doi: 10.1146/annurev.immunol.22.012703.104639. [DOI] [PubMed] [Google Scholar]
- 40.Vanhoutte PM, Mombouli JV. Vascular endothelium: Vasoactive mediators. Prog Cardiovasc Dis. 1996;39:229–238. doi: 10.1016/s0033-0620(96)80003-x. [DOI] [PubMed] [Google Scholar]
- 41.Jin H, et al. Histamine-induced vasodilation and vasoconstriction in the mesenteric resistance artery of the rat. Eur J Pharmacol. 2006;529:136–144. doi: 10.1016/j.ejphar.2005.10.060. [DOI] [PubMed] [Google Scholar]
- 42.Rondaij MG, Bierings R, Kragt A, van Mourik JA, Voorberg J. Dynamics and plasticity of Weibel-Palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1002–1007. doi: 10.1161/01.ATV.0000209501.56852.6c. [DOI] [PubMed] [Google Scholar]
- 43.Hol J, Wilhelmsen L, Haraldsen G. The murine IL-8 homologues KC, MIP-2, and LIX are found in endothelial cytoplasmic granules but not in Weibel-Palade bodies. J Leukoc Biol. 2010;87:501–508. doi: 10.1189/jlb.0809532. [DOI] [PubMed] [Google Scholar]
- 44.Hattori R, Hamilton KK, Fugate RD, McEver RP, Sims PJ. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem. 1989;264:7768–7771. [PubMed] [Google Scholar]
- 45.Yong T, Zheng MQ, Linthicum DS. Local histamine release increases leukocyte rolling in the cerebral microcirculation of the mouse. Brain Inj. 1997;11:765–774. doi: 10.1080/026990597123142. [DOI] [PubMed] [Google Scholar]
- 46.Kerfoot SM, Kubes P. Overlapping roles of P-selectin and alpha 4 integrin to recruit leukocytes to the central nervous system in experimental autoimmune encephalomyelitis. J Immunol. 2002;169:1000–1006. doi: 10.4049/jimmunol.169.2.1000. [DOI] [PubMed] [Google Scholar]
- 47.Comi G. Treatment of multiple sclerosis: Role of natalizumab. Neurol Sci. 2009;30(Suppl 2):S155–S158. doi: 10.1007/s10072-009-0147-2. [DOI] [PubMed] [Google Scholar]
- 48.Inoue I, et al. Impaired locomotor activity and exploratory behavior in mice lacking histamine H1 receptors. Proc Natl Acad Sci USA. 1996;93:13316–13320. doi: 10.1073/pnas.93.23.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu C, et al. Pertussis toxin induces angiogenesis in brain microvascular endothelial cells. J Neurosci Res. 2008;86:2624–2640. doi: 10.1002/jnr.21716. [DOI] [PubMed] [Google Scholar]
- 50.Teuscher C, et al. Evidence that the Y chromosome influences autoimmune disease in male and female mice. Proc Natl Acad Sci USA. 2006;103:8024–8029. doi: 10.1073/pnas.0600536103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.