Abstract
Larger brains have an increasingly folded cerebral cortex whose white matter scales up faster than the gray matter. Here we analyze the cellular composition of the subcortical white matter in 11 primate species, including humans, and one Scandentia, and show that the mass of the white matter scales linearly across species with its number of nonneuronal cells, which is expected to be proportional to the total length of myelinated axons in the white matter. This result implies that the average axonal cross-section area in the white matter, a, does not scale significantly with the number of neurons in the gray matter, N. The surface area of the white matter increases with N0.87, not N1.0. Because this surface can be defined as the product of N, a, and the fraction n of cortical neurons connected through the white matter, we deduce that connectivity decreases in larger cerebral cortices as a slowly diminishing fraction of neurons, which varies with N−0.16, sends myelinated axons into the white matter. Decreased connectivity is compatible with previous suggestions that neurons in the cerebral cortex are connected as a small-world network and should slow down the increase in global conduction delay in cortices with larger numbers of neurons. Further, a simple model shows that connectivity and cortical folding are directly related across species. We offer a white matter-based mechanism to account for increased cortical folding across species, which we propose to be driven by connectivity-related tension in the white matter, pulling down on the gray matter.
Keywords: brain size, number of neurons, small-world networks, evolution
Larger mammalian brains have relatively larger cerebral cortices that become increasingly folded, such that the overall cortical surface increases more quickly than the exposed cortical surface, presumably as a result of fast expansion of the gray matter (1). The fastest expanding structure, however, is not the cortical gray matter (GM), but the subcortical white matter (WM), which contains the axons that interconnect nearby as well as distant areas in the GM and their subcortical targets. Across mammalian species, the WM comprises as little as 5% of the cerebral cortex in the smallest insectivores, but >40% of the cerebral cortex of dolphins, whales, elephants, and humans (2). The faster increase in WM volume VW than in GM volume VG is to be expected from their spatial characteristics, one as the core, and the other as the shell, of the cortex; if the WM were a perfect sphere surrounded by a spherical shell of GM of constant thickness, VW should increase with VG3/2 or VG1.5. However, WM has been found to increase more slowly than expected across mammalian species, with VG1.22 (3), VG1.24 (4), VG1.33 (2), or VG1.23 (5), which raises the possibility that connectivity through the WM does not increase proportionally with increases in GM.
It has been argued that an exponent of 1.23 would follow naturally once the exponent of 1.33, or 4/3, consequence of the local uniformity of the cortex (in number of neurons beneath a unit surface area), and of the requirement for compact arrangement of long axonal fibers, is corrected to account for variations in cortical thickness (5). This model considered explicitly that a constant fraction of cortical neurons sends axons into the WM, that is, that connectivity does not scale with brain size. Alternatively, another model, which also considered the number of neurons beneath a cortical surface unit to be constant, and additionally assumed that synaptic density is invariant, estimated that the connectivity between neurons and cortical areas should decrease with increasing brain size (6). Such models with opposing views on the scaling of connectivity considered the distribution of neurons in the GM to be uniform out of necessity, as a means to estimate scaling of the total number of neurons in the GM and hence scaling of the number of fibers in the WM.
Assuming neuronal uniformity in the cortex, however, is no longer necessary or appropriate to model cortical connectivity. By means of a method that we developed, the isotropic fractionator (7), we have recently been able to determine the number of neurons in the cortical gray matter of a number of primate species (8), including humans (9). A comparative analysis of the ratio between the total number of neurons in the GM and the total GM surface showed that the number of neurons beneath a unit surface area varies by as much as 3×, depending on neuronal density in a manner that does not correlate with brain size (because, in primates, neuronal density does not vary significantly with cortical size), and therefore cannot be considered uniform (10).
Here we examine how cortical WM size and connectivity scale with the number of neurons in the cortex without making assumptions about its cellular composition or fraction of neurons with axons in the WM, by determining the cellular composition of the subcortical white matter of various primate brains and investigating how it scales with the number of neurons in the gray matter (10). Because the number of myelinating oligodendrocytes is considered to increase linearly with axon length (11), and the density of nonneuronal cells has been shown not to vary with brain size (8), we use the number of nonneuronal cells that compose the subcortical white matter to determine how total axon length in the white matter scales with the number of cortical neurons, to infer whether and how average axonal diameter varies with cortical size, to deduce how the fraction of cortical neurons with axons in the WM scales with GM size and number of neurons, and to examine how changes in connectivity relate to changes in cortical folding.
Results
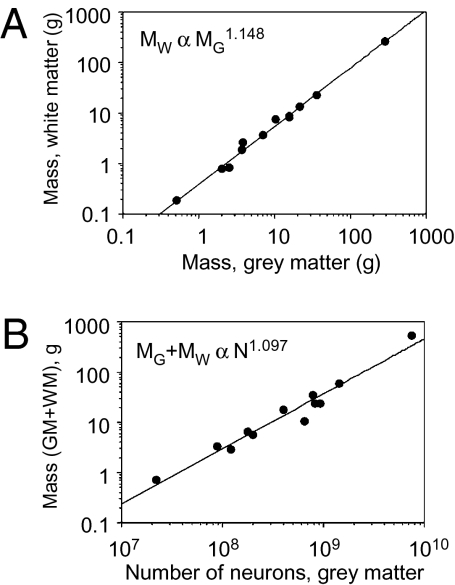
All analyses reported refer to the 11 species in the dataset and the closely related Tupaia, because its exclusion from the dataset had only negligible effects on the results (Table S1). Because scaling exponents were little affected by accounting for phylogenetic relatedness in the dataset (Table S1), the exponents reported below refer to the uncorrected relationships. Across species, we find that WM mass scales with GM mass raised to an exponent of 1.148 ± 0.037 including humans (P < 0.0001; from here on  ± σa signifies expected exponent
± σa signifies expected exponent  with SD σa; Fig. 1A) or 1.147 ± 0.055 (without humans; P < 0.0001), confirming that WM size scales faster than GM size in the larger primate brains in our sample. Accordingly, the relative size of the WM increases with cortical size from 27.0% of cortical mass in the tree shrew to 47.8% in humans (Table S2). WM volume VW also scales faster than GM volume VG, with VG1.184±0.063 (P < 0.0001; does not include humans and macaque monkeys, for which VG was not available). Because mass and volume for each structure were found to be linearly related (GM, r2 = 0.982, P < 0.001; WM, r2 = 0.896, P < 0.0001), and measurements of mass, but not volume, were available for all species, heretofore we use solely GM and WM mass in the analysis and interchangeably with VW and VG where called for in the models.
with SD σa; Fig. 1A) or 1.147 ± 0.055 (without humans; P < 0.0001), confirming that WM size scales faster than GM size in the larger primate brains in our sample. Accordingly, the relative size of the WM increases with cortical size from 27.0% of cortical mass in the tree shrew to 47.8% in humans (Table S2). WM volume VW also scales faster than GM volume VG, with VG1.184±0.063 (P < 0.0001; does not include humans and macaque monkeys, for which VG was not available). Because mass and volume for each structure were found to be linearly related (GM, r2 = 0.982, P < 0.001; WM, r2 = 0.896, P < 0.0001), and measurements of mass, but not volume, were available for all species, heretofore we use solely GM and WM mass in the analysis and interchangeably with VW and VG where called for in the models.
Fig. 1.
White matter scales faster than gray matter. (A) White matter mass (MW) increases faster than gray matter mass (MG), with MG1.148 (P < 0.0001). (B) Total cortical mass (MW + MG) increases with the number of GM neurons N1.097 (P < 0.0001). Each point indicates the average for one species. Power functions include the human datapoint.
As reported previously (10), cortical GM increases linearly in mass as a function of its number of neurons, N. This relationship is linear even when humans are included in the comparison (the exponent is 1.043 ± 0.073, P < 0.0001 and r2 = 0.991 including humans, and 0.956 ± 0.084, P < 0.0001 and r2 = 0.896 excluding humans). Whole cortical mass (GM + WM) increases as a power of the number of neurons in the GM, with exponents 1.097 ± 0.081 if we include humans (P < 0.0001; Fig. 1B), or 1.000 ± 0.091 if humans are excluded (P < 0.0001).
White Matter Scaling.
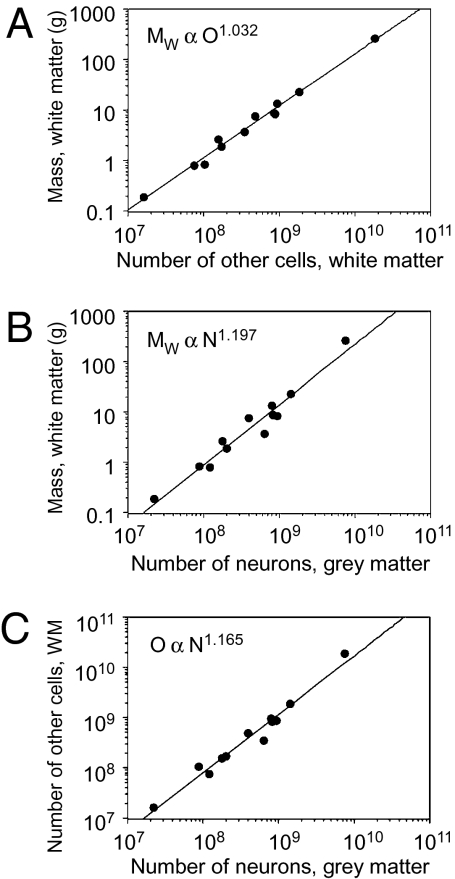
White matter mass, MW, increases across primate brains as a linear function of its number of nonneuronal cells (“other cells,” O) whether humans are included (exponent, 1.032 ± 0.040; P < 0.0001; Fig. 2A) or excluded from the comparison (exponent, 1.019 ± 0.057, P < 0.0001; linear regression, r2 = 1.000 and 0.970, respectively, P < 0.0001). MW increases as a power function of the number of neurons in the gray matter N raised to an exponent of 1.197 ± 0.091 if humans are included (P < 0.0001; Fig. 2B) or 1.096 ± 0.111 if humans are excluded from the comparison (P < 0.0001).
Fig. 2.
White matter scaling. (A) White matter mass (MW) increases linearly with the number of nonneuronal (other) cells in the WM (O), with O1.032 (P < 0.0001). (B) White matter mass (MW) increases faster than the number of neurons N in the GM, with N1.197 (P < 0.0001). (C) The number of nonneuronal cells (O) in the WM increases faster than the number of neurons N in the GM, with N1.165 (P < 0.0001). Each point indicates the average for one species. Power functions include the human datapoint.
The number of nonneuronal cells in the subcortical WM increases as a power law of N with exponent 1.165 ± 0.070 (including humans, P < 0.0001; Fig. 2C) or 1.081 ± 0.074 if humans are excluded from the comparison (P < 0.0001). Assuming the number of nonneuronal cells in the WM, O (presumably mainly oligodendrocytes), varies linearly with total myelinated axonal length (L) in the WM (11), we can therefore infer that, for primates including humans, L ∝ O ∝ N1.165±0.040.
Scaling of Average Axon Cross-Sectional Area.
The average cross-sectional area a of myelinated axons in the WM can be estimated by using the same assumption about the proportionality between L and O. Considering that VW ∝ n.N.a.l (where n is the fraction of gray matter neurons connected through the GM and l is the average axonal length in the WM) and L = n.N.l, it follows that a ∝ VW/L ∝ VW/O. Using the observed relations for VW and O as (very similar) power laws of N shown above, we obtain a ∝ Nα, where α = 0.032 ± 0.049, i.e., close to, and statistically indistinguishable from, zero. In other words, because VW is linearly related to O, it follows that VW/O is constant, that is, that a is invariant for primate brains. The same relationship holds if we consider O to be proportional to total axonal surface, rather than length (SI Methods). We can infer, therefore, that the average myelinated cross-sectional area in the white matter does not vary significantly with increasing number of neurons in the gray matter.
Scaling of Gray Matter Connectivity Through the White Matter.
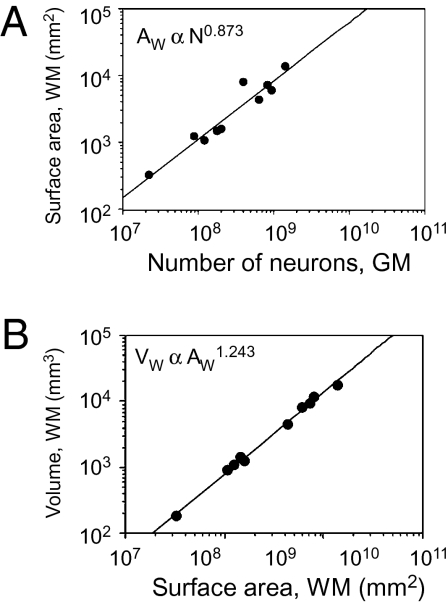
The surface area of the GM/WM interface, AW, can be defined as AW ∝ n.N.a. Given that a ∝ Nα, then AW ∝ N1+c+α. We find that AW ∝ N0.873±0.102 (P < 0.0001; Fig. 3A), which implies that n is not invariant with N, but rather changes with N−0.159±0.113. This result suggests that gray matter connectivity through the white matter, n, decreases slightly with increasing number of neurons in the GM, as n ∝ Nc, such that c = −0.159 ± 0.113.
Fig. 3.
Scaling of the white matter surface area. (A) White matter surface area (AW) increases with the number of neurons in the GM with N0.873 (P < 0.0001). Each point indicates the average for one species. (B) White matter volume (VW) increases with WM area AW more slowly than expected for an isometric surface, with AW1.243 (P < 0.0001) instead of AW1.5, as if growing under tension. Power functions do not include human or rhesus datapoints, for which measurements of AW are lacking.
Scaling of WM Volume, Surface, and Radius.
Visual inspection of brain sections of different species shows that the surface of the WM is convoluted and does not scale isometrically with cortical size. Were this the case, the volume of the WM, VW, would scale with AW3/2. In contrast, we find that VW ∝ AW1.243±0.036 (P < 0.0001; Fig. 3B), with an exponent significantly <1.5, which means that the volume of the WM increases significantly more slowly than expected, as if growing under tension. This scaling relationship implies that the WM surface AW grows faster than its volume—that is, it becomes more convoluted as the cortex grows.
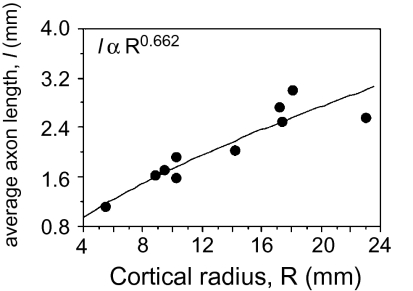
Under very general conditions (SI Methods), the average length of myelinated axons in the WM is given by l = 2VW/(p.AW), where p is approximately the average of the cosine of the incidence angle of the axons into the GM–WM interface. An economically built brain (i.e., one folded only as much as it needs to be able to accommodate all its WM axons as tightly packed as possible) would have p ≈ 1, so we assume that p is close to unity and constant across species. In any case, because p ≤ 1, 2.VW/AW can always be taken as a lower bound for the value of l. Models of cortical scaling often assume that l varies linearly as a function of the cortex radius R [a length scale defined as (3V/4π)1/3]. Instead, we find that l varies with Rλ, with λ = 0.662 ± 0.186 (P < 0.0001; Fig. 4), with an exponent well below linearity, and therefore with N0.242±0.085. This result strongly suggests that, contrary to expectations, the average axonal length in the WM grows more slowly than the radius of the cerebral cortex, again as expected if its axons were under tension.
Fig. 4.
Scaling of average axonal length, l. The lower bound for average axonal length l, calculated as 2.VW/AW, increases with approximate cortical radius R0.662 [where R is defined as (3V/4π)1/3, for one hemisphere only; P < 0.0001]. Each point indicates the average for one species. Power function does not include human or rhesus datapoints, for which measurements of AW are lacking.
Cortical Folding.
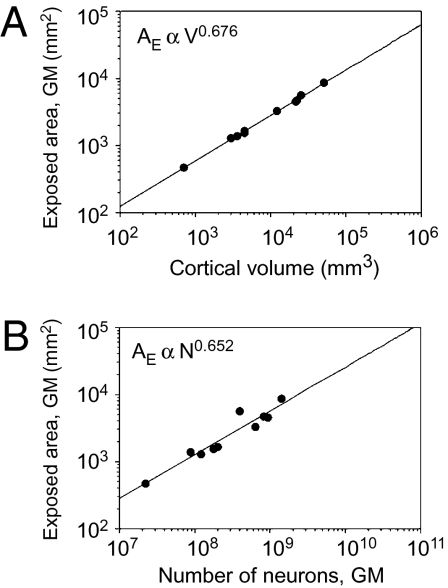
The degree to which the WM becomes convoluted as the GM gains neurons can be expressed by its folding index FW, which we define as the ratio AW/AE (where AE is the exposed surface of the cerebral cortex). As expected from the approximately isometric external shape of the brain, AE is found to vary in our sample with V0.676±0.010 (P < 0.0001; Fig. 5A) and N0.652±0.079 (P < 0.0001; Fig. 5B). Given these similar exponents and considering that V varies approximately linearly with N (V ∝ N1.097±0.081), AE can be considered to scale as N2/3 and R to scale as N1/3. FW, defined as AW/AE, scales approximately as n.a.N/N2/3∝ n.a.R. Because a does not vary systematically with N, FW thus increases linearly with the product of cortical radius and connectivity through the WM. The larger the number of cortical neurons interconnected through the WM is, the more the WM surface is folded.
Fig. 5.
Scaling of the exposed cortical surface area. (A) Exposed GM surface area (AE) increases with total cerebral cortical volume V as expected for an isometric surface, with V0.676 (P < 0.0001). (B) Exposed GM surface area (AE) increases with the number of neurons in the GM with N0.652 (P < 0.0001). Each point indicates the average for one species. Power functions do not include human or rhesus datapoints, for which measurements of AW are lacking.
Applying the relationship FW ∝ N1/3+c+α, we find that in hypothetical cortices in which FW did not change with N (that is, in cortices that gained neurons in the GM without becoming more convoluted), cortical connectivity n would decrease steeply with c = −1/3. In contrast, in hypothetical primate brains in which cortical connectivity did not change with N (that is, with c = 0), FW should vary with N1/3. In other words, increasing the WM without increasing the folding of its surface can occur only in the face of a steep decline in cortical connectivity through the WM; on the other hand, increasing the WM without decreasing cortical connectivity through it, or with an actual increase in cortical connectivity, would be accompanied by in a sharp increase in WM folding. Therefore, the higher degree of folding of the WM in larger cortices can be seen as a feature that accompanies either maintenance of connectivity or only a slight decrease in it.
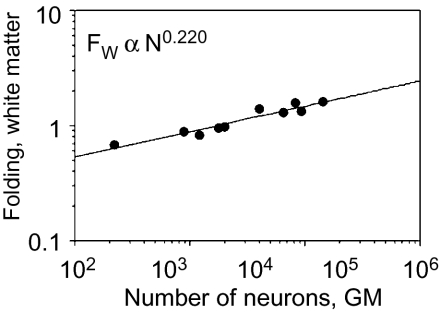
In our primate sample, we find that FW ∝ N0.220±0.026 (P < 0.0001; Fig. 6), with an exponent that is both significantly >0 and <1/3. Given the relationship FW ∝ N1/3+c+α, this exponent suggests that cortical connectivity decreases in larger primate brains with c = −0.145, consistent with our previous estimates of c = −0.159 ± 0.113.
Fig. 6.
Folding of the white matter as a function of cortical neurons. Folding of the WM surface (FW) increases with the number of cortical neurons N0.220 (P < 0.0001). Each point indicates the average for one species. Power functions do not include human or rhesus datapoints, for which measurements of AW are lacking.
Considering that the WM is a structure under tension because of the axons that compose it (12), and that this tension might force the folding of the WM surface AW in a way that increases together with the number of cortical neurons interconnected through the WM, the folding of AW might consequently force the folding of the external surface of the GM. In this manner, the folding index of the external surface of the GM, FG, would be expected to follow as a consequence of the folding of AW. Using our model, it can be shown that FW and FG should be related by the formula FW = FG + (T/R)FG[(T/R)FG − 2] (SI Methods and Fig. S1). We find that the values of FW obtained by applying this formula are very close to the measured values of FW: FW = 1.000 × expected FW1.007 (P < 0.0001), indicating that the formula that relates FW to FG is accurate despite being based on a very simple model. This coordinated increase in FG and FW is consistent with our proposition that the folding of the external surface of the cerebral cortex is a consequence of the folding of the WM surface, which is in turn proportional to GM connectivity through the WM.
Discussion
Making no assumptions about cortical uniformity, neuronal composition of the cerebral cortex, the fraction of GM neurons connected through the WM, or how average axonal length in the WM scales with brain size, and assuming only that the number of nonneuronal cells in the WM is proportional to its total length of myelinated axons, here we show that, for primate brains, average axon length in WM increases more slowly than cortical radius, and total axonal length in WM increases more slowly than expected if a constant fraction of neurons had axons in the WM. This result implies that GM connectivity (n, or the fraction of GM neurons that sends an axon through the WM) decreases as the GM gains neurons, in a manner that we estimate as n ∝ N−0.159. Supposing, for the sake of argument, that 50% of all cortical neurons were connected through the white matter in the marmoset, a decrease in n as N−0.159 would imply that in a monkey-sized cortex with 10 times more neurons than the marmoset, WM connectivity would fall to 10−0.159 = 0.59 × 50% = 30% of all neurons; and a human-sized cortex with about 100 times more neurons than a marmoset would have only 18% of its neurons interconnected through the white matter.
Note that decreased connectivity occurs in the face of an increased total number of axons in the WM, which is proportional to n.N, or N1+c. In the exercise scenario above, the total number of axons in the WM would increase from ∼122 million in the marmoset, to 510 million in the macaque, to 2.9 billion in the human cortex. Larger primate cortices, therefore, increase in size proportionally to N1 neurons in the GM, of which a number proportional to N0.841 send axons into the WM. Given that the average axonal length in the WM increases with N0.242, and given our inference that the average axonal diameter does not change appreciably with N, WM volume (being proportional to N.n.a.l) is expected to increase with N1.N−0.159.N0.032.N0.242 = N1.114, which is close to the exponent obtained empirically.
Decreasing Connectivity in Small-World Networks.
The decrease in connectivity in larger cortices is compatible with the decrease predicted by previous models of cortical scaling (6, 13–15) and calls into question popular scaling models that assume constant cortical connectivity (5, 16, 17). Such a decrease in connectivity in larger cerebral cortices is also compatible with the view that the cerebral cortex displays among its neurons the connectivity properties of a small-world network (18, 19), even though the cerebral cortex may be densely connected at the level of functional areas (20). A small-world network is a network in which distance between nodes (neurons) is small and grows with the addition of mostly local connectivity (through horizontal connections in the GM) and only a relatively small number of long-range connections (21) (through long fibers in the WM, which still guarantees fast global communication) (6, 22–25). A decrease in neuronal connectivity is, indeed, an expected feature of growing small-world networks (26).
Constant Axon Diameter.
From the relationship between n, N, and A, we deduce that average axon cross-sectional area a in the WM does not scale with N or brain size and is therefore approximately constant across the species in our sample. This result is compatible with the finding that the distribution of fiber diameters in the splenium of the corpus callosum is conserved across species of different mammalian orders (27). In that study, Olivares et al. found that, across species, the average diameter of the widest callosal axons (of up to 0.8 μm in diameter, which are a minority of callosal fibers) increases with brain size, albeit slowly, with brain size raised to an exponent of 0.2. In a more recent study (17), Wang et al. described much larger axons (up to >10 μm in diameter) in two other regions of the callosum, but the distribution of the widest axons across a variety of species with brains of the size of the macaque and larger did not seem to accompany brain size (their figure 2d), despite the authors’ claim that the diameters of the widest callosal axons increase (across all species, including least shrew, mouse, and rat) with brain diameter.
An increase in the axon diameter of a small subpopulation of WM axons, such as callosal axons, has been considered as a requirement to allow synchronous activity at the level of the whole neocortex (28). The alternative of maintaining constant conduction delays in larger brains by widening all axons would result in an unsustainable increase in the volume of the WM (29) and is clearly not the case in primates. Our finding of an invariant average axonal diameter in the WM as a whole implies that, if an increase in axonal diameter does occur, it must apply to only very few WM axons, such that the average axonal diameter is not altered even in large brains such as ours. Alternatively, if one considers the evidence that the corpus callosum increases in volume more slowly than intrahemispheric WM (30), our finding of no scaling of average axonal diameter combined with the decrease in connectivity points to the possibility that it is mostly the longest-range (callosal) connectivity that decreases in larger cortices (which would balance out the increase in widest axon diameter of a few fibers).
White Matter Scaling.
Here we use the described linear relationship between axonal length and numbers of myelinating oligodendrocytes (11, 31) to infer, from the finding of a linear scaling between WM volume and number of nonneuronal cells in the WM, that total axonal length in the WM also increases linearly with VW. We presume, like scaling models usually do (5, 32), that, at least in our sample of primate brains, the majority of axons in the WM are myelinated (∼70% of all axons) (27), that the relative number of myelinated fibers does not vary significantly across species of different brain sizes (27), and that most of the WM volume amounts to myelinated axons (17). In this scenario, our findings regarding the scaling of the cellular composition and total myelinated axonal length of the WM can be considered representative of the whole WM, even though they do not take into consideration the volume of WM that is occupied by unmyelinated fibers.
Implications for Scaling of Conduction Velocity.
Average conduction delay T in the brain scales with average length of global connections l and average axon diameter D such that T ∝ l/D. A previous model of scaling of conduction times, which assumed a fixed number of connections per neuron and therefore presumed that volume of the whole brain (or WM) is proportional to N.D2.l, estimated that T increases with N0.5 (32). Considering our finding that global connectivity through the WM decreases as a function of N, our updated estimate is that global T for the whole cerebral cortex actually increases more slowly, with N0.242±0.085. Decreased connectivity in larger cortices, therefore, effectively decreases average conduction delays along global connections.
Cortical Folding and Connectivity.
The increasing folding of the cerebral cortex in larger brains has been attributed to various factors such as space limitations inside the skull (1), the growth of the cortical sheet relative to its subcortical core, and the sheer expansion of the cortical sheet (33). However, partial removal of the skull during development does not have a dramatic effect on the fissure pattern, and lesion experiments suggest that cortical folding is not primarily dependent on a disproportionate growth between cortical and subcortical structures (33). Thus, the primary source of fissure formation must be sought in factors within the cortex itself.
One probable such factor to promote cortical folding is the mechanical tension along the axons that course in the WM, such that more densely interconnected cortical areas would tend to buckle together, forming a gyrus between them (12). The qualitative, tension-based theory of morphogenesis that takes into consideration the patterns of connectivity between cortical areas, proposed by David Van Essen, accounts for the consistent formation of convolutions in a species-specific pattern (12), but does not explain the increased cortical folding that accompanies increasing cortical size across species.
We propose an extension of Van Essen's tension-based theory of cortical folding that takes into consideration how GM connectivity through the WM changes across primate species to explain how increased folding accompanies increasing cortical size across primate species. In our view, rather than driving the folding of the WM surface, the folding of the external surface of the GM results from folding of the WM surface, which, in turn, results from increased tension within the WM due to increased numbers of axons composing the WM (although other contributing factors such as cortical growth and molecular factors cannot yet be ruled out). According to our model, cortical folding begins in the WM as a consequence of the elastic tension of its axons and proceeds as a function of both the number of axons that it contains and their length: the larger the number of axons (which varies as a function of N and c) is, the larger the tension in the WM, the more folded the WM surface, and, therefore, the more folded the GM surface. Given the different scaling relationships observed between the number of cortical neurons and cortical size across mammalian orders (8, 34, 35), such a connectivity-based model of cortical folding might account for the differences in the relationship between cortical folding and brain size across orders (36).
Methods
Animals.
We analyzed the cellular composition of the WM, the WM surface area, and its folding in the same cortical hemispheres for which we had determined the cellular composition of the GM before (10), namely tree shrews (Tupaia belangeri, n = 2), galagos (Otolemur garnetti, n = 2), marmosets (Callithrix jacchus, n = 3), owl monkeys (Aotus trivirgatus, n = 3), squirrel monkeys (Saimiri sciureus, n = 3), capuchin monkeys (Cebus apella, n = 2), baboons (Papio sp., n = 2), one Goeldi's marmoset (Callimico goeldii), one long-tailed macaque monkey (Macaca fascicularis), and one bonnet macaque monkey (Macaca radiata). Additionally, one rhesus monkey (Macaca mulatta) cerebral cortical hemisphere was also analyzed (8), although we could not subject it to topological or volumetric analyses because we received it already cut into pieces. All animals were young adults at the time of the experiments (10). Data for the human brain were obtained from ref. 9 (no surface or volume measurements available). All GM values are from ref. 10.
Dissection.
All animals were killed by a lethal injection of sodium pentobarbital, weighed, and perfused transcardially with 0.9% PBS followed by 4% phosphate-buffered paraformaldehyde. Brains were removed from the skull, weighed, and postfixed for 2 wk to 18 mo by immersion in 4% phosphate-buffered paraformaldehyde. Only one cerebral hemisphere of each animal (13 right hemispheres, 7 left hemispheres) was available for analysis.
Cerebral Cortical Reconstruction.
Reconstruction of the WM volume and surface areas was performed in the same 2-mm-thick serial coronal brain sections for which GM volume and surface areas were determined before (10). All sections had their anterior surface digitalized in a 1,200-dpi desktop scanner. Subcortical white matter was defined as the entire WM contained between the inferior surface of the GM and the external surface of the striatum. Surface area AW of the WM was estimated as 2 × the sum of all perimeters of the WM in the sections, and WM volume (VW) was estimated as the sum of the coronal WM area of all sections multiplied by 2 mm.
Isotropic Fractionator.
Once all sections were scanned, the white matter in the coronal sections was dissected away from the gray matter under a stereoscope and weighed to determine WM mass (MW). Total numbers of neurons in the GM were estimated separately in the GM and WM and considered jointly as the total number of cortical neurons (N) to avoid loss of gray matter neurons due to imprecision in the dissection (10). Total numbers of nonneuronal cells in the white matter of each cortical hemisphere were estimated as described previously using the isotropic fractionator method (7).
Data Analysis.
Statistical analyses and regressions were performed in Statview (SAS), using the average values obtained from the individuals of each species. Least-squares regressions of the data to linear and power functions were calculated and reported. The uncertainties in the model parameters were propagated in the usual way from the variance associated with the power law best fit for the experimental variables, assuming uncorrelated residues and the existence of power law relations between the various quantities.
Phylogenetic Analysis.
Phylogenetic independent contrasts were calculated to examine the scaling of the primate brain structures as a function of their cellular composition in the expanded dataset of 12 primate species, including humans, while controlling for effects of phylogenetic relatedness in the dataset (37). Standardized independent contrasts were calculated using the PDAP:PDTREE module of Mesquite software version 2.7 (38). Contrasts were calculated from both log-transformed and raw data, to evaluate how well they are described by power and linear functions, respectively. Phylogenetic relationships, shown in Fig. S2, are based on ref. 39. Branch lengths were transformed according to the method of Pagel (40), which assigns all branch lengths to 1 with the constraint that tips are contemporaneous. The reported values for the linear regressions of independent contrasts on log-transformed or raw data are reduced major axis (RMA) slope, r2, and P value.
Supplementary Material
Acknowledgments
We thank Christine Collins for collecting most of the brains used and for continued collaboration; Alexandre Valotta, Esper Cavalheiro (Universidade Federal de São Paulo, São Paulo, Brazil), and Laila Torres (Instituto Evandro Chagas, Belém, Brazil) for donating the hemispheres of one Cebus and one Callimico; and Drs. Terrence Sejnowski, Henry Kennedy, Jan Karbowski, Dmitri Chklovskii, and Georg Striedter for their comments on the manuscript. This work was supported by Fundação de Amparo á Pesquisa do Estado do Rio de Janeiro (FAPERJ)-Jovem Cientista and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)–Edital Universal (S.H.-H.), FAPERJ (B.M.), and National Eye Institute Grant 002686 (to J.H.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012590107/-/DCSupplemental.
References
- 1.Welker W. In: Cerebral Cortex. Comparative Structure and Evolution of Cerebral Cortex. Jones EG, Peters A, editors. NY: Plenum Press; 1990. Vol 8B, Part II, pp 3–10. [Google Scholar]
- 2.Hofman MA. Size and shape of the cerebral cortex in mammals. I. The cortical surface. Brain Behav Evol. 1985;27:28–40. doi: 10.1159/000118718. [DOI] [PubMed] [Google Scholar]
- 3.Schlenska G. Measurements of volume and surface area of the brain of various mammals in comparison to a model (Translated from German) J Hirnforsch. 1974;15:401–408. [Google Scholar]
- 4.Frahm HD, Stephan H, Stephan M. Comparison of brain structure volumes in Insectivora and Primates. I. Neocortex. J Hirnforsch. 1982;23:375–389. [PubMed] [Google Scholar]
- 5.Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proc Natl Acad Sci USA. 2000;97:5621–5626. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karbowski J. How does connectivity between cortical areas depend on brain size? Implications for efficient computation. J Comput Neurosci. 2003;15:347–356. doi: 10.1023/a:1027467911225. [DOI] [PubMed] [Google Scholar]
- 7.Herculano-Houzel S, Lent R. Isotropic fractionator: A simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci. 2005;25:2518–2521. doi: 10.1523/JNEUROSCI.4526-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herculano-Houzel S, Collins CE, Wong P, Kaas JH. Cellular scaling rules for primate brains. Proc Natl Acad Sci USA. 2007;104:3562–3567. doi: 10.1073/pnas.0611396104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azevedo FAC, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 10.Herculano-Houzel S, Collins CE, Wong P, Kaas JH, Lent R. The basic nonuniformity of the cerebral cortex. Proc Natl Acad Sci USA. 2008;105:12593–12598. doi: 10.1073/pnas.0805417105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barres BA, Raff MC. Axonal control of oligodendrocyte development. J Cell Biol. 1999;147:1123–1128. doi: 10.1083/jcb.147.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- 13.Stevens CF. How cortical interconnectedness varies with network size. Neural Comput. 1989;1:473–479. [Google Scholar]
- 14.Ringo JL. Neuronal interconnection as a function of brain size. Brain Behav Evol. 1991;38:1–6. doi: 10.1159/000114375. [DOI] [PubMed] [Google Scholar]
- 15.Karbowski J. Optimal wiring principle and plateaus in the degree of separation for cortical neurons. Phys Rev Lett. 2001;86:3674–3677. doi: 10.1103/PhysRevLett.86.3674. [DOI] [PubMed] [Google Scholar]
- 16.Prothero J. Scaling of cortical neuron density and white matter volume in mammals. J Hirnforsch. 1997;38:513–524. [PubMed] [Google Scholar]
- 17.Wang SSH, et al. Functional trade-offs in white matter axonal scaling. J Neurosci. 2008;28:4047–4056. doi: 10.1523/JNEUROSCI.5559-05.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grinstein G, Linsker R. Synchronous neural activity in scale-free network models versus random network models. Proc Natl Acad Sci USA. 2005;102:9948–9953. doi: 10.1073/pnas.0504127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassett DS, Meyer-Lindenberg A, Achard S, Duke T, Bullmore E. Adaptive reconfiguration of fractal small-world human brain functional networks. Proc Natl Acad Sci USA. 2006;103:19518–19523. doi: 10.1073/pnas.0606005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markov NT, et al. The tribal networks of the cerebral cortex. In: Chalupa LM, Berardi N, Caleo M, Galli-Resta L, Pizzorusso T, editors. Cerebral Plasticity. Cambirdge, MA: MIT Press; 2010. [Google Scholar]
- 21.Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- 22.Changizi MA. Principles underlying mammalian neocortical scaling. Biol Cybern. 2001;84:207–215. doi: 10.1007/s004220000205. [DOI] [PubMed] [Google Scholar]
- 23.Harrison KH, Hof PR, Wang SS. Scaling laws in the mammalian neocortex: Does form provide clues to function? J Neurocytol. 2002;31:289–298. doi: 10.1023/a:1024178127195. [DOI] [PubMed] [Google Scholar]
- 24.Sporns O, Kötter R. Motifs in brain networks. PLoS Biol. 2004;2:e369. doi: 10.1371/journal.pbio.0020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sporns O, Zwi JD. The small world of the cerebral cortex. Neuroinformatics. 2004;2:145–162. doi: 10.1385/NI:2:2:145. [DOI] [PubMed] [Google Scholar]
- 26.Argollo de Menezes M, Moukarzel C, Penna TJP. First-order transition in small-world networks. Europhys Lett. 2000;50:574–579. [Google Scholar]
- 27.Olivares R, Montiel J, Aboitiz F. Species differences and similarities in the fine structure of the mammalian corpus callosum. Brain Behav Evol. 2001;57:98–105. doi: 10.1159/000047229. [DOI] [PubMed] [Google Scholar]
- 28.Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: Phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 29.Ringo JL, Doty RW, Demeter S, Simard PY. Time is of the essence: A conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb Cortex. 1994;4:331–343. doi: 10.1093/cercor/4.4.331. [DOI] [PubMed] [Google Scholar]
- 30.Rilling JK, Insel TR. Differential expansion of neural projection systems in primate brain evolution. Neuroreport. 1999;10:1453–1459. doi: 10.1097/00001756-199905140-00012. [DOI] [PubMed] [Google Scholar]
- 31.Barres BA, Raff MC. Control of oligodendrocyte number in the developing rat optic nerve. Neuron. 1994;12:935–942. doi: 10.1016/0896-6273(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 32.Wen Q, Chklovskii DB. Segregation of the brain into gray and white matter: A design minimizing conduction delays. PLoS Comput Biol. 2005;1:e78. doi: 10.1371/journal.pcbi.0010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaas JH. Encyclopedia of Neuroscience. Amsterdam: Elsevier; 2009. pp. 793–800. [Google Scholar]
- 34.Herculano-Houzel S, Mota B, Lent R. Cellular scaling rules for rodent brains. Proc Natl Acad Sci USA. 2006;103:12138–12143. doi: 10.1073/pnas.0604911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarko DK, Catania KC, Leitch DB, Kaas JH, Herculano-Houzel S. Cellular scaling rules of insectivore brains. Front Neuroanat. 2009;3:8. doi: 10.3389/neuro.05.008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pillay P, Manger PR. Order-specific quantitative patterns of cortical gyrification. Eur J Neurosci. 2007;25:2705–2712. doi: 10.1111/j.1460-9568.2007.05524.x. [DOI] [PubMed] [Google Scholar]
- 37.Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- 38.Maddison WP, Maddison DR. 2005. Mesquite: A modular system for evolutionary analysis. Version 2.7. Available at http://mesquiteproject.org. [Google Scholar]
- 39.Purvis A. A composite estimate of primate phylogeny. Philos Trans R Soc Lond B Biol Sci. 1995;348:405–421. doi: 10.1098/rstb.1995.0078. [DOI] [PubMed] [Google Scholar]
- 40.Pagel MD. A method for the analysis of comparative data. J Theor Biol. 1992;156:431–442. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.