Abstract
Public goods cooperation abounds in nature, occurring in organisms ranging from bacteria to humans. Although previous research focused on the behavioral and ecological conditions favoring cooperation, the question of whether the molecular and regulatory properties of the public good itself can influence selection for cooperation has received little attention. Using a metapopulation model, we show that extended molecular durability of a public good—allowing multiple reuse across generations—greatly reduces selection for cheating if (and only if) the production of the public good is facultatively regulated. To test the apparent synergy between public goods durability and facultative regulation, we examined the production of iron-scavenging pyoverdin molecules by the bacterium Pseudomonas aeruginosa, a cooperative behavior that is facultatively regulated in response to iron availability. We show that pyoverdin is a very durable public good and that extended durability significantly enhances fitness. Consistent with our model, we found that nonsiderophore-producing mutants (cheats) had a relative fitness advantage over siderophore producers (cooperators) when pyoverdin durability was low but not when durability was high. This was because cooperators facultatively reduced their investment in pyoverdin production when enough pyoverdin had accumulated in the media—a cost-saving strategy that minimized the ability of cheats to invade. These findings show how molecular properties of cooperative acts can shape the costs and benefits of cooperation.
Keywords: extracellular products, siderophores, public goods durability, inclusive fitness, microbes
The joint contribution of individuals to a public good that benefits the local community is ubiquitous in nature and occurs in numerous organisms ranging from bacteria to humans (1–3). However, explaining the evolution of such cooperation is difficult, because public goods, although beneficial to the community, can be exploited by cheating individuals that refrain from making the costly contribution while still reaping the benefits (4–7). Despite this dilemma, which predicts the breakdown of cooperation, public goods cooperation often prevails in nature.
Although numerous behavioral and ecological factors have been proposed that can provide either direct (self) or indirect (kin-selected) benefits to cooperators (7–9), the question of whether the molecular and regulatory properties of the public good itself can influence selection for cooperation has received little attention. Recently, Brown and Taddei (10) showed that the dynamics of cooperation and cheating are affected when the durability of the public good (i.e., the extent of multiple reuse of a public good across generations) is altered. For instance, increased durability introduced oscillations between cooperators and cheats, characterized by alternate multigenerational epochs of dominance by cooperators and cheats. However, whether and under what conditions molecular durability influences selection for cooperation in nature remains unknown. Addressing this issue is crucial, because many public goods are durable and persist across generations [e.g., microbial exoproducts (3), nest constructions in social animals (11), and educational, health, and national defense institutions in human societies (12)]. Furthermore, the results of Brown and Taddei (10) imply that, all else being equal, evolutionary innovations generating more durable public goods variants will be selected against, because they allow greater proliferation by cheats. However, what if, in contrast to the assumptions of ref. 10, the public good is facultatively produced?
To address these issues, we focus on a model system of microbial public goods provision, the production of the iron-scavenging pyoverdin molecule by the bacterium Pseudomonas aeruginosa. Pyoverdin production is well-understood to be facultatively regulated in response to the severity of iron limitation (13, 14). Iron is a major limiting growth factor and is actively withheld by hosts during infections (15, 16). When free iron is scarce, the σ factor PvdS triggers pyoverdin synthesis, whereas the intracellular accumulation of iron results in the binding of iron to the ferric uptake regulator (Fur) protein, which represses pvdS promoter activity and pyoverdin synthesis (17, 18). Pyoverdin can be recycled (19) and used multiple times (20), which suggests considerable durability of this public good. Pyoverdin production is a cooperative trait, because pyoverdin molecules can be shared among neighboring cells, providing benefits to cells other than a focal producer (21–28). Consequently, pyoverdin can be exploited by cheats that avoid the cost of production (29) while reaping the benefits by taking up iron in complex with pyoverdin produced by others.
Using a mix of theory and experiment, we show that pyoverdin is highly durable and readily recyclable after bacterial use. We further show that selection for increased durability and cooperation critically depends on the ability of producers to concurrently modify their production effort, particularly through the facultative regulation of production.
Results
Theoretical Model.
To understand the interaction between pyoverdin durability and its facultative regulation, we developed an ecological metapopulation model tracking the dynamics of cooperators (pyoverdin producers) and defectors (nonpyoverdin-producing cheats), with and without facultative production, over a range of public goods durability (Fig. S1). We assume that only cooperators are able to colonize empty patches, whereas cooperator patches are in turn vulnerable to colonization and takeover by migrating or de novo-arising defectors (30).
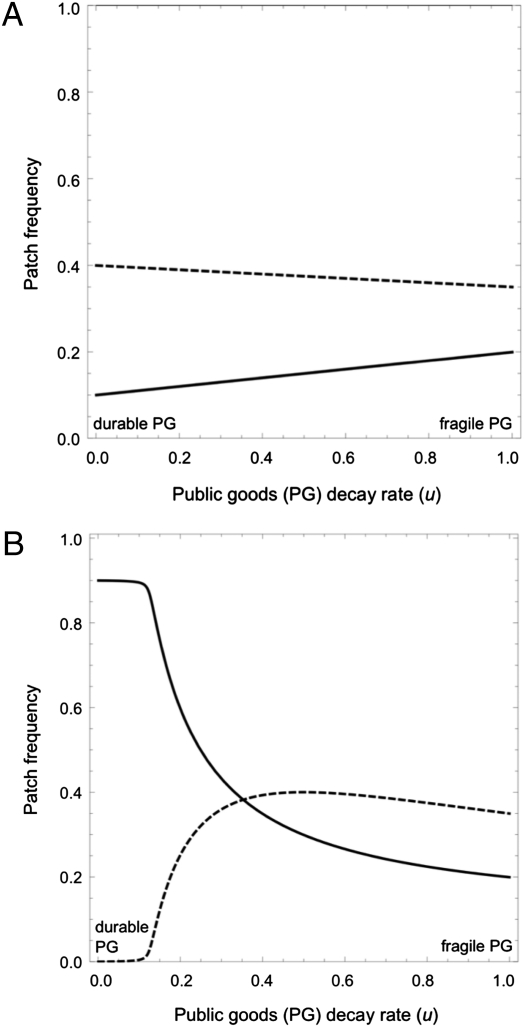
When pyoverdin production is constitutive (Fig. 1A), we find that the prevalence of cooperators across the metapopulation is maximized for the most fragile public goods, whereas more durable public goods increase the life expectancy of defector patches and the total prevalence of defectors. Thus, we anticipate the evolution of a molecular planned obsolescence (31) where the public good is built to fail so as to minimize exploitation by defectors. Our constitutive model (Fig. 1A) assumes a constancy of production effort, following an innovation in molecular design. Thus, more durable molecules would lead to a greater equilibrium density of public good. In contrast, our facultative model (Fig. 1B) assumes a constancy of production outcome, and thus, more durable molecules would immediately induce lower production rates and therefore, a constant equilibrium density of public good. With facultative regulation, we find that very durable public goods now offer greater security against invasion by cheats, because the costs of cooperation are minimized (Fig. 1B). In contrast, as durability decreases, the cost to producers increases (becoming equivalent to the constitutive model when the public goods decay rate u = 1) as well as the advantage to cheats.
Fig. 1.
Increased durability favors cooperation in a structured population if production is facultative. Frequency of cooperator patches (solid lines) and defector patches (dashed lines) as a function of the rate of public goods decay, u. (A) Constitutive production by cooperators and (B) facultative production by cooperators. Parameters are c = d = d0 = 10, e = 1, and m = m0 = 0.01.
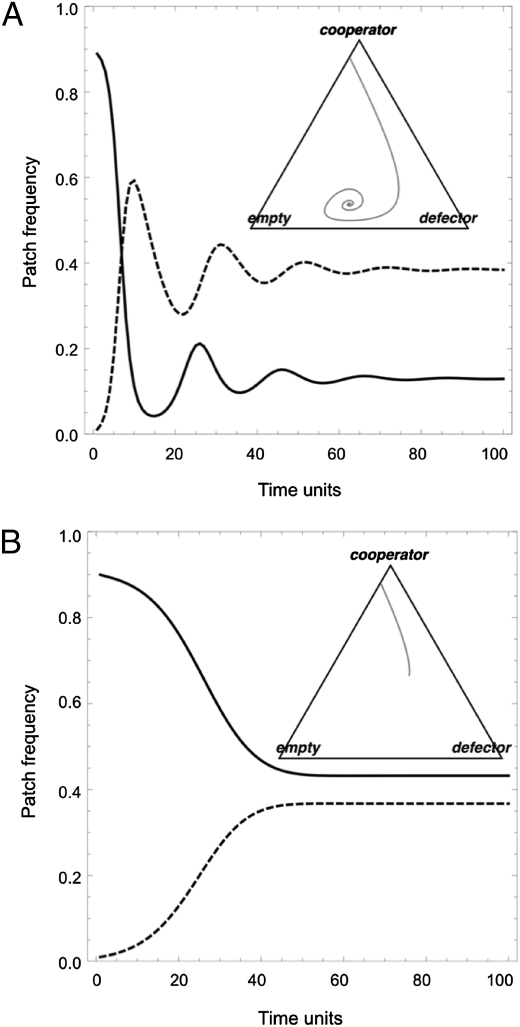
In Fig. 2, we turn to the temporal dynamics of cooperator–defector competition across a metapopulation. We begin our simulations with a metapopulation consisting solely of cooperators (producing a relatively durable public good, u = 0.3) and empty patches at equilibrium. We now introduce a small proportion of defectors and track their fate over time. When production is constitutive (Fig. 2A), defectors have a great initial advantage because of the accumulation of public goods in each patch and the continued investment in public goods by cooperators. Rapid growth of defectors is followed by collapse, because the public goods that they are reliant on eventually expire; this pattern of boom and bust continues as a series of damped oscillations until the equilibrium point is achieved. In contrast, when production is facultative (Fig. 2B), the pattern of boom and bust is damped by the ability of cooperators to respond to their social environment and reduce their costly production in response to the accumulation of the public good in the environment.
Fig. 2.
Facultative producers of durable public goods are more resistant to oscillations and cheats in a structured population. Time series of cooperator (solid line) and defector (dashed line) patch frequencies across a metapopulation. (A) Constitutive production by cooperators and (B) facultative production by cooperators. Parameters are as for Fig. 1, with u = 0.3 and metapopulation seeded with 89% cooperator patches and 1% defector patches. Simulations are redrawn as phase diagrams in triangular Inset.
Experimental Results.
We examined 11 different P. aeruginosa strains originating from different environmental and clinical backgrounds (Table S1). Six of these strains produce pyoverdin type I, whereas three and two strains produce pyoverdin type II and type III, respectively (three main pyoverdin types, which differ in the amino acid sequence of their peptide chain, have been characterized for P. aeruginosa so far) (32, 33). For each of these 11 strains, we were in possession of a cheating mutant that produces no or reduced amounts of pyoverdin (27) but is able to take up the pyoverdin of the corresponding wild-type strain.
Across-strain comparison of pyoverdin durability.
Pyoverdin fluoresces green and can be quantified in solution as relative fluorescence units (RFU) (34). We isolated pyoverdin from culture supernatants and followed RFU over time. We found high levels of pyoverdin fluorescence being maintained after 48 h for all three pyoverdin types (type I = 90.4% ± 0.8%; type II = 90.7% ± 1.1%; type III = 81.5% ± 1.8%) (Fig. S2), suggesting high durability of pyoverdin. Despite this overall slow decay, durability was significantly lower in strains with pyoverdin type III than in strains with pyoverdin type I [combined analysis for measures after 6, 24, and 48 h; linear mixed model (LMM): t7 = −4.81, P = 0.0019] and type II (LMM: t7 = −5.13, P = 0.0014), whereas there was no significant difference between strains with pyoverdin type I and II (LMM: t7 = 0.32, P = 0.76).
To test whether the persistence of fluorescence levels over time goes along with the retention of pyoverdin functionality, we conducted growth stimulation assays using pyoverdin of different ages. We added pyoverdin supernatant on the day of its extraction as well as 24 and 48 h after the extraction to fresh iron-limited media (Casamino acids supplemented with human apo-transferrin to bind free iron)—conditions that require pyoverdin for growth—and inoculated 105 cells from pyoverdin-defective cheating strains. We found that pyoverdin remained fully functional, because there was no significant difference in growth (optical density = OD600 ± SE) between cultures supplemented with fresh (0.157 ± 0.033), 24-h-old (0.158 ± 0.026), and 48-h-old (0.177 ± 0.037) pyoverdin (LMM across all three pyoverdin types: 0.28 < t39 < 1.98, all P > 0.05).
Pyoverdin durability in different environments.
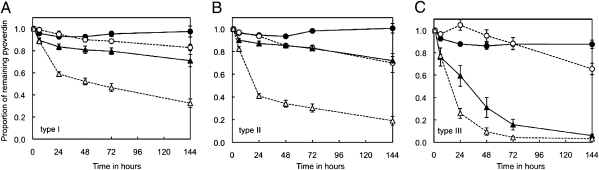
To test whether pyoverdin durability is affected by environmental conditions, we measured the durability in environments varying in (i) the presence or absence of nonpyoverdin-producing cheats (i.e., when the pyoverdin molecule is or is not taken up and recycled by bacteria), and (ii) low vs. high iron availability, with pyoverdin not being needed in iron-supplemented (50 μM FeCl3) media. We found that the durability of pyoverdin was significantly influenced by both the presence of cheats and the supplementation of iron (Fig. 3). Over a 144-h assay, pyoverdin durability was significantly lower when iron was supplemented (LMM for pyoverdin type I: t42 = 6.06, P < 0.0001; for type II: t42 = 5.38, P < 0.0001; for type III: t42 = 9.89, P < 0.0001) and was significantly lower in the presence of cheats for pyoverdin type II (LMM: t42 = 4.26, P = 0.0001) but not for pyoverdin type I (t42 = 1.70, P = 0.096) and type III (t42 = 1.28, P = 0.21). Moreover, there was a significant interaction between the presence of cheats and iron supplementation, whereby the supplementation of iron decreased the durability much more in the presence than in the absence of cheats (LMM for type I: t42 = 3.79, P = 0.0005; for type II: t42 = 4.40, P < 0.0001; for type III: t42 = 2.99, P = 0.0046).
Fig. 3.
Pyoverdin durability alters with usage and iron availability. The proportion of pyoverdin (±95% confidence interval) remaining in the medium after 24 h in iron-limited (circles) and 50 μM iron (FeCl3)-supplemented (triangles) cultures, inoculated with either no bacteria (filled symbols) or nonpyoverdin-producing bacteria (open symbols). (A) Pyoverdin type I is from strain 1, (B) pyoverdin type II is from strain 11, and (C) pyoverdin type III is from strain 7. Pyoverdin durability was significantly lower when iron was supplemented, and it decreased significantly stronger in the presence than in the absence of cheats.
Fitness consequences of pyoverdin durability.
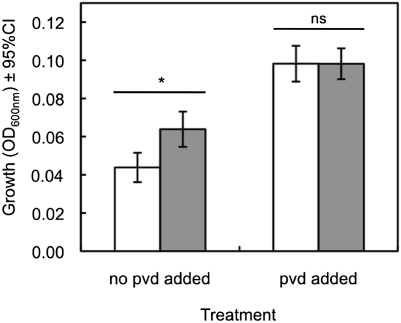
We simulated extended pyoverdin durability by supplementing pyoverdin to the growth medium, which mimics the presence of durable pyoverdin produced by a previous generation. In monocultures, we found that pyoverdin supplementation significantly increased culture growth in all 11 strains (LMM across strains: t129 = 20.3, P < 0.0001) but increased growth more in cheat than in cooperator monocultures (significant interaction between strain type and pyoverdin supplementation level: t129 = 5.3, P < 0.0001) (Fig. 4). Crucially, we found that cooperators in pyoverdin-supplemented cultures reduced investment in pyoverdin production by 87.9% ± 1.9% (mean ± SE across 11 strains) compared with cooperator cultures with no pyoverdin supplementation. This estimate was obtained by comparing the extra pyoverdin produced per cell in pyoverdin-supplemented cooperator cultures with the pyoverdin production per cell in nonsupplemented cooperator cultures (details in Materials and Methods).
Fig. 4.
Increased durability enhances fitness. The effect of pyoverdin (pvd) supplementation—which mimics the presence of durable pyoverdin produced by a previous generation—on the growth of cooperator (gray bars) and cheat (white bars) monocultures. Pyoverdin supplementation significantly increased growth, with growth stimulation being more pronounced in cheat than in cooperator monocultures in all 11 strain pairs. *P < 0.05. ns, not significant.
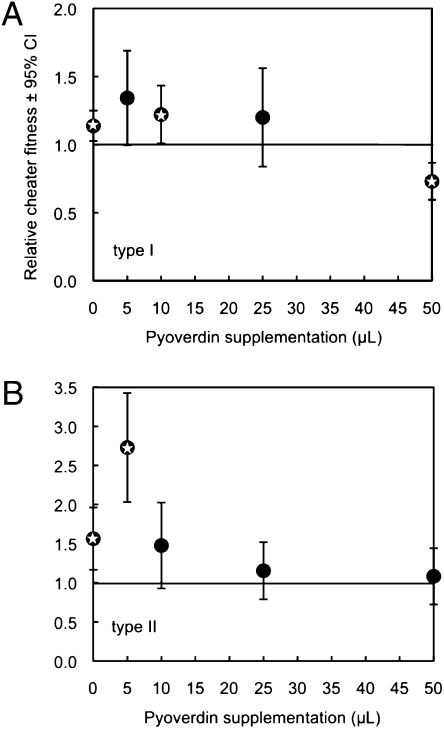
In mixed cultures, the relative fitness of cheats and cooperators depended on the amount of pyoverdin supplemented (Fig. 5). Specifically, cheats did significantly better than cooperators in cultures mimicking low durability of pyoverdin (low quantities of pyoverdin supplemented). In contrast, cheats lost their competitive advantage when higher quantities of pyoverdin were supplemented (Fig. 5).
Fig. 5.
Increased durability reduces selection for cheats. Outcome of 24-h competition assays between cheats and cooperators in cultures supplemented with different quantities of pyoverdin (i.e., mimicking different durability): (A) for strain 1, pyoverdin type I, and (B) for strain 11, pyoverdin type II. Stars inside symbols indicate values significantly different from 1.
Discussion
We show that pyoverdin is an extremely durable and fitness-enhancing public good that remains functional for long periods of time. Experimentally extended pyoverdin durability resulted in the down-regulation of pyoverdin production by bacteria—a cost-saving strategy that eliminated the fitness advantage that nonpyoverdin-producing cheats normally experience in mixed cultures with cooperative pyoverdin producers. Together with the findings of our model, our data highlight that facultative production of a durable public good represents a powerful mechanism to reduce selection for cheating, because it minimizes the cost (c) of cooperation while maintaining its benefits (b) (35) and thereby, contributes to satisfying Hamilton's rule for the evolution of cooperation: rb > c, where r is the relatedness between the actor and the beneficiary of a cooperative act (36). This two-pronged mechanism (durability plus facultative control) could potentially contribute to the evolutionary stability of numerous bacterial extracellular public goods (3).
Producing something that is durable and can continue to deliver benefits would seem obviously preferable to producing something ephemeral. In a social context, the benefits of durability may even be magnified, because the benefits can accrue not only to contemporary kin but also to descendent kin that are not yet even born (37). However, our model shows that when all else is equal, the constitutive production of more durable public goods does not favor cooperation, because defectors can thrive on their patches for extended periods of time without suffering a loss of social benefits (Fig. 1A) (10). This leads to the counterintuitive result that the constitutive production of something more durable is unfavorable in a social context. We then show that this problem can be solved when the public good is facultatively produced (Fig. 1B). The reason for this is that the production costs (proportional to c in Hamilton's rule) can now be immediately mitigated after the innovation of a more durable public good such that they need only to be paid briefly on colonization of an empty patch. Thus, regulatory control can temporally decouple investment into the public good by cooperators (early in colonization when r is high and benefits of cooperation accrue to producers exclusively) and subsequent competition between cooperators and cheats (i.e., when r is low). Consequently, after the cost for the public good has been paid during colonization, the resident cooperators are competitively in a strong position to withstand the challenge of any defector mutant or migrant. Empirical findings suggest that a decoupling between investment into the public good and effective competition is actually taking place: the highest investment in pyoverdin production occurs at low cell densities (i.e., on colonization) (14), conditions under which cheats have limited access to the public good and consequently, have a hard time competing and invading (26).
Our results reveal that durability of pyoverdin type III is significantly reduced compared with pyoverdin types I and II (Fig. S2). Interestingly, this pattern correlates with the abundance of the pyoverdin types across both natural and clinical isolates, with the more fragile type III pyoverdins being the least common (29, 33). This raises the question of whether increased durability provides a competitive advantage. It has been suggested that the pyoverdin locus is under diversifying selection (32), with altered pyoverdin structures possibly being a measure to limit access to the public good to close relatives (i.e., clone mates). Any structural changes to pyoverdin may, in turn, modify the robustness of the molecule and thus, contribute to the diversity in durability among types I–III. It is also possible that durability is itself under direct selection, with the direction of selection depending on prevailing ecological conditions and also the nature of regulatory control over pyoverdin production. The synergy between the benefits of regulation and durability entails that neither trait can be properly considered in isolation, and we have a multidimensional social dilemma (38), with increasing durability only favored when regulatory control of the public good is sufficiently developed. By introducing variable degrees of regulatory efficiency (SI Text), we show that the regulatory threshold for durability selection depends on the rate of disturbance. Specifically, when the risk of environmental perturbation is low (e.g., chronic infections) and therefore, the burden of cheats is high (39), very precise regulation is required before selection can favor more durable public goods (Fig. S3).
We found that the durability of pyoverdin was not only influenced by its molecular design (pyoverdin type) but also by environmental factors such as iron concentration (Fig. 3). The more rapid degradation of pyoverdin in iron-rich media in the presence of bacteria suggests that bacteria consume pyoverdin when the molecule becomes redundant. Consumption of a public good by individuals of the same or a different species may be an important factor, reducing its durability in environmental settings. Furthermore, other factors such as a high diffusion rate might significantly reduce reusability even when the public good is molecularly durable, because diffusion leaches the public good away from its producers (24, 40). Ecological conditions that determine public goods diffusion are, therefore, likely to determine reusability and the selection for cooperation. For instance, our previous work showed that media viscosity reduces pyoverdin diffusion, thereby increasing selection for cooperation (24). More generally, understanding how production, consumption, degradation, and diffusion combine to shape the dynamics of microbial public goods promises a range of insights into the ecology and evolution of microbial cooperation. Given the coupling between microbial cooperation and virulence (22, 41, 42), more detailed understanding of the dynamics of microbial social behaviors can, in turn, open strategies of pathogen control (43).
Materials and Methods
Strains.
We used 11 different P. aeruginosa strains originating from different environmental and clinical backgrounds (Table S1). Six of these strains produce pyoverdin type I, whereas three and two strains produce pyoverdin type II and type III, respectively. Pyoverdin consists of a conserved fluorescent chromophore linked to a short peptide, with the pyoverdin types I, II, and III differing in the amino composition of their peptide chain (18). For each of these 11 strains, we were in possession of a cheating mutant that produced no or reduced amounts of pyoverdin (27). As the reference wild type–mutant (i.e., cooperator–cheat) pair, we used strain PAO1 (pyoverdin type I, ATCC 15692; ATCC) and the knockout mutant (PAO1ΔpvdD), which was directly derived from PAO1 and is unable to produce pyoverdin because the peptide synthetase (pvdD) is knocked out (44). Moreover, we used the strain pair PAO6049-PAO9(PAO6609), where PAO6049 is a methione auxotrophe derivate from PAO1 but a wild-type pyoverdin producer and PAO9 is a pyoverdin-deficient mutant derived by UV mutagenesis from PAO6049 (45). For the other nine wild types, spontaneous pyoverdin-defective mutants have been isolated and characterized by Jiricny et al. (27).
Extraction of Pyoverdin.
To stimulate pyoverdin production, we grew wild-type strains for 24 h at 37 °C in a shaken incubator in 30-mL glass vials containing 6 mL minimal-iron casamino acids (CAA) media (5 g casamino acids, 1.18 g K2HPO4*3 H2O, 0.25 g MgSO4*7 H2O per liter) supplemented with 20 mM NaHCO3 (sodium bicarbonate) and 100 μg/mL human apo-transferrin (Sigma). Apo-transferrin is a powerful natural iron chelator that binds free Fe(III) in the presence of bicarbonate and prevents nonsiderophore-mediated uptake of iron by bacteria (46). After growth, we centrifuged aliquots of 1 mL in Eppendorf tubes at 13,835 × g for 10 min and passed the supernatant containing pyoverdin through a microfilter (Sartorius Minisart, pore size = 0.2 μm; Sartorius) for sterilization. Besides pyoverdin, this sterile supernatant contains many other metabolic compounds secreted by bacteria, and measuring pyoverdin durability under these biochemical conditions reflects the natural situation.
Measuring Pyoverdin Durability.
Pyoverdin fluoresces green and can be quantified in solution as RFU using a fluorimetre (excitation: 400 nm, emission: 460 nm; SpectraMax M2; Molecular Devices) (34). We transferred 100 μL of the filtered supernatant into individual wells on a 96-well microtitre plate, incubated the plate at 37 °C (the optimal growth temperature of P. aeruginosa at which pyoverdin is produced abundantly in iron-limited medium) in a static incubator, and measured RFU at incubation time followed by measures after 1, 6, 24, and 48 h. RFU measurements were taken from nine (1, 6, and 24 h) and three (48 h) independent replicates for all 11 cooperator strains. We measured the durability of pyoverdin as RFUt = x/RFUt = 1, which represents the proportion of pyoverdin remaining in the medium over a given time interval (x − 1 h). Preliminary experiments revealed that pyoverdin is highly sensitive to shaking [mean proportion of maintained pyoverdin fluorescence after 6 h: shaken at 1.1 × g = 0.35 ± 0.02, static = 0.95 ± 0.01, LMM (linear mixed model): t8 = 34.0, P < 0.0001], and when we studied decay in static culture (the biologically relevant condition), we discarded the first hour after sample preparation involving shaking.
To test whether pyoverdin remains functional over time, we conducted growth stimulation assays using pyoverdin of different ages. We added 100 μL pyoverdin supernatant on the day of its extraction as well as 24 and 48 h after the extraction (stored at 37 °C) to 100 μL fresh CAA media inoculated with 105 cells of the corresponding cheater strain. We measured culture growth 24 h after inoculation at 600 nm using SpectraMax M2 and compared growth between the different age classes of pyoverdin.
Measuring Pyoverdin Durability in Different Environments.
We measured the durability of pyoverdin under different environmental conditions by varying (i) the presence or absence of cheats, and (ii) the iron content of the media (50 μM FeCl3 vs. no iron supplementation). Each treatment was independently replicated three times for all three pyoverdin types: type I (strain 1), type II (strain 11), and type III (strain 7) (details in Table S1). We transferred 100 μL of the filtered supernatant into individual wells on a 96-well microtitre plate with 100 μL fresh CAA media and supplemented wells with (i) 105 cells of the cheater strain from overnight CAA culture, (ii) 50 μM FeCl3, (iii) 105 cheater cells and 50 μM FeCl3, or (iv) nothing. We incubated the plate at 37 °C in a static incubator and measured RFU at incubation time, followed by measures after 1, 6, 24, 48, 72, and 144 h. Evaporation of the medium occurring during incubation results in increasing overestimations of RFU with longer incubation times. This effect could particularly be seen in treatment where the effect of pyoverdin decay on RFU was weaker than the effect of evaporation. Pyoverdin of all three types decayed at a constant rate under the condition where pyoverdin is taken up and recycled by bacteria in iron-limited media (linear regression on logarithmically transformed decay values; type I: R2 = 0.854, F1,13 = 82.6, P < 0.0001; type II: R2 = 0.862, F1,13 = 88.3, P < 0.0001; type III: R2 = 0.837, F1,13 = 72.7, P < 0.0001).
Effects of Pyoverdin Durability on Monoculture Fitness.
To assess the consequences of extended pyoverdin durability (mimicked by pyoverdin supplementation) on fitness of cooperator and cheat strains in monocultures, we carried out an experiment with four treatments: (i) 200 μL fresh CAA media inoculated with 105 cooperator cells, (ii) 200 μL fresh CAA media inoculated with 105 cheat cells, (iii) 50 μL of pyoverdin supernatant + 150 μL fresh CAA media with 105 cooperator cells, or (iv) 50 μL of pyoverdin supernatant + 150 μL fresh CAA media with 105 cheat cells. This experiment involved four replications for all 11 cooperator–cheat strain pairs. Culture growth was measured as optical density at 600 nm after 24 h.
To investigate whether the supplementation of pyoverdin resulted in reduced pyoverdin investment by cooperators, we compared RFUt = 24 /OD600 nm (i.e., an estimate of the amount of pyoverdin produced per cell) (14) in nonpyoverdin-supplemented cooperator monocultures with the extra pyoverdin produced per cell in pyoverdin-supplemented cooperator monocultures. A proxy for the extra pyoverdin produced per cooperator cells in pyoverdin-supplemented cultures is given by [(RFUt = 24 in pyoverdin-supplemented cooperator cultures) − (RFUt = 24 in pyoverdin-supplemented cheat cultures)]/(OD600 nm in pyoverdin-supplemented cooperator cultures). OD is significantly positively correlated with cfu per milliliter across the OD range used here (Pearson's product moment correlation: r = 0.973, df = 30, P < 0.0001).
Effects of Pyoverdin Durability on Fitness in Mixed Cultures.
We conducted competition assays between cooperator and cheat strains by inoculating 105 bacteria at a 1:1 ratio into CAA supplemented with various amounts of pyoverdin. Specifically, we supplemented 0, 5, 10, 25, and 50 μL of pyoverdin supernatant to 200, 195, 190, 175, and 150 μL CAA, respectively. Each experimental treatment was independently replicated 8 to 12 times for all three pyoverdin types (type I: strain 1; type II: strain 11; type III: strain 7). After a 24-h competition period, cultures were individually diluted and plated on King's B medium (KB) and incubated overnight in a static incubator at 37 °C. We then quantified the number of cooperator and cheat cfu on each plate and calculated the relative fitness of cheats: v = [x2 (1 − x1)]/[x1 (1 − x2)], where x1 is the initial proportion of cheats and x2 is their final proportion (23). Colonies of cooperators and cheats can usually be distinguished based on color differences: cooperator colonies are green because of the presence of fluorescent pyoverdin molecules, whereas cheater colonies are whitish because of the absence of pyoverdin (24, 27). Unfortunately, we were unable to reliably tell apart the colonies of strain 7 and its mutant, because the pyoverdin-defective mutant of strain 7 turned out to be phenotypically polymorphic.
Statistical Analyses.
We used LMM to test whether pyoverdin durability differs between pyoverdin types, environmental conditions, and iron-supplementation treatments. Before analysis, we arcsine-transformed our estimates of pyoverdin durability to account for the proportional nature of our variable and hence, to render the variance independent of the mean. Whenever applicable, we introduced the strain identification, replicate number, or time interval as a random factor into our models to control for the fact that repeated measures were taken from the same pyoverdin type, strain, or culture, respectively. We further introduced RFUt = 1 as a covariate in our models. This was done, because RFUt = 1 measures from supernatants varied between strains (RFUt = 1: mean ± SE = 1,627 ± 74, range = 1,194–2,038). However, RFUt = 1 had no significant effect on any of our response variables (P ≥ 0.32 for all analysis), showing that differences in RFUt = 1 did not influence our results. Finally, we used the false-discovery rate (FDR) control method to adjust the nominal = 0.05 in posthoc pair-wise comparisons. All statistical computations were carried out with R 2.10.1 (http://www.r-project.org/).
Model.
Our model tracks the dynamics of three distinct patch types characterized by their occupants, empty (with prevalence E), cooperator only (with prevalence C), and defector only (with prevalence D), building on the metapopulation models by Levins (47) describing the dynamics of subpopulations within a metapopulation (30, 47). We assume that defectors always displace cooperators if they co-occur within a patch (i.e., the patches are well-mixed, and the cooperative provision of the public good entails a direct cost, ensuring a local tragedy of the commons) (4, 5) and that the dynamics of strain replacement are sufficiently fast that we can reduce within-patch dynamics to simple transition rates among the single strain states. Our model tracks three classes of transition events: colonization of empty patches (by cooperators), extinction of occupied patches (at a higher rate for defector patches), and replacement of cooperators by defectors (because of both transmission of defectors and de novo mutation).
These transition events define the following system of ordinary differential equations, controlled by cooperator and defector transmission rates c and d, patch extinction rate e, cooperator to defector mutation and replacement rate m, and public good decay rate u (Eqs. 1–4):
To introduce facultative production of a public good, we consider that the rates of within-patch cooperator replacement by defectors (whether arising from spontaneous mutation or colonization) will decrease for more durable public goods, because the costs of production (driving cheat replacement) will only be paid intermittently. Specifically, we assume that d and m are positive functions of u so that fragile public goods ensure a high (i.e., constant) production cost and therefore, high rates of replacement by cheats. In Fig. 1B, we specify that d = d0 u and m = m0 u, ensuring coexistence whenever c > e + m0 u. More detailed model exposition and analysis are presented in SI Text.
Supplementary Material
Acknowledgments
We thank Natalie Jiricny (University of Oxford, Oxford) for providing strains and Craig Maclean, Ashleigh Griffin, Stuart West, and two anonymous referees for their helpful comments. This work was funded by the Swiss National Science Foundation, a Marie Curie Intra-European Fellowship (to R.K.), and Wellcome Trust Grant 082273/Z/07/Z (to S.P.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011154107/-/DCSupplemental.
References
- 1.Ledyard JO. In: The Handbook of Experimental Economics. Kagel JH, Roth AE, editors. Princeton: Princeton University Press; 1995. [Google Scholar]
- 2.Frank SA. Foundations of Social Evolution. Princeton: Princeton University Press; 1998. [Google Scholar]
- 3.West SA, Diggle SP, Buckling A, Gardner A, Griffin AS. The social lives of microbes. Annu Rev Ecol Evol Syst. 2007;38:53–77. [Google Scholar]
- 4.Hardin G. The tragedy of the commons. Science. 1968;162:1243–1248. [PubMed] [Google Scholar]
- 5.Rankin DJ, Bargum K, Kokko H. The tragedy of the commons in evolutionary biology. Trends Ecol Evol. 2007;22:643–651. doi: 10.1016/j.tree.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Frank SA. A general model of the public goods dilemma. J Evol Biol. 2010;23:1245–1250. doi: 10.1111/j.1420-9101.2010.01986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West SA, Griffin AS, Gardner A. Evolutionary explanations for cooperation. Curr Biol. 2007;17:R661–R672. doi: 10.1016/j.cub.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann L, Keller L. The evolution of cooperation and altruism—a general framework and a classification of models. J Evol Biol. 2006;19:1365–1376. doi: 10.1111/j.1420-9101.2006.01119.x. [DOI] [PubMed] [Google Scholar]
- 9.West SA, Griffin AS, Gardner A. Social semantics: Altruism, cooperation, mutualism, strong reciprocity and group selection. J Evol Biol. 2007;20:415–432. doi: 10.1111/j.1420-9101.2006.01258.x. [DOI] [PubMed] [Google Scholar]
- 10.Brown SP, Taddei F. The durability of public goods changes the dynamics and nature of social dilemmas. PLoS ONE. 2007;2:e593. doi: 10.1371/journal.pone.0000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hölldobler B, Wilson EO. The Ants. Berlin: Springer; 1990. [Google Scholar]
- 12.Ostrom E. Governing the Commons: The Evolution of Institutions for Collective Action. New York: Cambridge University Press; 1990. [Google Scholar]
- 13.Tiburzi F, Imperi F, Visca P. Intracellular levels and activity of PvdS, the major iron starvation sigma factor of Pseudomonas aeruginosa. Mol Microbiol. 2008;67:213–227. doi: 10.1111/j.1365-2958.2007.06051.x. [DOI] [PubMed] [Google Scholar]
- 14.Kümmerli R, Jiricny N, Clarke LS, West SA, Griffin AS. Phenotypic plasticity of a cooperative behaviour in bacteria. J Evol Biol. 2009;22:589–598. doi: 10.1111/j.1420-9101.2008.01666.x. [DOI] [PubMed] [Google Scholar]
- 15.Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annu Rev Microbiol. 2000;54:881–941. doi: 10.1146/annurev.micro.54.1.881. [DOI] [PubMed] [Google Scholar]
- 16.Miethke M, Marahiel MA. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev. 2007;71:413–451. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escolar L, Pérez-Martín J, de Lorenzo V. Opening the iron box: Transcriptional metalloregulation by the Fur protein. J Bacteriol. 1999;181:6223–6229. doi: 10.1128/jb.181.20.6223-6229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visca P, Imperi F, Lamont IL. Pyoverdine siderophores: From biogenesis to biosignificance. Trends Microbiol. 2007;15:22–30. doi: 10.1016/j.tim.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Imperi F, Tiburzi F, Visca P. Molecular basis of pyoverdine siderophore recycling in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2009;106:20440–20445. doi: 10.1073/pnas.0908760106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faraldo-Gómez JD, Sansom MSP. Acquisition of siderophores in gram-negative bacteria. Nat Rev Mol Cell Biol. 2003;4:105–116. doi: 10.1038/nrm1015. [DOI] [PubMed] [Google Scholar]
- 21.Griffin AS, West SA, Buckling A. Cooperation and competition in pathogenic bacteria. Nature. 2004;430:1024–1027. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- 22.Harrison F, Browning LE, Vos M, Buckling A. Cooperation and virulence in acute Pseudomonas aeruginosa infections. BMC Biol. 2006 doi: 10.1186/1741-7007-4-21. 10.1186/1741-7007-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross-Gillespie A, Gardner A, West SA, Griffin AS. Frequency dependence and cooperation: Theory and a test with bacteria. Am Nat. 2007;170:331–342. doi: 10.1086/519860. [DOI] [PubMed] [Google Scholar]
- 24.Kümmerli R, Griffin AS, West SA, Buckling A, Harrison F. Viscous medium promotes cooperation in the pathogenic bacterium Pseudomonas aeruginosa. Proc Biol Sci. 2009;276:3531–3538. doi: 10.1098/rspb.2009.0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kümmerli R, Gardner A, West SA, Griffin AS. Limited dispersal, budding dispersal, and cooperation: An experimental study. Evolution. 2009;63:939–949. doi: 10.1111/j.1558-5646.2008.00548.x. [DOI] [PubMed] [Google Scholar]
- 26.Ross-Gillespie A, Gardner A, Buckling A, West SA, Griffin AS. Density dependence and cooperation: Theory and a test with bacteria. Evolution. 2009;63:2315–2325. doi: 10.1111/j.1558-5646.2009.00723.x. [DOI] [PubMed] [Google Scholar]
- 27.Jiricny N, et al. Fitness correlates with the extent of cheating in a bacterium. J Evol Biol. 2010;23:738–747. doi: 10.1111/j.1420-9101.2010.01939.x. [DOI] [PubMed] [Google Scholar]
- 28.Kümmerli R, van den Berg P, Griffin AS, West SA, Gardner A. Repression of competition favours cooperation: Experimental evidence from bacteria. J Evol Biol. 2010;23:699–706. doi: 10.1111/j.1420-9101.2010.01936.x. [DOI] [PubMed] [Google Scholar]
- 29.De Vos D, et al. Study of pyoverdine type and production by Pseudomonas aeruginosa isolated from cystic fibrosis patients: Prevalence of type II pyoverdine isolates and accumulation of pyoverdine-negative mutations. Arch Microbiol. 2001;175:384–388. doi: 10.1007/s002030100278. [DOI] [PubMed] [Google Scholar]
- 30.Maynard Smith J. Group selection. Q Rev Biol. 1976;51:277–283. [Google Scholar]
- 31.Waldman M. Durable goods theory for real world markets. J Econ Perspect. 2003;17:131–154. [Google Scholar]
- 32.Smith EE, Sims EH, Spencer DH, Kaul R, Olson MV. Evidence for diversifying selection at the pyoverdine locus of Pseudomonas aeruginosa. J Bacteriol. 2005;187:2138–2147. doi: 10.1128/JB.187.6.2138-2147.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer JM, et al. Use of siderophores to type pseudomonads: The three Pseudomonas aeruginosa pyoverdine systems. Microbiology. 1997;143:35–43. doi: 10.1099/00221287-143-1-35. [DOI] [PubMed] [Google Scholar]
- 34.Ankenbauer R, Sriyosachati S, Cox CD. Effects of siderophores on the growth of Pseudomonas aeruginosa in human serum and transferrin. Infect Immun. 1985;49:132–140. doi: 10.1128/iai.49.1.132-140.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brockhurst MA, Buckling A, Racey D, Gardner A. Resource supply and the evolution of public-goods cooperation in bacteria. BMC Biol. 2008 doi: 10.1186/1741-7007-6-20. 10.1186/1741-7007-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamilton WD. The genetical evolution of social behaviour. I. J Theor Biol. 1964;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 37.Lehmann L. The evolution of trans-generational altruism: Kin selection meets niche construction. J Evol Biol. 2007;20:181–189. doi: 10.1111/j.1420-9101.2006.01202.x. [DOI] [PubMed] [Google Scholar]
- 38.Brown SP, Taylor PD. Joint evolution of multiple social traits: A kin selection analysis. Proc Biol Sci. 2010;277:415–422. doi: 10.1098/rspb.2009.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brockhurst MA, Buckling A, Gardner A. Cooperation peaks at intermediate disturbance. Curr Biol. 2007;17:761–765. doi: 10.1016/j.cub.2007.02.057. [DOI] [PubMed] [Google Scholar]
- 40.Le Gac M, Doebeli M. Environmental viscosity does not affect the evolution of cooperation during experimental evolution of colicigenic bacteria. Evolution. 2010;64:522–533. doi: 10.1111/j.1558-5646.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- 41.Nogueira T, et al. Horizontal gene transfer of the secretome drives the evolution of bacterial cooperation and virulence. Curr Biol. 2009;19:1683–1691. doi: 10.1016/j.cub.2009.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rumbaugh KP, et al. Quorum sensing and the social evolution of bacterial virulence. Curr Biol. 2009;19:341–345. doi: 10.1016/j.cub.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 43.Brown SP, West SA, Diggle SP, Griffin AS. Social evolution in micro-organisms and a Trojan horse approach to medical intervention strategies. Philos Trans R Soc Lond B Biol Sci. 2009;364:3157–3168. doi: 10.1098/rstb.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghysels B, et al. FpvB, an alternative type I ferripyoverdine receptor of Pseudomonas aeruginosa. Microbiology. 2004;150:1671–1680. doi: 10.1099/mic.0.27035-0. [DOI] [PubMed] [Google Scholar]
- 45.Hohnadel D, Haas D, Meyer JM. Mapping of mutations affecting pyoverdine production in Pseudomonas aeruginosa. FEMS Microbiol Lett. 1986;36:195–199. [Google Scholar]
- 46.Meyer J-M, Neely A, Stintzi A, Georges C, Holder IA. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun. 1996;64:518–523. doi: 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levins R. Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull Entomol Soc Am. 1969;15:237–240. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.