Abstract
Thiamine-deficient encephalopathy is characterized by morphologic lesions in the brainstem and less extensively in the cerebellum, but the early neurologic signs reverse rapidly and fully with thiamine, indicating a metabolic disorder. The suggested causal mechanisms of the encephalopathy involve two thiamine-dependent enzymes: (a) impairment of pyruvate decarboxylase activity with decreased cerebral energy (ATP) synthesis, and (b) reduction of transketolase activity with possible impairment of the hexose monophosphate shunt and subsequent decrease in NADPH formation. The latter may be important in maintaining glutathione in a reduced form (GSH), which apparently functions by keeping enzymes in a reduced (active) conformation.
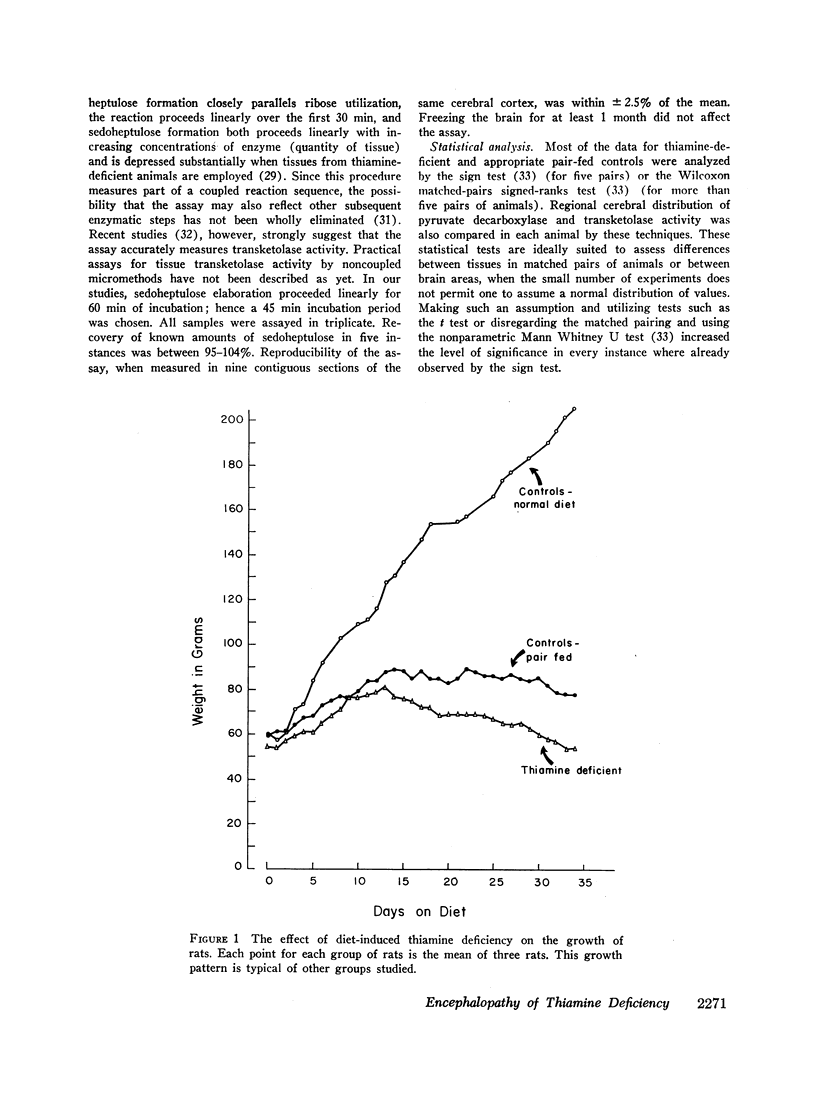
To examine some of these postulated mechanisms, in this study we measured pyruvate decarboxylase and transketolase activity, lactate, ATP and GSH levels in the cerebral cortex, cerebellum, and brainstem, and thiamine concentration in whole brain of rats with diet-induced low thiamine encephalopathy. Pair-fed and normally fed asymptomatic control animals were similarly investigated. To assess the functional importance of some of our results, we repeated the studies in rats, immediately (16-36 hr) after reversal of the neurological signs with thiamine administration.
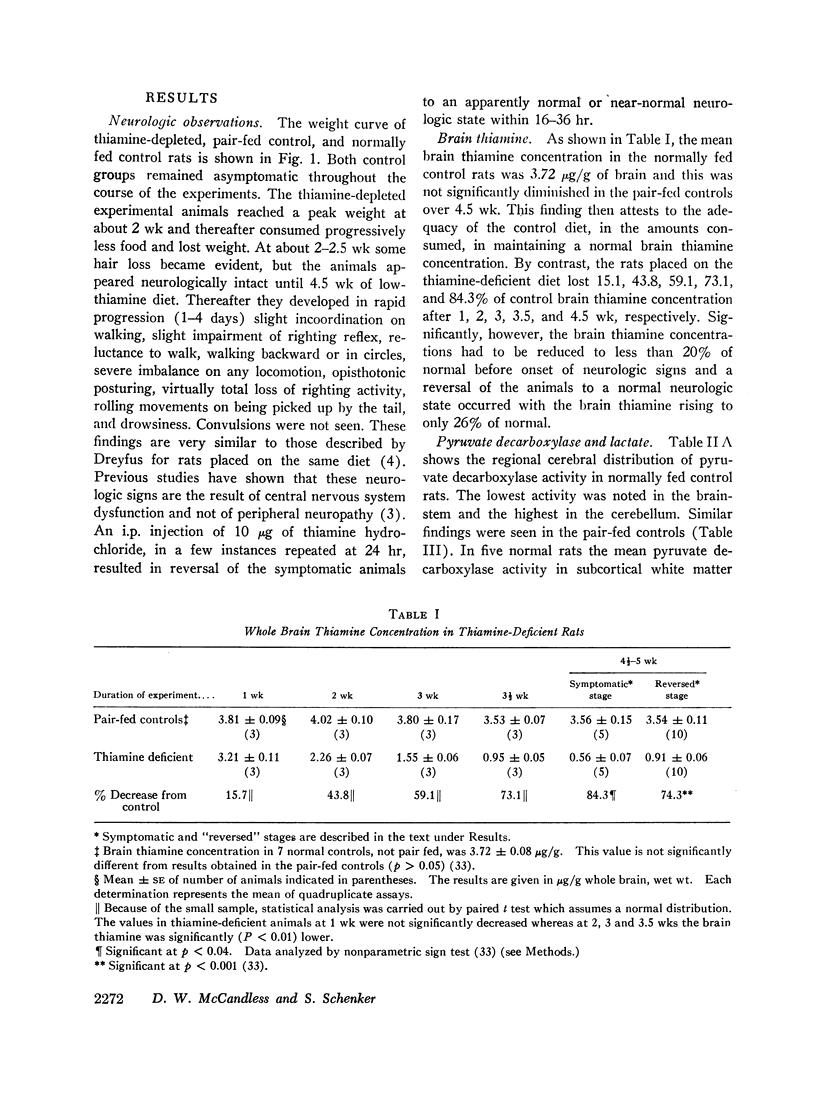
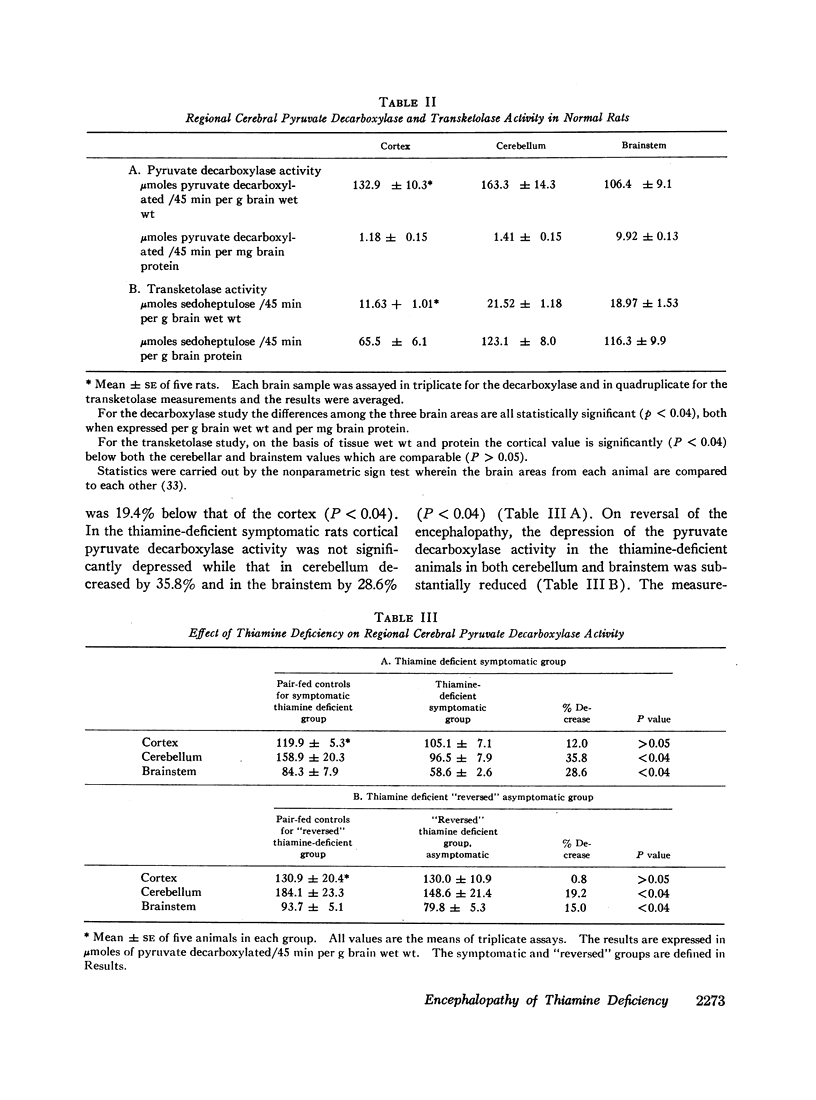
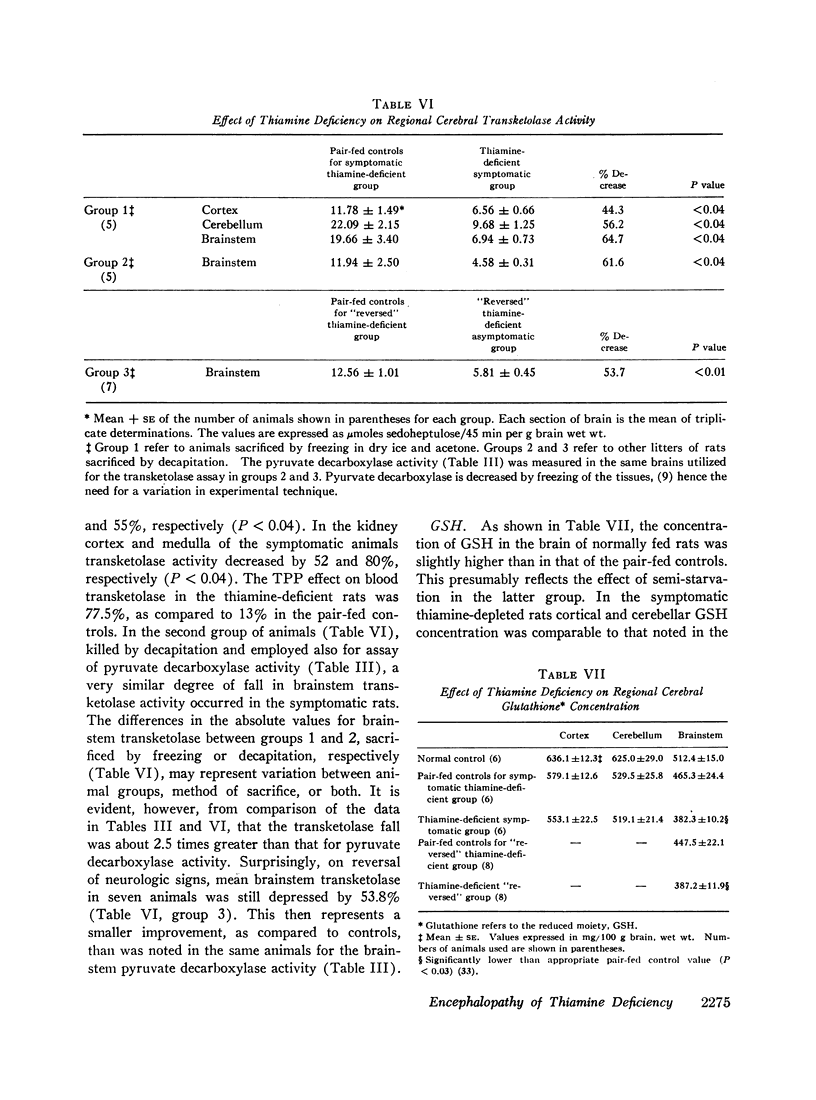
The data obtained led to the following conclusions: (a) Brain contains a substantial reserve of thiamine in that thiamine level has to fall to below 20% of normal before the onset of overt encephalopathy and an increase in brain thiamine to only 26% of normal results in rapid reversal of neurologic signs. (b) Both cerebral transketolase and pyruvate decarboxylase activities are impaired in low thiamine encephalopathy and the abnormality in the pyruvate decarboxylase is reflected in a rise in brain lactate. These biochemical abnormalities occur primarily in the brainstem and cerebellum, the sites of the morphologic changes. (c) Although the fall in cerebral transketolase is about twofold greater than that of pyruvate decarboxylase activity during encephalopathy, both enzymes rise on reversal of neurologic signs and the degree of the transketolase rise is slight. Accordingly, this study cannot ascertain the relative functional importance of these two pathways in the induction of the encephalopathy. The data suggest, however, that the depression of transketolase is not functionally important per se, but may only be an index of some other critical aspect of the hexose monophosphate shunt. (d) The normal cerebral ATP concentration and small GSH fall during encephalopathy, with little GSH rise on reversal of neurologic signs, suggest that a depletion of neither substance is instrumental in inducing thiamine-deficient encephalopathy.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEUTLER E., DURON O., KELLY B. M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963 May;61:882–888. [PubMed] [Google Scholar]
- Balaghi M., Pearson W. N. Tissue and intracellular distribution of radioactive thiamine in normal and thiamine-deficient rats. J Nutr. 1966 Jun;89(2):127–132. doi: 10.1093/jn/89.2.127. [DOI] [PubMed] [Google Scholar]
- Brin M. The oxidation of C14-pyruvate and of C14-ribose in thiamine deficient intact rats. Isr J Med Sci. 1967 Nov-Dec;3(6):792–799. [PubMed] [Google Scholar]
- Collins G. H. Glial cell changes in the brain stem of thiamine-deficient rats. Am J Pathol. 1967 May;50(5):791–814. [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- DREYFUS P. M. Clinical application of blood transketolase determinations. N Engl J Med. 1962 Sep 20;267:596–598. doi: 10.1056/NEJM196209202671204. [DOI] [PubMed] [Google Scholar]
- DREYFUS P. M. THE REGIONAL DISTRIBUTION OF TRANSKETOLASE IN THE NORMAL AND THE THIAMINE DEFICIENT NERVOUS SYSTEM. J Neuropathol Exp Neurol. 1965 Jan;24:119–129. doi: 10.1097/00005072-196501000-00011. [DOI] [PubMed] [Google Scholar]
- DREYFUS P. M. The quantitative histochemical distribution of thiamine in deficient rat brain. J Neurochem. 1961 Nov;8:139–145. doi: 10.1111/j.1471-4159.1961.tb13535.x. [DOI] [PubMed] [Google Scholar]
- Dreyfus P. M., Hauser G. The effect of thiamine deficiency on the pyruvate decarboxylase system of the central nervous system. Biochim Biophys Acta. 1965 Jun 15;104(1):78–84. doi: 10.1016/0304-4165(65)90222-9. [DOI] [PubMed] [Google Scholar]
- FRIEDE R. L., FLEMING L. M., KNOLLER M. A comparative mapping of enzymes involved in hexosemonophosphate shunt and citric acid cycle in the brain. J Neurochem. 1963 Apr;10:263–277. doi: 10.1111/j.1471-4159.1963.tb05042.x. [DOI] [PubMed] [Google Scholar]
- GUBLER C. J. Studies on the physiological functions of thiamine. I. The effects of thiamine deficiency and thiamine antagonists on the oxidation of alpha-keto acids by rat tissues. J Biol Chem. 1961 Dec;236:3112–3120. [PubMed] [Google Scholar]
- HAUGEN H. N. The determination of thiamine and thiamine phosphates in blood and tissues by the thiochrome method. Scand J Clin Lab Invest. 1961;13:50–56. doi: 10.3109/00365516109137248. [DOI] [PubMed] [Google Scholar]
- HSU J. M., CHOW B. F. Effect of thiamine deficiency on glutathione contents of erythrocytes and tissues in the rat. Proc Soc Exp Biol Med. 1960 Jun;104:178–180. doi: 10.3181/00379727-104-25771. [DOI] [PubMed] [Google Scholar]
- KOEPPE R. E., O'NEAL R. M., HAHN C. H. PYRUVATE DECARBOXYLATION IN THIAMINE DEFICIENT BRAIN. J Neurochem. 1964 Sep;11:695–699. doi: 10.1111/j.1471-4159.1964.tb06153.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H. Biochemical evidence of nutritional status. Physiol Rev. 1952 Oct;32(4):431–448. doi: 10.1152/physrev.1952.32.4.431. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewin L. M., Wei R. Microassay of thiamine and its phosphate esters after separation by paper chromatography. Anal Biochem. 1966 Jul;16(1):29–35. doi: 10.1016/0003-2697(66)90077-7. [DOI] [PubMed] [Google Scholar]
- Novello F., McLean P. The pentose phosphate pathway of glucose metabolism. Measurement of the non-oxidative reactions of the cycle. Biochem J. 1968 May;107(6):775–791. doi: 10.1042/bj1070775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'brien J. R., Peters R. A. Vitamin B(1) deficiency in the rat's brain. J Physiol. 1935 Dec 16;85(4):454–463. doi: 10.1113/jphysiol.1935.sp003332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS G. B., VICTOR M., ADAMS R. D., DAVIDSON C. S. A study of the nutritional defect in Wernicke's syndrome; the effect of a purified diet, thiamine, and other vitamins on the clinical manifestations. J Clin Invest. 1952 Oct;31(10):859–871. doi: 10.1172/JCI102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALL T. W., LEHNINGER A. L. Glutathione reductase of animal tissues. J Biol Chem. 1952 Jan;194(1):119–130. [PubMed] [Google Scholar]
- Robertson D. M., Wasan S. M., Skinner D. B. Ultrastructural features of early brain stem lesions of thiamine-deficient rats. Am J Pathol. 1968 May;52(5):1081–1097. [PMC free article] [PubMed] [Google Scholar]
- SCHENKER S., MENDELSON J. H. CEREBRAL ADENOSINE TRIPHOSPHATE IN RATS WITH AMMONIA-INDUCED COMA. Am J Physiol. 1964 May;206:1173–1176. doi: 10.1152/ajplegacy.1964.206.5.1173. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Schenker S., McCandless D. W., Brophy E., Lewis M. S. Studies on the intracerebral toxicity of ammonia. J Clin Invest. 1967 May;46(5):838–848. doi: 10.1172/JCI105583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VICTOR M., ADAMS R. D. On the etiology of the alcoholic neurologic diseases with special reference to the role of nutrition. Am J Clin Nutr. 1961 Jul-Aug;9:379–397. doi: 10.1093/ajcn/9.4.379. [DOI] [PubMed] [Google Scholar]


