Abstract
The in vivo metabolism of purified third component of complement labeled with 125-iodine (C′3-125I) was studied in normal subjects and in patients with acquired hemolytic anemias. 27 such studies were performed; in addition, three studies were performed using C′3i, the biologically inactive reaction product of C′3.
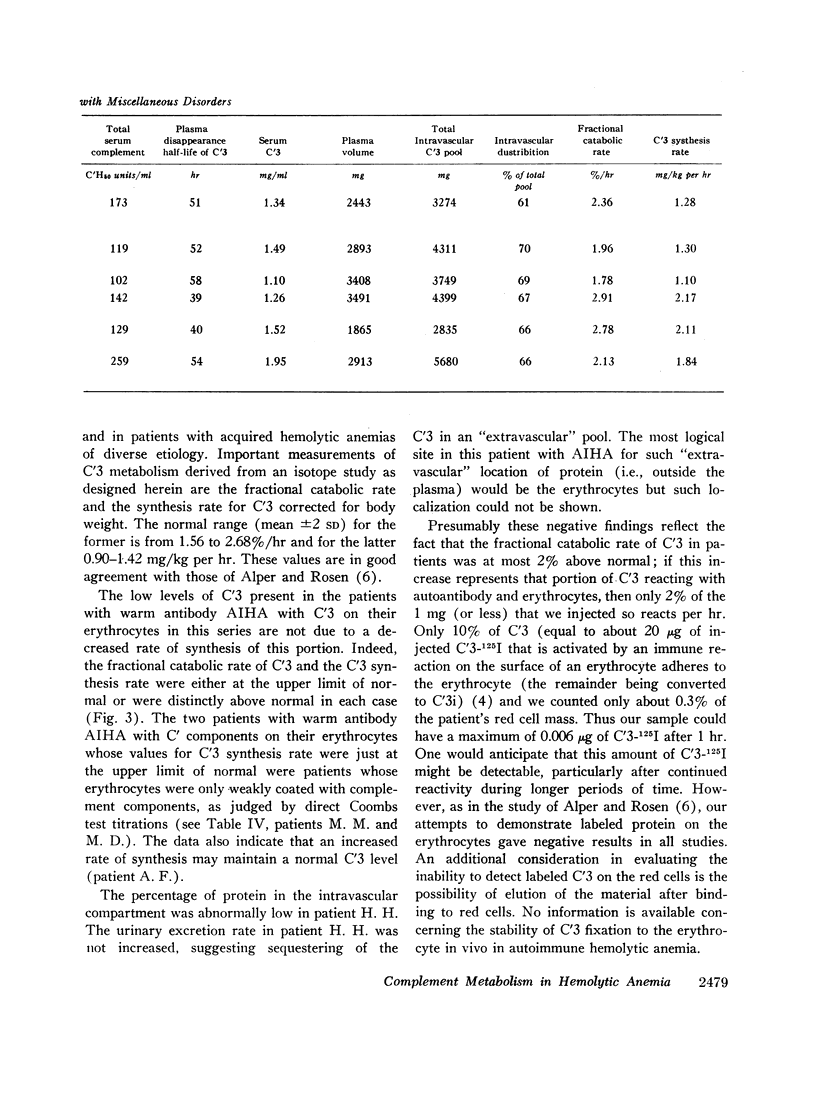
In normal subjects the mean fractional catabolic rate of C′3 was 2.12%/hr and the normal range (defined throughout as the mean ± 2 SD) was from 1.56 to 2.68. The mean percentage of C′3 that was intravascular was 66.6% and the normal range was from 51 to 83. The C′3 synthesis rate averaged 1.16 mg/kg per hr with a normal range of from 0.90 to 1.42. The mean serum concentration of C′3 was 1.43 mg/ml with a normal range of from 1.00 to 1.87.
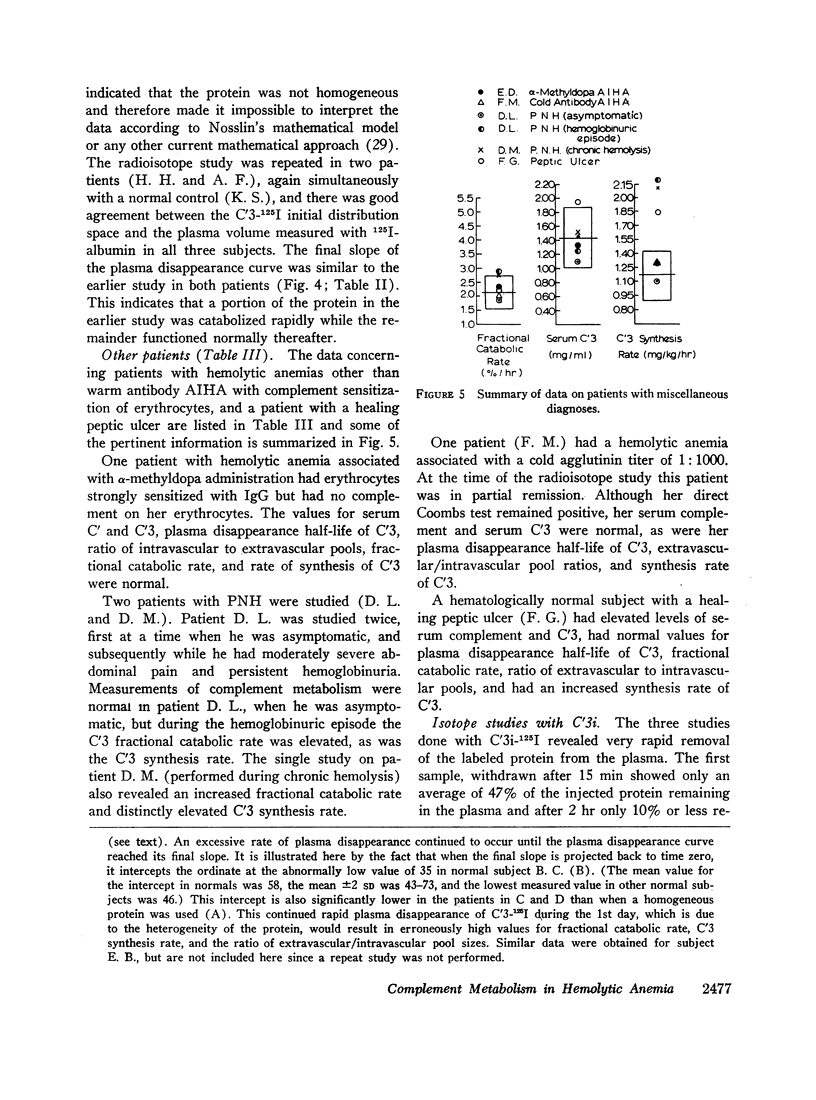
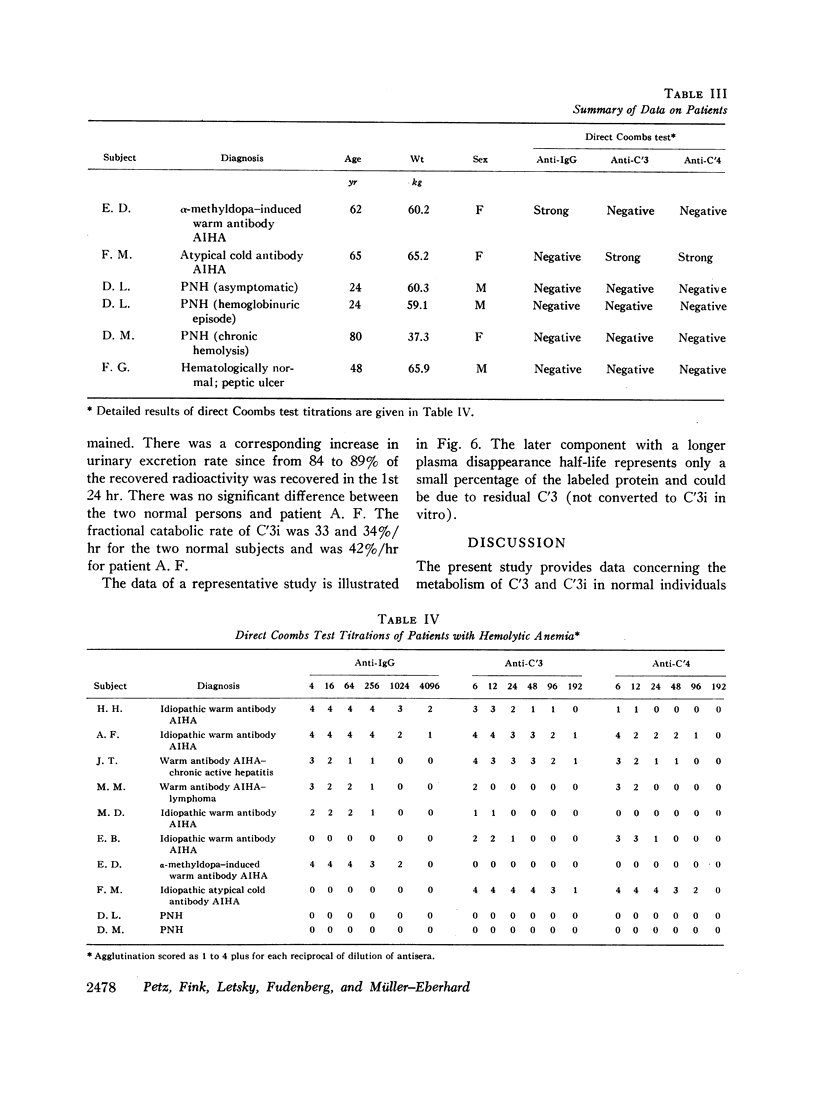
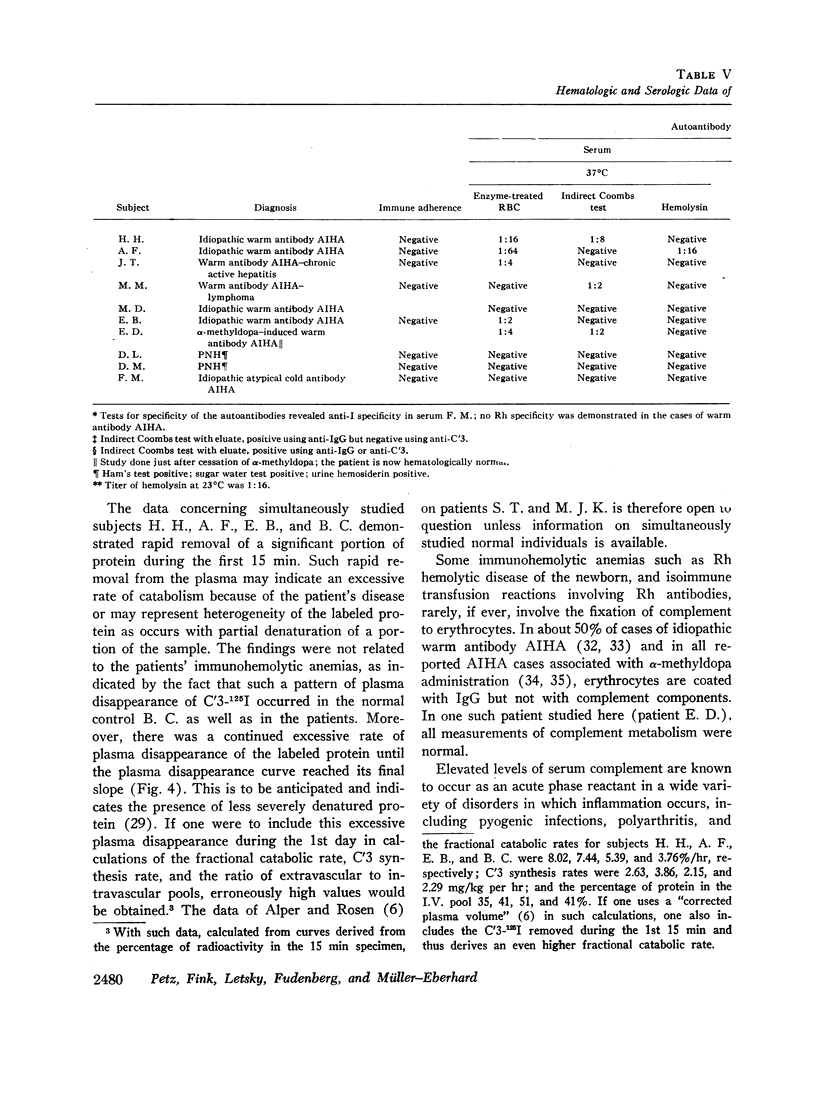
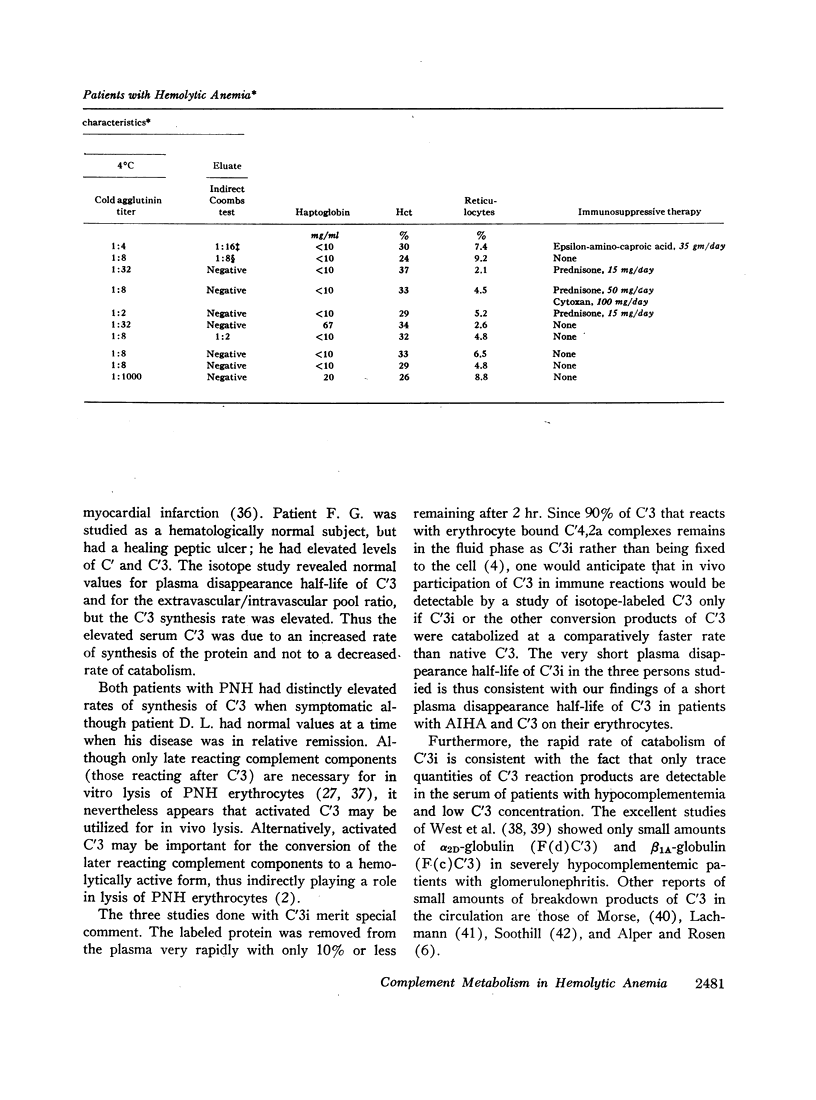
The fractional catabolic rate and synthesis rate of C′3 were at the upper limit of normal or were increased above normal in patients who had warm antibody autoimmune hemolytic anemia with complement on their erythrocytes and in patients with paroxysmal nocturnal hemoglobinuria studied during periods of active hemolysis. An increased C′3 synthesis rate was also found in one patient who was hematologically normal but had an active peptic ulcer and elevated serum concentration of C′3.
A normal fractional catabolic rate and C′3 synthesis rate were found in patients with autoimmune hemolytic anemia associated with α-methyldopa administration, atypical cold antibody autoimmune hemolytic anemia, and in paroxysmal nocturnal hemoglobinuria during an asymptomatic interval.
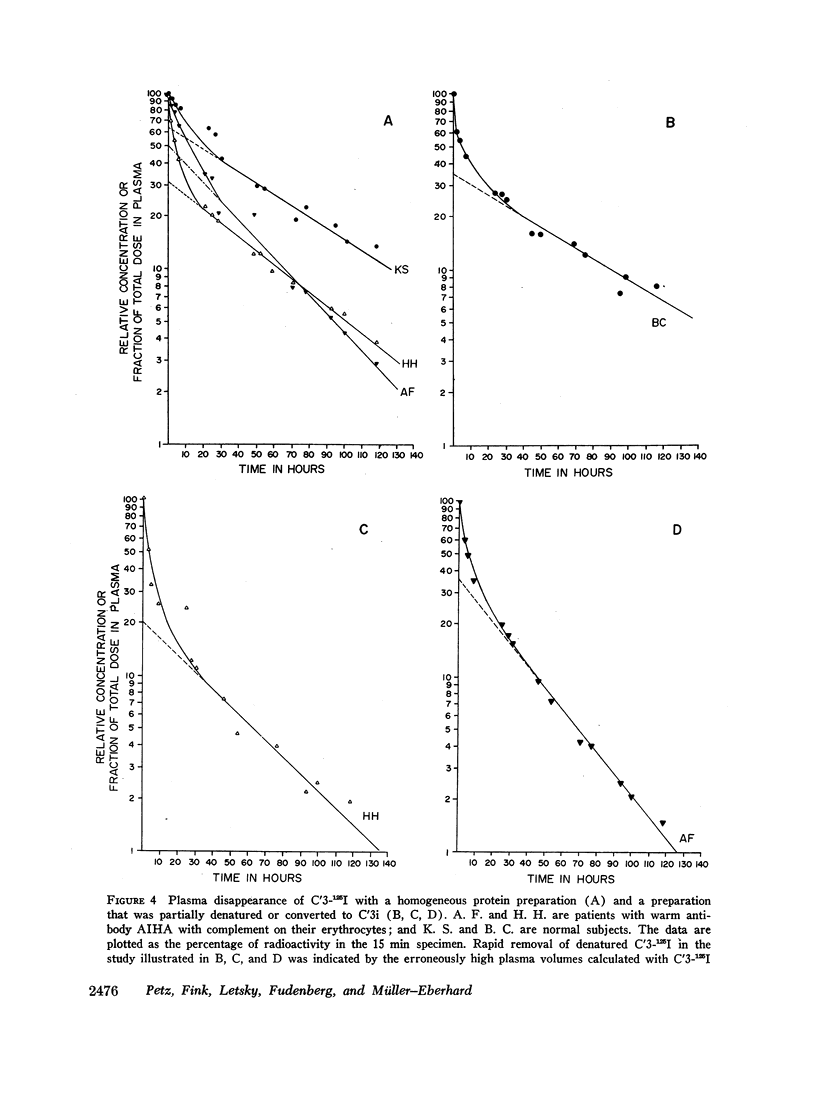
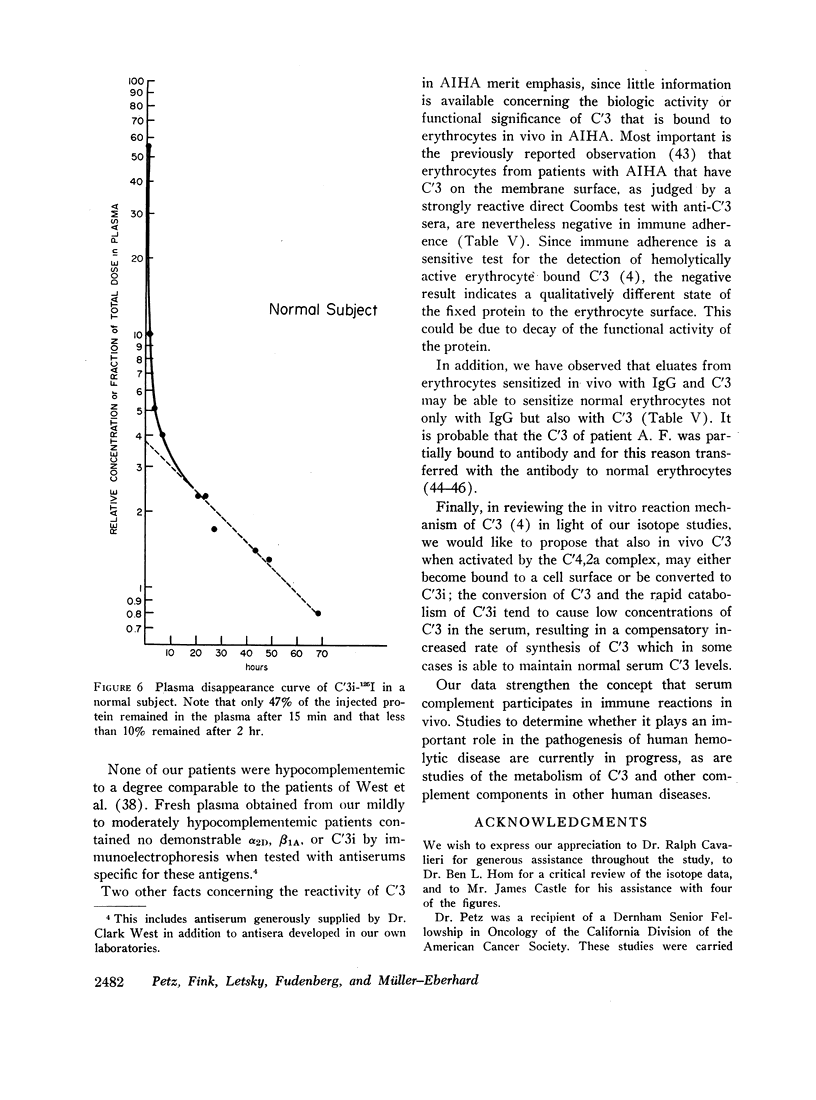
The three studies with C′3i-125I revealed a very rapid removal of the labeled protein from the plasma with less than 10% remaining after 2 hr and with a corresponding increase in urinary excretion rate of the label. The fractional catabolic rate of C′3i averaged 37%/hr.
The findings are consistent with the previously elucidated in vitro reaction mechanism of C′3 and strengthen the concept that serum complement participates in immune reactions in vivo.
Full text
PDF


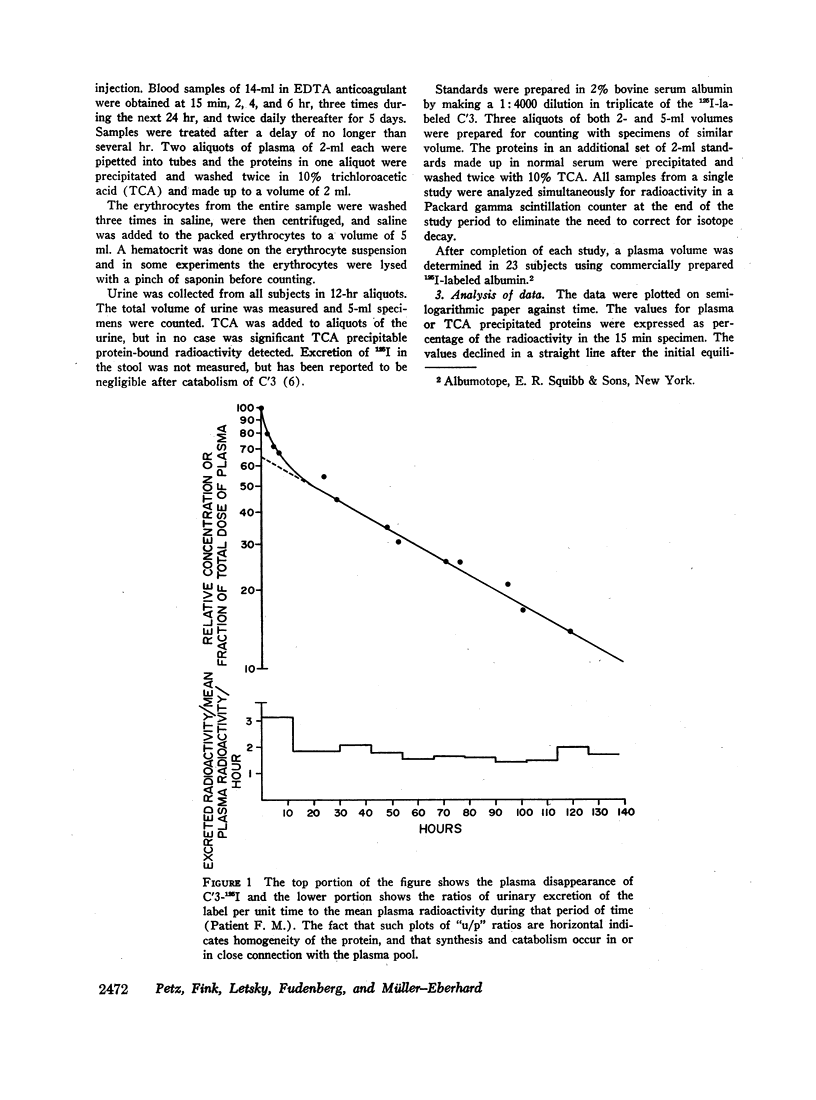
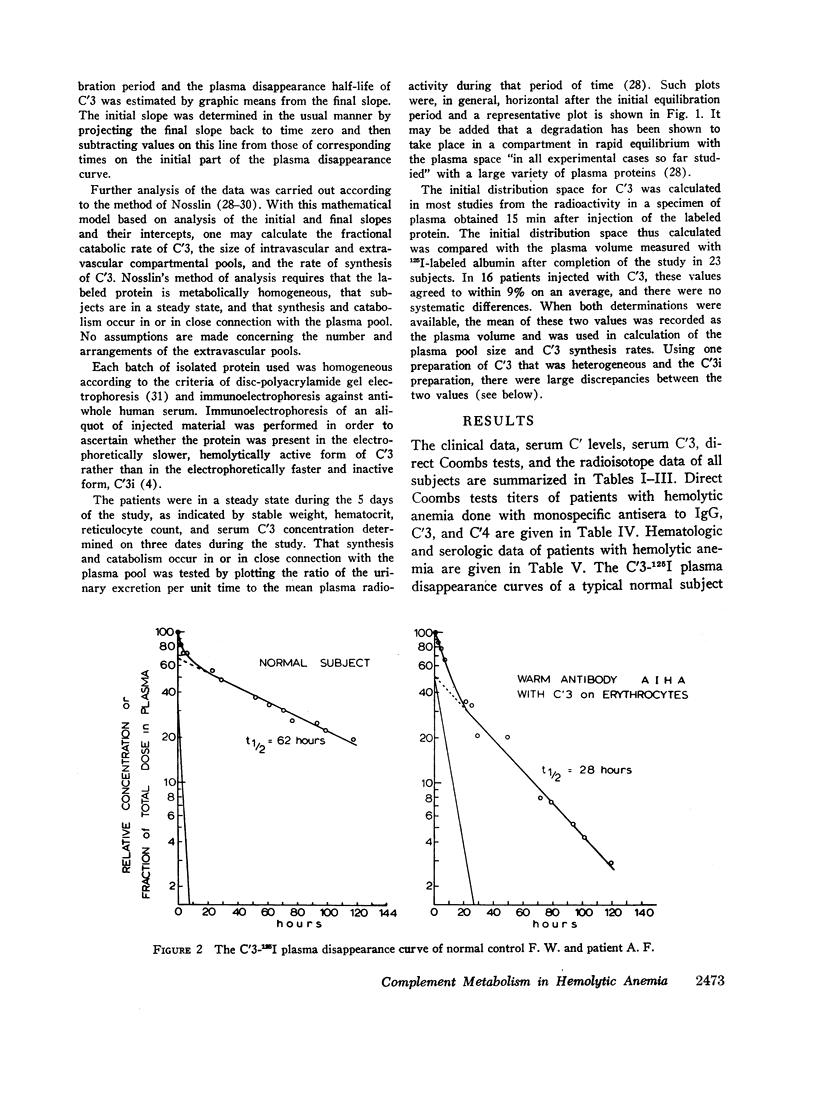
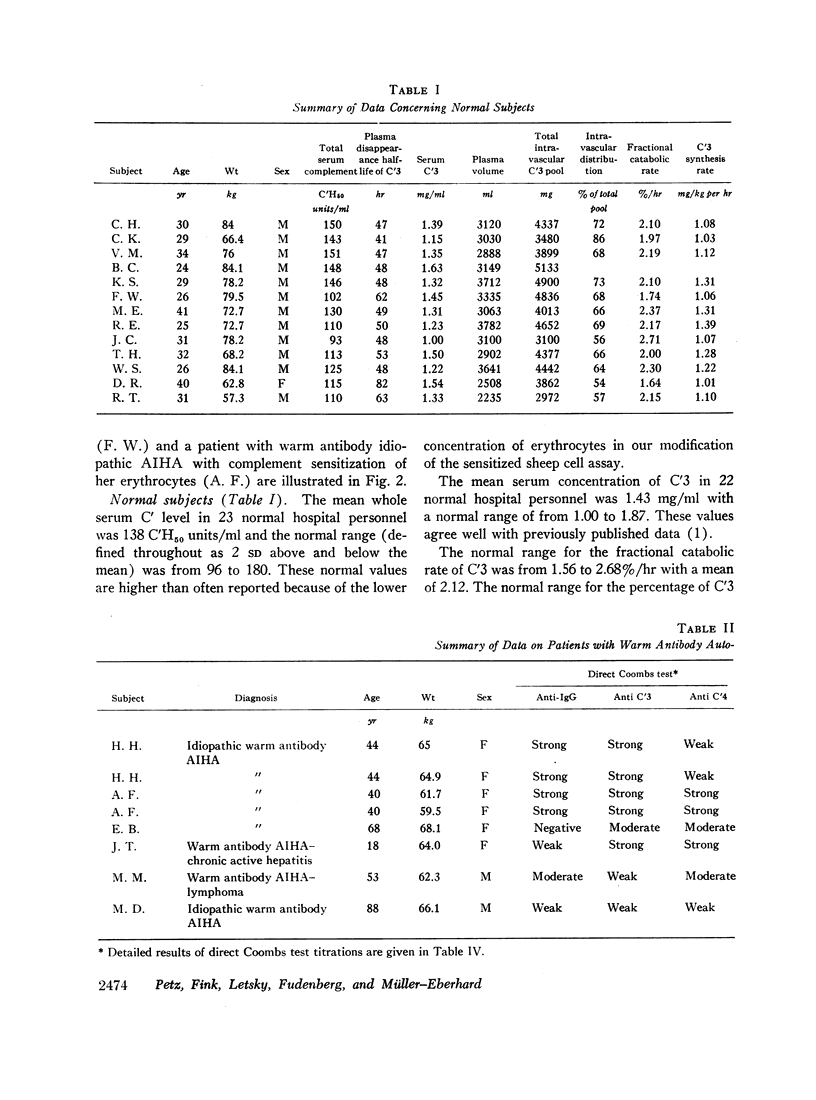
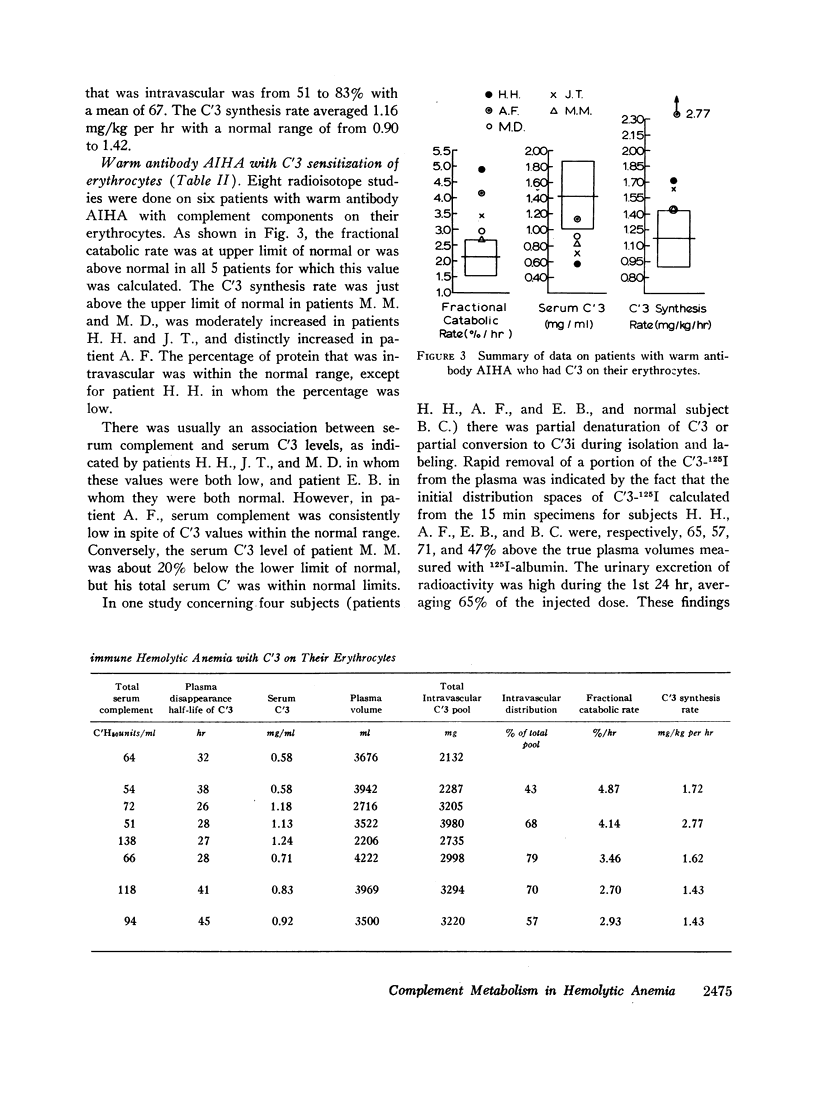


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper C. A., Levin A. S., Rosen F. S. Beta-1C-globulin: metabolism in glomerulonephritis. Science. 1966 Jul 8;153(3732):180–182. doi: 10.1126/science.153.3732.180. [DOI] [PubMed] [Google Scholar]
- Alper C. A., Rosen F. S. Alper CA, Rosen FS: Studies of the in vivo behavior of human C'3 in normal subjects and patients. J Clin Invest. 1967 Dec;46(12):2021–2034. doi: 10.1172/JCI105691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARBOE M., MUELLER-EBERHARD H. J., FUDENBERG H., POLLY M. J., MOLLISON P. L. IDENTIFICATION OF THE COMPONENTS OF COMPLEMENT PARTICIPATING IN THE ANTIGLOBULIN REACTION. Immunology. 1963 Jul;6:412–420. [PMC free article] [PubMed] [Google Scholar]
- Hartmann R. C., Jenkins D. E. The "sugar-water" test for paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1966 Jul 21;275(3):155–157. doi: 10.1056/NEJM196607212750308. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LoBuglio A. F., Jandl J. H. The nature of the alpha-methyldopa red-cell antibody. N Engl J Med. 1967 Mar 23;276(12):658–665. doi: 10.1056/NEJM196703232761203. [DOI] [PubMed] [Google Scholar]
- MORSE J. H., MULLER-EBERHARD H. J., KUNKEL H. G. Anti-nuclear factors and serum complement in systemic lupus erythematosus. Bull N Y Acad Med. 1962 Oct;38:641–651. [PMC free article] [PubMed] [Google Scholar]
- MUELLER-EBERHARD H. J., BIRO C. E. ISOLATION AND DESCRIPTION OF THE FOURTH COMPONENT OF HUMAN COMPLEMENT. J Exp Med. 1963 Sep 1;118:447–466. doi: 10.1084/jem.118.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Muller-Eberhard H. J., Nilsson U. R., Dalmasso A. P., Polley M. J., Calcott M. A. A molecular concept of immune cytolysis. Arch Pathol. 1966 Sep;82(3):205–217. [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Polley M. J., Calcott M. A. Formation and functional significance of a molecular complex derived from the second and the fourth component of human complement. J Exp Med. 1967 Feb 1;125(2):359–380. doi: 10.1084/jem.125.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllerèberhard H. J., Dalmasso A. P., Calcott M. A. The reaction mechanism of beta-1C-globulin (C'3) in immune hemolysis. J Exp Med. 1966 Jan 1;123(1):33–54. doi: 10.1084/jem.123.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILSSON U. R., MUELLER-EBERHARD H. J. ISOLATION OF BETA IF-GLOBULIN FROM HUMAN SERUM AND ITS CHARACTERIZATION AS THE FIFTH COMPONENT OF COMPLEMENT. J Exp Med. 1965 Aug 1;122:277–298. doi: 10.1084/jem.122.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OWEN J. A., BETTER F. C., HOBAN J. A simple method for the determination of serum haptoglobins. J Clin Pathol. 1960 Mar;13:163–164. doi: 10.1136/jcp.13.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBIN H. Antibody elution from red blood cells. J Clin Pathol. 1963 Jan;16:70–73. doi: 10.1136/jcp.16.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp H. J., Borsos T. Complement and Hemolysis. Science. 1963 Aug 23;141(3582):738–740. doi: 10.1126/science.141.3582.738. [DOI] [PubMed] [Google Scholar]
- Soothill J. F. Altered complement component C3A (beta-1C--beta-1A) in patients with glomerulonephritis. Clin Exp Immunol. 1967 Jan;2(1):83–92. [PMC free article] [PubMed] [Google Scholar]
- Townes A. S. Topics in clinical medicine. Complement levels in disease. Johns Hopkins Med J. 1967 May;120(5):337–343. [PubMed] [Google Scholar]
- WILLIAMS D. E., REISFELD R. A. DISC ELECTROPHORESIS IN POLYACRYLAMIDE GELS: EXTENSION TO NEW CONDITIONS OF PH AND BUFFER. Ann N Y Acad Sci. 1964 Dec 28;121:373–381. doi: 10.1111/j.1749-6632.1964.tb14210.x. [DOI] [PubMed] [Google Scholar]
- West C. D., Winter S., Forristal J., Davis N. C. Effect of ageing of serum on consumption of antibody by beta-1C-globulin determinants; evidence for circulating breakdown products in glomerulonephritis. Clin Exp Immunol. 1968 Jan;3(1):57–62. [PMC free article] [PubMed] [Google Scholar]
- West C. D., Winter S., Forristal J., McConville J. M., Davis N. C. Evidence for in vivo breakdown of beta-10-globulin in hypocomplementemic glomerulonephritis. J Clin Invest. 1967 Apr;46(4):539–548. doi: 10.1172/JCI105555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C., Davis N. C., Forristal J., Herbst J., Spitzer R. Antigenic determinants of human beta-1c and beta-1g-globulins. J Immunol. 1966 Apr;96(4):650–658. [PubMed] [Google Scholar]
- Worlledge S. M., Carstairs K. C., Dacie J. V. Autoimmune haemolytic anaemia associated with alpha-methyldopa therapy. Lancet. 1966 Jul 16;2(7455):135–139. doi: 10.1016/s0140-6736(66)92423-8. [DOI] [PubMed] [Google Scholar]
- YACHNIN S. THE HEMOLYSIS OF RED CELLS FROM PATIENTS WITH PAROXYSMAL NOCTURNAL HEMOGLOBINURIA BY PARTIALLY PURIFIED SUB-COMPONENTS OF THE THIRD COMPLEMENT COMPONENT. J Clin Invest. 1965 Sep;44:1534–1546. doi: 10.1172/JCI105260. [DOI] [PMC free article] [PubMed] [Google Scholar]


