Abstract
The mechanisms of hemoglobin precipitation into Heinz bodies and hemolytic anemia that characterize congenital Heinz body hemolytic anemia (CHBHA) were studied in patients with the unstable hemoglobins, Köln (β-98 valine → methionine) and Hammersmith (β-42 phenylalanine → serine). The cysteines in the 93rd position of the β-chains of CHBHA hemoglobins bound glutathione excessively in mixed disulfide linkage. The resulting diminished “free” GSH within the cell accelerated hexose monophosphate shunt metabolism. The unique precipitability of CHBHA hemoglobins when heated at 50°C could be induced in normal hemoglobin A by artificially blockading its sulfhydryl groups with paramercuribenzoate (PMB).
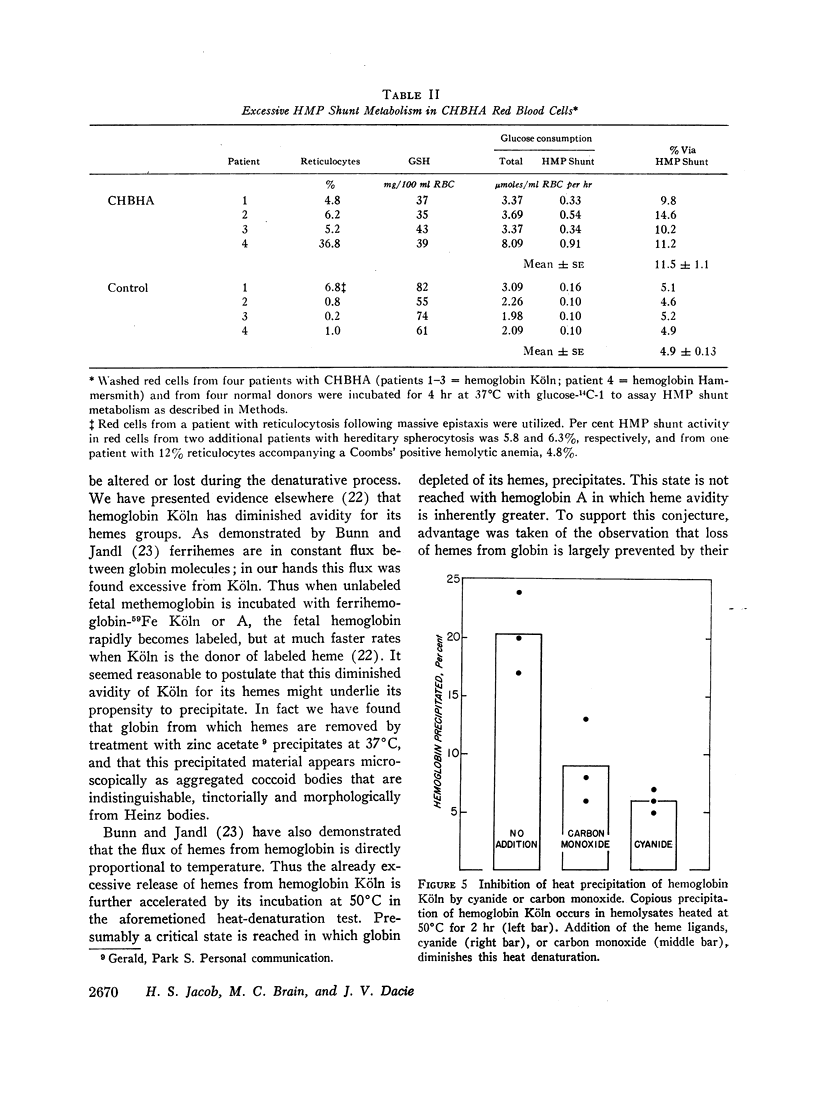
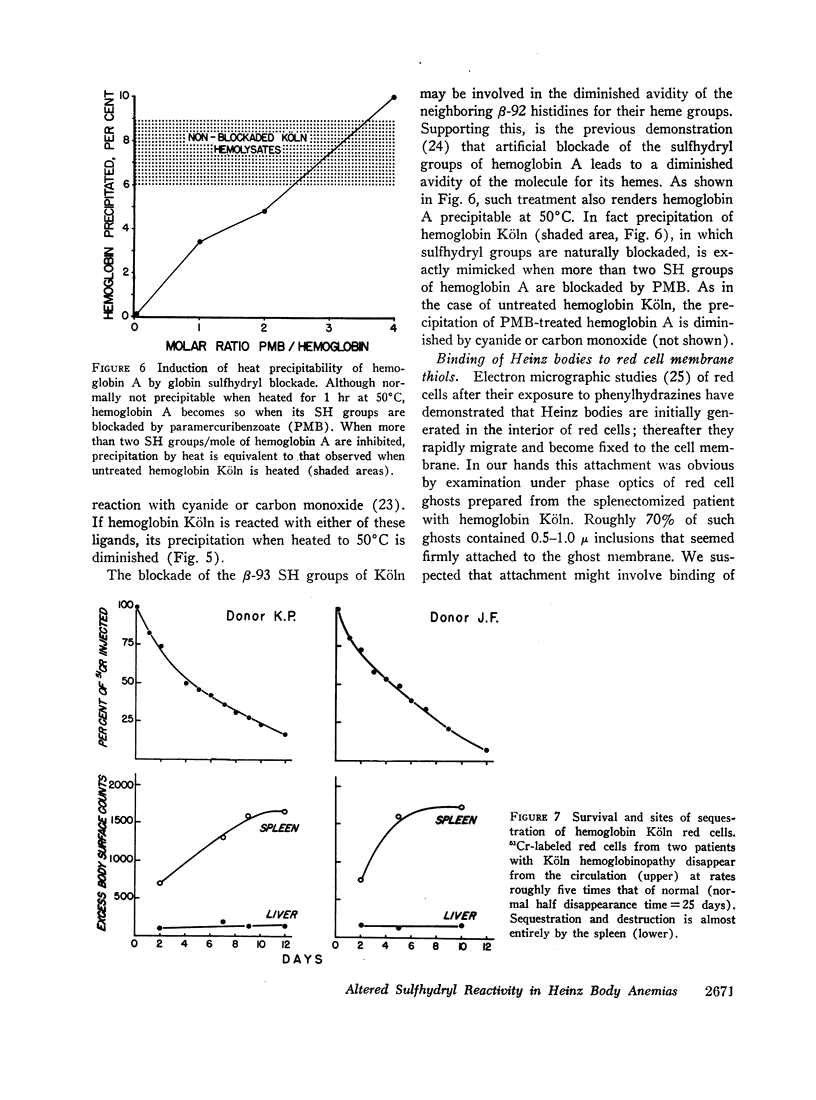
Reflecting the previously reported excessive flux of hemes from hemoglobin Köln, the expected heme/globin ratio in this hemoglobin was reduced by 30%. The further increment in heme loss that occurs with heat (50°C) underlies the unique heat precipitability of CHBHA hemoglobins; it was retarded if detachment of heme was inhibited by cyanide or carbon monoxide.
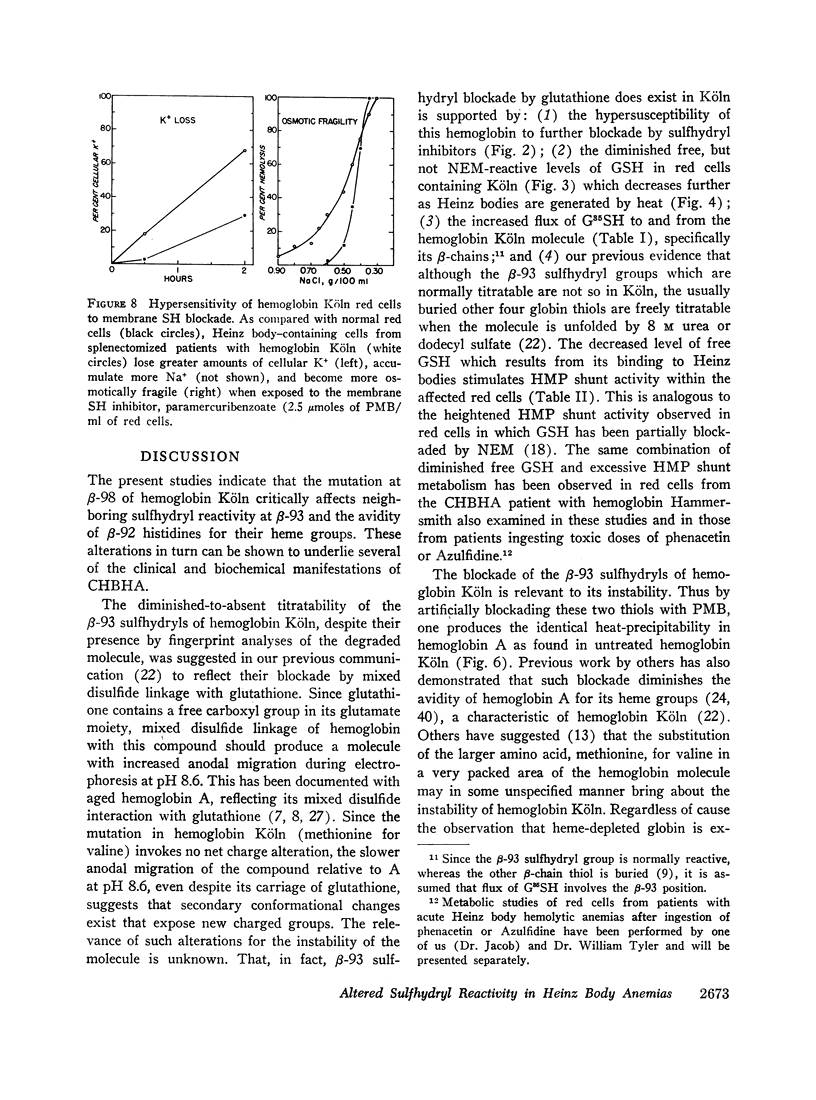
Heinz bodies were attached to red cell membrane thiol groups presumably through mixed disulfide bonds, being released by mercaptoethanol. Binding of hemoglobin Köln-59Fe to red cell ghosts, which was markedly enhanced when Heinz bodies were generated at 50°C, was inhibited if membrane thiols were preblockaded by PMB. The depletion of membrane thiols by their reaction with Heinz bodies rendered CHBHA red cells hypersusceptible to membrane sulfhydryl inhibitors, as manifested by inordinate cation leakage, osmotic fragility, and autohemolysis.
We conclude that both cellular and membrane thiols bind β-93 sulfhydryls of CHBHA hemoglobins as mixed disulfides. Concomitantly, heme avidity to β-92 lessens, suggesting that degradation of the resulting excessively freed heme may produce the pigmented dipyrroluria of this syndrome. Heinz bodies, reflecting the heightend precipitability of heme-deficient globin, attach to, thereby depleting, membrane sulfhydryl groups. This, as shown previously, could underlie the hemolytic anemia of this syndrome by causing membrane hyperpermeability, premature splenic entrapment, and ultimately osmotic destruction of red blood cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN D. W., JANDL J. H. Oxidative hemolysis and precipitation of hemoglobin. II. Role of thiols in oxidant drug action. J Clin Invest. 1961 Mar;40:454–475. doi: 10.1172/JCI104273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley T. B., Jr, Wohl R. C., Rieder R. F. Hemoglobin Gun Hill: deletion of five amino acid residues and impaired heme-globin binding. Science. 1967 Sep 29;157(3796):1581–1583. doi: 10.1126/science.157.3796.1581. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Jandl J. H. Exchange of heme among hemoglobin molecules. Proc Natl Acad Sci U S A. 1966 Sep;56(3):974–978. doi: 10.1073/pnas.56.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Jandl J. H. Exchange of heme among hemoglobins and between hemoglobin and albumin. J Biol Chem. 1968 Feb 10;243(3):465–475. [PubMed] [Google Scholar]
- CARLIN H., HECHTER O. The disulfide-sulfhydryl interchange as a mechanism of insulin action. J Biol Chem. 1962 Apr;237:1371–1372. [PubMed] [Google Scholar]
- Carrell R. W., Lehmann H., Hutchison H. E. Haemoglobin Köln (beta-98 valine--methionine): an unstable protein causing inclusion-body anaemia. Nature. 1966 May 28;210(5039):915–916. doi: 10.1038/210915a0. [DOI] [PubMed] [Google Scholar]
- DACIE J. V., GRIMES A. J., MEISLER A., STEINGOLD L., HEMSTED E. H., BEAVEN G. H., WHITE J. C. HEREDITARY HEINZ-BODY ANAEMIA. A REPORT OF STUDIES ON FIVE PATIENTS WITH MILD ANAEMIA. Br J Haematol. 1964 Jul;10:388–402. doi: 10.1111/j.1365-2141.1964.tb00715.x. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dacie J. V., Shinton N. K., Gaffney P. J., Jr, Lehmann H. Haemoglobin Hammersmith (beta-42 (CDI) Phe replaced by ser). Nature. 1967 Nov 18;216(5116):663–665. doi: 10.1038/216663a0. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- FRICK P. G., HITZIG W. H., BETKE K. Hemoglobin Zurich. I. A new hemoglobin anomaly associated with acute hemolytic episodes with inclusion bodies after sulfonamide therapy. Blood. 1962 Sep;20:261–271. [PubMed] [Google Scholar]
- GRIMES A. J., MEISLER A., DACIE J. V. CONGENITAL HEINZ-BODY ANAEMIA. FURTHER EVIDENCE ON THE CAUSE OF HEINZ-BODY PRODUCTION IN RED CELLS. Br J Haematol. 1964 Jul;10:281–290. doi: 10.1111/j.1365-2141.1964.tb00704.x. [DOI] [PubMed] [Google Scholar]
- GUIDOTTI G., KONIGSBERG W. THE CHARACTERIZATION OF MODIFIED HUMAN HEMOGLOBIN. I. REACTION WITH IODOACETAMIDE AND N-ETHYLMALEIMIDE. J Biol Chem. 1964 May;239:1474–1484. [PubMed] [Google Scholar]
- Huishman T. H., Dozy A. M., Horton B. F., Nechtman C. M. Studies on the heterogeneity of hemoglobin. X. The nature of various minor hemoglobin components produced in human red blood cell hemolysates on aging. J Lab Clin Med. 1966 Mar;67(3):355–373. [PubMed] [Google Scholar]
- JACOB H. S., INGBAR S. H., JANDL J. H. OXIDATIVE HEMOLYSIS AND ERYTHROCYTE METABOLISM IN HEREDITARY ACATALASIA. J Clin Invest. 1965 Jul;44:1187–1199. doi: 10.1172/JCI105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. I. Mechanism of hemolysis. J Clin Invest. 1962 Apr;41:779–792. doi: 10.1172/JCI104536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. II. Studies in vivo. J Clin Invest. 1962 Jul;41:1514–1523. doi: 10.1172/JCI104607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. INCREASED CELL MEMBRANE PERMEABILITY IN THE PATHOGENESIS OF HEREDITARY SPHEROCYTOSIS. J Clin Invest. 1964 Aug;43:1704–1720. doi: 10.1172/JCI105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., ENGLE L. K., ALLEN D. W. Oxidative hemolysis and precipitation of hemoglobin. I. Heinz body anemias as an acceleration of red cell aging. J Clin Invest. 1960 Dec;39:1818–1836. doi: 10.1172/JCI104206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOSEPHSON A. M., WEINSTEIN H. G., YAKULIS V. J., SINGER L., HELLER P. A new variant of hemoglobin M disease: hemoglobin M-Chicago. J Lab Clin Med. 1962 Jun;59:918–925. [PubMed] [Google Scholar]
- Jacob H. S., Brain M. C., Dacie J. V., Carrell R. W., Lehmann H. Abnormal haem binding and globin SH group blockade in unstable haemoglobins. Nature. 1968 Jun 29;218(5148):1214–1217. doi: 10.1038/2181214a0. [DOI] [PubMed] [Google Scholar]
- Jacob H. S., Jandl J. H. Effects of sulfhydryl inhibition on red blood cells. 3. Glutathione in the regulation of the hexose monophosphate pathway. J Biol Chem. 1966 Sep 25;241(18):4243–4250. [PubMed] [Google Scholar]
- LANGE R. D., AKEROYD J. H. Congenital hemolytic anemia with abnormal pigment metabolism and red cell inclusion bodies: a new clinical syndrome. Blood. 1958 Oct;13(10):950–958. [PubMed] [Google Scholar]
- Miwa S., Kato H., Saito M., Chiba A., Irisawa K., Ohyama H. Congenital hemolytic anemia with abnormal pigment metabolism and red cell inclusion bodies. Report of a case and review of literature. Nihon Ketsueki Gakkai Zasshi. 1965 Oct;28(5):593–608. [PubMed] [Google Scholar]
- Nathan D. G., Gunn R. B. Thalassemia: the consequences of unbalanced hemoglobin synthesis. Am J Med. 1966 Nov;41(5):815–830. doi: 10.1016/0002-9343(66)90039-8. [DOI] [PubMed] [Google Scholar]
- RIFKIND R. A., DANON D. HEINZ BODY ANEMIA--AN ULTRASTRUCTURAL STUDY. I. HEINZ BODY FORMATION. Blood. 1965 Jun;25:885–896. [PubMed] [Google Scholar]
- RIGAS D. A., KOLER R. D. Erythrocyte enzymes and reduced glutathione (GSH) in hemoglobin H disease: relation to cell age and denaturation of hemoglobin H. J Lab Clin Med. 1961 Sep;58:417–424. [PubMed] [Google Scholar]
- Rasmussen H., Schwartz I. L., Schoessler M. A., Hochster G. STUDIES ON THE MECHANISM OF ACTION OF VASOPRESSIN. Proc Natl Acad Sci U S A. 1960 Oct;46(10):1278–1287. doi: 10.1073/pnas.46.10.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind R. A. Heinz body anemia: an ultrastructural study. II. Red cell sequestration and destruction. Blood. 1965 Oct;26(4):433–448. [PubMed] [Google Scholar]
- SHIBATA S., IUCHI I., MIYAJI T., UEDA S., TAKEDA I. HEMOLYTIC DISEASE ASSOCIATED WITH THE PRODUCTION OF ABNORMAL HEMOGLOBIN AND INTRAERYTHROCYTIC HEINZ BODIES. Nihon Ketsueki Gakkai Zasshi. 1963 Apr;26:164–173. [PubMed] [Google Scholar]
- SNYDER A. L., SCHMID R. THE CONVERSION OF HEMATIN TO BILE PIGMENT IN THE RAT. J Lab Clin Med. 1965 May;65:817–824. [PubMed] [Google Scholar]


