Abstract
High blood pressure increases the risks of stroke, dementia, and neurocognitive dysfunction. Although aerobic exercise and dietary modifications have been shown to reduce blood pressure, no randomized trials have examined the effects of aerobic exercise combined with dietary modification on neurocognitive functioning in individuals with high blood pressure (ie, prehypertension and stage 1 hypertension). As part of a larger investigation, 124 participants with elevated blood pressure (systolic blood pressure 130 to 159 mm Hg or diastolic blood pressure 85 to 99 mm Hg) who were sedentary and overweight or obese (body mass index: 25 to 40 kg/m2) were randomized to the Dietary Approaches to Stop Hypertension (DASH) diet alone, DASH combined with a behavioral weight management program including exercise and caloric restriction, or a usual diet control group. Participants completed a battery of neurocognitive tests of executive function-memory-learning and psychomotor speed at baseline and again after the 4-month intervention. Participants on the DASH diet combined with a behavioral weight management program exhibited greater improvements in executive function-memory-learning (Cohen’s D=0.562; P=0.008) and psychomotor speed (Cohen’s D=0.480; P=0.023), and DASH diet alone participants exhibited better psychomotor speed (Cohen’s D=0.440; P=0.036) compared with the usual diet control. Neurocognitive improvements appeared to be mediated by increased aerobic fitness and weight loss. Also, participants with greater intima-medial thickness and higher systolic blood pressure showed greater improvements in executive function-memory-learning in the group on the DASH diet combined with a behavioral weight management program. In conclusion, combining aerobic exercise with the DASH diet and caloric restriction improves neurocognitive function among sedentary and overweight/obese individuals with prehypertension and hypertension.
Keywords: hypertension, exercise, nutrition, clinical trial, neurocognition
It is estimated that 1 billion men and women worldwide1,2 experience prehypertension or hypertension. Blood pressure (BP) increases with age, with high BP (HBP) affecting 50% of adults aged ≥60 years3 and a lifetime prevalence of 90%.4 HBP is associated with increased risk for Alzheimer’s disease (AD),5 mild cognitive impairment,6 and vascular dementia,7 and the World Health Organization estimates that suboptimal BP (>115 mm Hg systolic BP [SBP]) is responsible for 62% of cerebrovascular disease.8 Furthermore, HBP is associated with subtle neurocognitive deficits,9–11 which may be potentiated by obesity12–14 and may further increase the risk of dementia.15 Although pharmacological treatments for HBP have been shown to effectively lower BP,1 a recent meta-analysis concluded that antihypertensive medications did not reliably reduce the incidence of dementia.16
Lifestyle modifications, including diet and exercise, have been shown to reduce BP17 and weight,18 improve neurocognitive function,19,20 and may protect against incident AD.21,22 However, to our knowledge, no randomized clinical trial has examined the combined effects of dietary modification and aerobic exercise on neurocognitive function among overweight individuals with HBP. Because previous randomized trials have demonstrated that aerobic exercise improves neurocognitive functioning,19 and recent observational studies have shown that dietary habits also may benefit neurocognition,21,22 we hypothesized that aerobic exercise combined with dietary modification would improve neurocognitive function and that diet alone also would be associated with improvement in neurocognition compared with a typical American diet without exercise or weight loss.
Methods
Participants
A total of 124 overweight (body mass index [BMI]: 25 to 40 kg/m2) men (N=45) and women (N=79) with HBP (SBP 130 to 159 mm Hg or diastolic BP [DBP] 85 to 99 mm Hg) who were enrolled in the Exercise and Nutrition Interventions for Cardiovascular Health (ENCORE) Study served as participants. The details of patient recruitment are described elsewhere.23 In brief, participants consisted of healthy but overweight (BMI: 25 to 40) and sedentary adults with HBP. Participants were eligible if they were not taking antihypertensive medication and had HBP on the basis of the average of 4 screening visits.
Assessment Procedures
Neurocognitive Measures
Participants completed a battery of neurocognitive tests to assess performance in the domains of executive function-memory-learning (EFML) and psychomotor speed before and after a 4-month treatment program (Table 1). Neurocognitive tests were selected for the availability of multiple test versions, well-established psychometric properties, and accepted clinical use.
Table 1.
Neurocognitive Test Descriptions
| Test | Description |
|---|---|
| EFML | |
| Trail making test B-A35 | Participants draw consecutive lines between numbers (part A: numbers 1 through 25) and then numbers and letters (part B: alternating between numbers [1 through 13] and letters [A through L]) as fast as possible. Score is the total time in seconds to complete section B minus the time to complete section A. |
| Stroop Interference36 | Test consists of word (W), color (C), and color-word (CW) sections, during which participants read aloud as many items as possible from a 100-item list during a 45-s time period. The CW section consists of color names in which the names of the items differ from the colors in which they are printed (eg, the word “RED” is printed in blue ink). The stroop interference score was used in the current analysis {CW−[(W*C)/(W+C)]}. |
| Digit span37 | Participants are asked to recall a list of consecutive numbers over 2 trials that become sequentially longer as they answer more trials correctly. On the first trial, numbers are recalled in the same order of presentation, and in the second trial numbers are recalled in reverse order. |
| Verbal fluency test (animal naming)38 | Participants generate as many words as possible from a semantic category (ie, animals) as quickly as possible in a 60-s time period. |
| Verbal paired associates37 | Participants learn 8 word pairs, half of which are highly associated (eg, baby-cries) and half of which are not associated (eg, school-grocery), across 4 consecutive trials. During each trial, participants are read the list aloud and then presented with 1 word from each pair and asked to recall the corresponding word. Score is the total number of correctly recalled word pairs. |
| Controlled oral Word Association Test38 | Participants generate as many words as possible beginning within 3 phonemic categories during a 60-s time period for each category. |
| Psychomotor speed | |
| Ruff 2 and 7 test (total correct)39 | Participants read through lines of letters (automatic detection) and numbers (controlled detection) and cross out specific targets (ie, digits 2 and 7) while ignoring other items. Score is the total number of correct cancellations in a 5-min time period. |
| Digit symbol substitution test37 | Participants are given a code table displaying the correspondence between pairs of digits (from 1 to 9) and symbols and are asked to draw as many symbols copied from the code table in a 90-s time limit. |
Clinic BP and Medical History
Clinic BP measurements were measured using standardized procedures.23 Medical history information was obtained from a standard medical examination. History of depression was assessed by self-report.
Cardiovascular Health Measures
Aerobic capacity was assessed by symptom-limited exercise treadmill testing using a modified Balke protocol.24 Vascular health was estimated using the Framingham Stroke Risk Profile (FSRP), which is a risk assessment tool used to assess the 10-year incidence of stroke,25 and was determined at the time of a baseline physical examination. Because age served as a covariate in our final analyses, it was not included in calculating FSRP scores. Carotid artery intima-medial thickness (IMT) of the left and right common carotid arteries was measured using high-resolution B-mode ultrasound. Longitudinal images spanning 2 cm proximal to the carotid bulb were acquired, and far wall IMT was measured over a 1-cm segment using edge detection software. Body weight and height were measured using a standard balance scale. Left ventricular mass index was assessed at end diastole from 2D echocardiogram images, and left ventricular mass was calculated as left ventricular mass/height2.7 to adjust for variations in heart size because of differences in body size. Body composition and fat distribution were assessed by dual energy absorptiometry, which was used to estimate abdominal adiposity. All of the neurocognitive and cardiovascular assessments were conducted by research staff blinded to treatment assignment.
Interventions
The Exercise and Nutrition Interventions for Cardiovascular Health Study was designed to examine the effects of the DASH diet in reducing BP and improving cardiovascular biomarkers among individuals with HBP in an outpatient setting.23 To determine whether the DASH diet alone or when combined with exercise and caloric restriction would reduce BP, participants were randomly assigned to 1 of 3 groups: DASH diet alone (DASH-A), DASH+weight management (DASH+WM), or to usual care control (UC). Participants in the DASH-A condition received instruction in modifying the content of their diet to meet DASH guidelines but did not exercise or lose weight. The DASH+WM group also received the DASH dietary intervention and also participated in a behavioral weight management program consisting of supervised aerobic exercise and behavior modification. Participants engaged in a 30-minute supervised aerobic exercise program 3 times per week and received weekly counseling sessions delivered in a group setting focused on teaching behavioral strategies for weight loss. Patients in the UC group maintained their usual dietary habits and did not lose weight or exercise for 4 months.
Statistical Analysis
Data Reduction
To minimize the number of statistical tests in examining the effects of treatment on neurocognitive performance, we used principle axis factor analysis to combine information from the 8 individual neuro-psychological tests into 2 cognitive domain scores using a Scree test, a minimum loading of 0.40, and a Promax rotation. On the basis of these results, we created unit-weighted composite scores (eg, z scores) by standardizing the individual neuropsychological test scores and then summing all of the subtests relevant to a given domain. This score was then scaled using the pretreatment sample SD for both pretreatment and posttreatment test composite scores, providing an index of change in neurocognitive performance in standardized effect sizes (ESs). These composites were then used as the criterion variable in the linear models described below. The use of composites has been shown to reduce type I error rates in studies using multiple outcomes.
Primary Analyses
Separate analyses were conducted to examine the following: (1) the effects of treatment on neurocognitive function; (2) potential moderators of treatment outcome; and (3) mediators of any observed treatment effects. Separate general linear models were used to examine the effects of treatment on each cognitive domain. Each model included group assignment as the primary predictor with posttreatment cognitive performance (the linear composites derived from the factor analysis) as the outcome variable. Models also included the corresponding pretreatment cognitive performance composite, age, years of education, IMT, FSRP, and abdominal adiposity as adjustment variables. All of the participants with pretreatment neurocognitive data were included in analyses, regardless of their adherence to the study protocol. In the few instances (n=4) when posttreatment values were not available for analysis, pretreatment values were used. Within each model, planned contrasts were used to compare the DASH+WM group with UC controls and the DASH-A group with UC. We hypothesized that the DASH+WM and DASH-A groups would each exhibit significant improvements in neurocognition relative to UC participants. To examine treatment mediation, 2 dummy variables were created corresponding with these planned contrasts. To provide an interpretable measure of the clinical significance of any observed treatment effects, we used available standardized regression-based modeling strategies26,27 to estimate changes in predicted age using pretreatment and then posttreatment neurocognitive performance, holding education and demographic factors constant.
Mediation was established if the regression coefficients for these dummy variables were substantially reduced after the inclusion of the mediating variable. To provide a statistical test for mediation, we used a Sobel test in which SEs were estimated using bootstrap resampling techniques.28 On the basis of previous work,29–31 we examined 3 cardiovascular mediators of neurocognitive improvements: peak exercise maximal oxygen uptake, SBP, and weight. Treatment moderation was assessed by examining the interaction term between pretreatment characteristics and treatment group assignment. Three pretreatment health indices were examined a priori as possible moderators: SBP, FSRP, and IMT.
Model Assumptions and Power
Model assumptions of additivity, linearity, and distribution of residuals were evaluated and found to be adequate before analysis. Regression coefficients for continuous predictor variables were scaled using the interquartile range of the predictor variable. The design and sample size were based on the primary outcomes.23 The present sample provided 80% power to detect a treatment difference of an ≈0.32 SD between groups on any given neurocognitive outcome at an α of 0.05.
Results
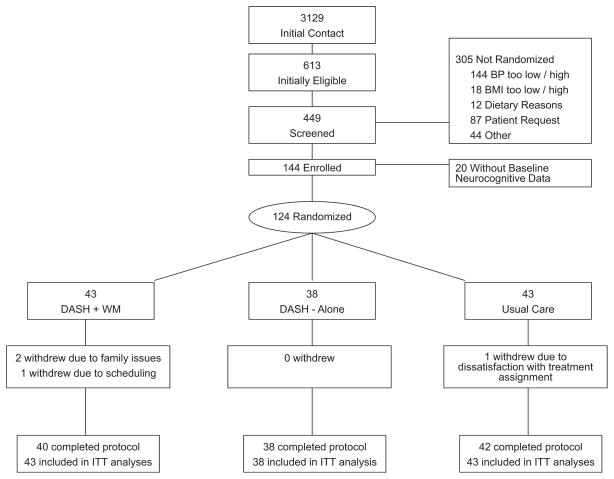
Neurocognitive data were obtained from 124 (79 women and 45 men) of the original 144 participants (86%; Figure 1). Four participants with baseline neurocognitive data were not available for testing at posttreatment: 3 participants dropped out (2 from DASH+WM and 1 from UC), and 1 participant (from DASH+WM) did not complete neurocognitive posttreatment assessments, but completed all other posttreatment assessments. Participants were generally middle aged, white (61%) or black (38%), had mildly to moderately elevated SBP and DBP (mean SBP: 138.3, SD=8.4; mean DBP: 86.1, SD=6.5), and were overweight (mean BMI: 32.8, SD=3.8; Table 2). No participants reported a history of stroke, whereas 4 participants reported a diagnosis of attention deficit hyperactivity disorder, and 5 reported a history of major depression.
Figure 1.
Flowchart of study participants.
Table 2.
Background Characteristics of Sample
| Variable | Treatment Group |
|||
|---|---|---|---|---|
| DASH+WM (n=43) | DASH-A (n=38) | Control (n=38) | Full Cohort (n=124) | |
| Men, n (%) | 15 (35) | 15 (39) | 15 (35) | 45 (36) |
| Whites, n (%) | 29 (74) | 17 (47) | 22 (54) | 68 (59) |
| Age, y | 52.9 (10.4) | 52.3 (9.5) | 51.7 (9.0) | 52.3 (9.6) |
| College degree, n (%) | 27 (63) | 23 (61) | 18 (42) | 68 (55) |
| IMT, mm | 0.68 (0.17) | 0.70 (0.11) | 0.71 (0.13) | 0.70 (0.14) |
| FSRP | 6.0 (2.6) | 6.2 (3.2) | 5.8 (2.5) | 6.0 (2.8) |
| Clinic SBP, mm Hg | 138.6 (8.1) | 137.5 (8.5) | 138.6 (8.7) | 138.3 (8.4) |
| Clinic DBP, mm Hg | 85.4 (7.2) | 87.2 (6.4) | 85.7 (5.9) | 86.1 (6.5) |
| Peak oxygen consumption, mL/kg per min | 23.7 (6.5) | 23.5 (6.7) | 23.6 (6.0) | 23.6 (6.3) |
| Weight, kg | 93.9 (14.5) | 93.9 (13.2) | 92.8 (15.2) | 93.5 (14.3) |
| BMI, kg/m2 | 32.8 (4.1) | 32.8 (3.4) | 32.7 (3.9) | 32.8 (3.8) |
Data are given as mean (SD) unless otherwise indicated. FSRP indicates Framingham Stroke Risk Profile.
Treatment Adherence
Examination of changes in the Healthy Eating Index32 showed that the DASH+WM and DASH-A groups exhibited significant dietary improvements relative to the UC group. DASH dietary class attendance was excellent, with participants attending 92% of classes in the DASH+WM and DASH-A groups. DASH+WM participants were adherent to their exercise prescription, attending 90% of their exercise sessions and exhibiting heart rate levels in their target heart rate range on 94% of random checks.
Changes in Cardiovascular Health
Participants in the DASH+WM group exhibited improved cardiovascular fitness, lower weight, and reduced BP (Table 3). As reported previously,23 both DASH+WM and DASH-A groups achieved lower clinic BPs compared with UC, and the DASH+WM group achieved greater reductions compared with the DASH-A group; as expected, the DASH+WM group also achieved greater weight loss and improved aerobic capacity relative to the other groups.
Table 3.
Changes in Weight, Aerobic Fitness, BP, and Neurocognition
| Variable | Treatment Time | Treatment Group* |
Contrast P† |
|||
|---|---|---|---|---|---|---|
| DASH+WM | DASH-A | Control | DASH+WM vs Control | DASH-A vs Control | ||
| Weight, kg | Before | 93.9 (14.5) | 93.9 (13.2) | 92.8 (15.2) | 0.841 | 0.828 |
| After | 85.0 (13.1) | 93.1 (13.6) | 92.8 (15.2) | <0.0001 | 0.505 | |
| BMI, kg/m2 | Before | 33.3 (4.4) | 33.3 (3.9) | 33.2 (4.4) | 0.943 | 0.981 |
| After | 30.0 (4.2) | 32.9 (3.4) | 33.1 (3.9) | <0.0001 | 0.483 | |
| Peak VO2, mL/kg per min | Before | 23.8 (6.6) | 23.5 (6.7) | 23.6 (6.0) | 0.884 | 0.954 |
| After | 29.0 (9.0) | 23.1 (6.7) | 22.5 (5.7) | <0.0001 | 0.482 | |
| SBP, mm Hg | Before | 138.6 (8.1) | 137.5 (8.5) | 138.6 (8.7) | 0.881 | 0.604 |
| After | 125.1 (12.3) | 127.8 (13.9) | 136.2 (15.2) | <0.001 | 0.033 | |
| DBP, mm Hg | Before | 85.4 (7.2) | 87.2 (6.4) | 85.7 (5.9) | 0.907 | 0.375 |
| After | 77.2 (9.1) | 79.5 (8.3) | 82.7 (8.2) | 0.003 | 0.033 | |
| EFML composite | Before | 0.06 (1.02) | −0.20 (1.14) | 0.14 (0.89) | 0.739 | 0.135 |
| After | 0.14 (1.09) | −0.19 (1.11) | 0.05 (0.85) | 0.014 | 0.266 | |
| Psychomotor speed composite | Before | 0.14 (0.95) | −0.21 (1.12) | 0.07 (0.97) | 0.774 | 0.206 |
| After | 0.24 (1.04) | −0.15 (1.05) | −0.07 (1.0) | 0.023 | 0.036 | |
| DSST | Before | 60.2 (8.9) | 54.2 (13.3) | 57.4 (9.7) | 0.225 | 0.184 |
| After | 62.6 (11.2) | 56.9 (12.4) | 57.9 (10.8) | 0.599 | 0.715 | |
| Ruff 2 and 7 | Before | 236.0 (53.7) | 230.8 (46.1) | 243.2 (50.5) | 0.513 | 0.278 |
| After | 250.0 (53.6) | 241.4 (48.4) | 240.2 (51.6) | 0.006 | 0.021 | |
| Trail’s B-A | Before | 44.0 (34.7) | 47.2 (37.0) | 37.6 (27.8) | 0.372 | 0.198 |
| After | 40.3 (30.1) | 45.4 (32.7) | 40.0 (28.4) | 0.026 | 0.696 | |
| VPA | Before | 17.3 (3.2) | 17.0 (3.9) | 17.2 (4.5) | 0.883 | 0.786 |
| After | 18.5 (3.3) | 17.6 (3.5) | 17.5 (4.0) | 0.045 | 0.541 | |
| COWAT | Before | 39.0 (11.7) | 37.8 (11.4) | 38.0 (10.7) | 0.689 | 0.932 |
| After | 40.4 (12.9) | 39.0 (9.9) | 40.3 (10.5) | 0.986 | 0.699 | |
| Animals | Before | 21.2 (5.6) | 20.5 (5.3) | 21.7 (5.5) | 0.650 | 0.357 |
| After | 21.3 (5.5) | 21.2 (6.0) | 21.5 (4.8) | 0.853 | 0.447 | |
| Stroop | Before | −1.4 (8.2) | −4.1 (7.3) | −0.3 (7.7) | 0.515 | 0.031 |
| After | 0.52 (9.3) | −3.3 (7.3) | −2.4 (8.2) | 0.024 | 0.523 | |
| Digit span | Before | 15.0 (3.4) | 14.8 (4.2) | 15.5 (3.3) | 0.536 | 0.389 |
| After | 15.5 (3.7) | 14.8 (3.7) | 16.5 (3.9) | 0.853 | 0.131 | |
COWAT indicates Controlled Oral Word Association Test; DSST, Digit Symbol Substitution Test; VPA, verbal paired associates.
Data are given as unadjusted mean (SD).
Contrasts for pretreatment means compare raw group means. Posttreatment values were compared after adjusting for pretreatment values, age, education, IMT, Framingham Stroke Risk Profile, and abdominal adiposity.
Neurocognitive Functioning
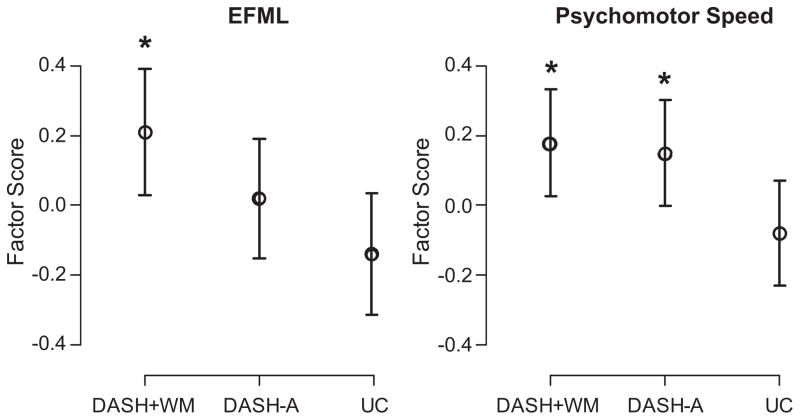
Examination of linear model revealed that the DASH+WM group demonstrated improved EFML relative to UC controls (ES: 0.21 [95% CI: 0.03 to 0.39]; Cohen’s D=0.562; P=0.008), although the DASH-A group did not improve relative to UC (ES: 0.02 [95% CI: −0.15 to 0.19]; Cohen’s D=0.260; P=0.214; Table 3 and Figure 2). Examination of changes in individual neurocognitive measures revealed that the DASH+WM group exhibited improvements in Trail Making Test B-A, Verbal Paired Associates, and the Stroop Interference Test, whereas performances on the Controlled Oral Word Association Test, Animal Naming, and the Digit Span were unchanged (for Figure displaying treatment effects on individual subtests please see the online Data Supplement at http://hyper.ahajournals.org). Using available standardized regression-based models, the observed improvements in the DASH+WM group were comparable to a 14.6-year improvement in predicted age for Trail Making Test B-A performance and a 6.1-year improvement for Stroop Interference Test performance. In contrast, the UC group’s performance was comparable to a 9.4-year poorer performance for Trail Making Test B-A and an 11.7-year poorer Stroop Interference Test performance. No available standardized regression-based precedents were available to perform these analyses for Verbal Paired Associates. In exploratory analyses requested by an anonymous reviewer, the DASH+WM and DASH-A groups showed similar improvements in EFML when compared directly (P=0.130).
Figure 2.
Posttreatment performance in EFML and psychomotor speed composites adjusted for baseline performance, age, education, IMT, Framingham Stroke Risk Profile, and abdominal adiposity. *Significantly different from control group at P=0.05. Error bars represent SEs.
Similar results were observed for psychomotor speed, with the DASH+WM (ES: 0.18 [95% CI: 0.02 to 0.33]; Cohen’s D=0.480; P=0.023) and DASH-A (ES: 0.15 [95% CI: 0.00 to 0.30]; Cohen’s D=0.440; P=0.036) groups exhibiting significant improvements relative to UC participants (Figure 2). Examination of changes in individual neurocognitive measures demonstrated that both the DASH+WM and DASH-A groups exhibited improvements in the Ruff 2 and 7 Test relative to UC participants. Using available standardized regression-based models, the observed improvements in the DASH+WM group were comparable to a 7.6-year improvement in automatic detection speed and an 8.7-year improvement in controlled detection speed. Improvements in the DASH-A group were comparable to an 8.3-year improvement in automatic detection speed and a 9.6-year improvement in controlled detection speed. The UC group exhibited relatively smaller improvements, exhibiting a 3.6-year improvement in controlled detection speed and a 0.6-year improvement in automatic detection speed. Neither the DASH+WM nor the DASH-A group exhibited improvements on the Digit Symbol Substitution Test. In exploratory analyses requested by an anonymous reviewer, the DASH+WM and DASH-A groups showed similar improvements in psychomotor speed when compared directly (P=0.932).
Moderation Analyses
Examination of individual differences in vascular health in response to treatment revealed an IMT-by-treatment-group interaction for EFML (P=0.033). Participants with higher IMT (eg, poorer vascular health) in the DASH+WM group exhibited larger cognitive gains compared with participants with lower IMT or participants in the UC and the DASH-A groups. Increasing baseline levels of IMT in the UC group were associated with reductions in neurocognitive performance from pretreatment to posttreatment (r=−0.31; P=0.077), whereas higher baseline IMT in the DASH+WM group was associated with neurocognitive improvements (r=0.38; P=0.030). Baseline IMT was not related to changes in neurocognition in the DASH-A group (r=−0.13; P=0.460), and treatment effects on EFML were not moderated by baseline FSRP levels (P=0.782). We also found a pretreatment-SBP-by-treatment-group interaction for SBP on EFML (P=0.020). Individuals with higher pretreatment SBP exhibited greater improvements in EFML (ES: 0.31) relative to participants with higher SBP in the DASH-A (ES: −0.18) and UC (ES: −0.03) groups. IMT (P=0.354), FSRP, (P=0.862), and SBP (P=0.118) did not moderate the effects of treatment on psychomotor speed.
Mediation Analyses
Results from our meditational analyses are presented in Table 4. Regression analyses demonstrated that the relationship between the DASH+WM group and improvements in EFML were attenuated once changes in peak oxygen volume were entered in our model (Sobel Z101=2.14; P=0.032). In contrast, there was no evidence of mediation for either weight (Sobel Z101=−0.62; P=0.533) or SBP (Sobel Z101=1.53; P=0.126) for EFML. Similarly, we found that the relationship between the DASH+WM group and improved psychomotor speed was attenuated when weight loss was included in our model (Sobel Z101=2.21; P=0.027) and approached significance for peak oxygen volume (Sobel Z101=1.72; P=0.085). Reductions in SBP did not mediate the effects of DASH+WM treatment on psychomotor speed (Sobel Z101=−0.13; P=0.894). Examination of mediators for the effects of DASH-A treatment on neurocognition showed that this effect was not attenuated after controlling for weight reduction (Sobel Z101=0.944; P=0.345), improvements in peak oxygen volume (Sobel Z101=0.72; P=0.475), or reductions in SBP (Sobel Z101=−0.13; P=0.894).
Table 4.
Mediators of Neurocognitive Change in EFML and Psychomotor Speed
| Mediator | ΔNeurocognitive Performance | Adjustment for Mediator | DASH+WM vs UC | DASH+WM Sobel Test | DASH-A vs UC | DASH-A Sobel Test |
|---|---|---|---|---|---|---|
| EFML | ||||||
| Δ weight, kg | −0.30‡ | Unadjusted | 0.35* | § | § | |
| Adjusted | 0.46* | Z101= −0.62 | ||||
| Δ peak V̇ O2, mL/kg per min | 0.19** | Unadjusted | 0.35* | § | § | |
| Adjusted | 0.10† | Z101=2.14† | ||||
| ΔSBP, mm Hg | −0.16† | Unadjusted | 0.35* | § | § | |
| Adjusted | 0.26‡ | Z101=1.53 | ||||
| Psychomotor speed | ||||||
| Δweight, kg | −0.36† | Unadjusted | 0.26† | 0.23† | Z101=0.944 | |
| Adjusted | −0.09 | Z101=2.21† | 0.20† | |||
| Δpeak V̇O2, mL/kg per min | 0.13† | Unadjusted | 0.26† | 0.23† | Z101=0.72 | |
| Adjusted | 0.10 | Z101=1.72‡ | 0.21† | |||
| ΔSBP, mm Hg | −0.04 | Unadjusted | 0.26† | 0.23† | Z101=−0.13 | |
| Adjusted | § | Z101=−0.13 | 0.23† | |||
Contrasts are adjusted for pretreatment neurocognitive performance, age, education, IMT, Framingham Stroke Risk Profile, and abdominal adiposity.
P<0.01.
P<0.05.
P<0.10.
Data show mediation analyses that were not conducted because either there was no main effect of treatment or changes in the mediating variable were not significantly related to neurocognitive outcomes.
Discussion
The DASH diet, combined with aerobic exercise and reduced calories, was associated with improved EFML and psychomotor speed performance relative to controls. The beneficial effects in the DASH+WM group were particularly pronounced for individuals with higher levels of IMT at baseline, a group at increased risk of stroke. Individuals who ate the DASH diet without losing weight or exercising exhibited improved psychomotor speed performance relative to controls, although EFML was not improved. We also observed that improvements in EFML in the DASH+WM group were mediated by improved cardiorespiratory fitness, whereas improvements in psychomotor speed were mediated by weight loss. It is unclear what mediated the effects of the improvements in psychomotor speed among the DASH-A group.
Several recent observational studies have reported that diet and exercise are related to improved neurocognition. Scarmeas et al21 demonstrated that physical activity and high adherence to the Mediterranean diet (similar in nutrient content to the DASH diet) were associated with reduced risk of AD and that exercise and diet had an additive effect. In addition, Feart et al22 demonstrated that better adherence to the Mediterranean diet was associated with slower cognitive decline on the Mini-Mental Status Examination but did not reduce the incidence of AD.
Aerobic exercise interventions generally have been found to result in modest neurocognitive improvements in attention and executive function, although the majority of individual trials have yielded null or equivocal findings.19 Interventional studies of dietary supplements and neurocognition, including antioxidants (vitamins C and E); vitamins B6, B12, and folate; fatty acids33; and caloric restriction34 also have provided negative or equivocal results. To our knowledge, the present study is the first randomized trial to examine the combined effects of diet and exercise on neurocognition in adults at risk for neurocognitive decline because of HBP.
We also explored potential treatment moderators and possible mechanisms of improvement in neurocognition. Our finding that improved aerobic fitness mediated improvements in EFML supports findings from several smaller interventions using neuroimaging, which have shown that improved peak oxygen volume increases anterior white matter integrity and gray matter volume.29 Our findings that both SBP and IMT moderated the effects of diet and exercise on neurocognition suggest that individuals with vascular disease may be especially likely to benefit from aerobic exercise and diet.
Limitations
Because our trial consisted of a 4-month intervention, the extent that these improvements could be maintained over time is not known. Second, the clinical significance of these improvements is not known, and a larger cohort followed over a longer time interval would be required to determine whether the intervention affected rates of AD. Third, the mechanisms for these improvements are not known, and it is possible that the observed improvements in neurocognitive function could be mediated by other factors, such as inflammation, growth factors, or other neurochemical changes not measured in the present study. Fourth, other diets, such as the Mediterranean diet alone or combined with exercise or weight loss, also could be beneficial. Fifth, because the current trial design did not use an exercise control group without dietary modification or a weight loss control group, it is unclear to what extent exercise or caloric restriction contributed to the observed pattern of findings. Although the DASH+WM and DASH-A groups were not statistically different when compared directly, our study was not powered to detect treatment group differences in neurocognitive performance specifically. Finally, although our sample included individuals with HBP who were not on medication, it is unclear whether our findings generalize to individuals with higher BP or more severe cardiovascular disease. Future studies should, therefore, examine the effects of aerobic exercise, dietary modification, and caloric restriction in other populations.
Perspectives
The results of this study indicated that the DASH diet, especially when associated with caloric restriction and aerobic exercise, improve neurocognitive performance among individuals with HBP. Improvements in neurocognitive performance were most pronounced among individuals with poorer vascular health. Dietary modification according to the DASH diet also appears to improve psychomotor functions. These improvements appear to be mediated by improved cardiorespiratory fitness and reduced body weight. The present findings could have important implications for improving neurocognitive function among older adults with HBP, at greater risk for cognitive decline and AD. Future studies should, therefore, examine the effects of diet and exercise in adults at elevated risk for dementia.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by grants from the National Heart, Lung, and Blood Institute (HL074103) and the General Clinical Research Center, National Institutes of Health (NIH) (M01-RR-30). This publication was made possible by grant number 5UL1RR024128-03 from the National Center for Research Resources (NCRR), a component of the NIH, and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Disclosures
None.
References
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi AI, Suri MF, Kirmani JF, Divani AA. Prevalence and trends of prehypertension and hypertension in United States: National Health and Nutrition Examination Surveys 1976 to 2000. Med Sci Monit. 2005;11:CR403–CR409. [PubMed] [Google Scholar]
- 3.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population: results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 4.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham Heart Study. JAMA. 2002;287:1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 5.Birkenhager WH, Staessen JA. Convergence of atherosclerosis and Alzheimer’s disease. Lancet. 2004;363:2091. doi: 10.1016/S0140-6736(04)16471-4. [DOI] [PubMed] [Google Scholar]
- 6.Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment: a 20-year follow-up of 999 men. Hypertension. 1998;31:780–786. doi: 10.1161/01.hyp.31.3.780. [DOI] [PubMed] [Google Scholar]
- 7.Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia: the Honolulu-Asia Aging Study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. World Health Report 2002: Reducing Risks, Promoting Healthy Life. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- 9.Duron E, Hanon O. Hypertension, cognitive decline and dementia. Arch Cardiovasc Dis. 2008;101:181–189. doi: 10.1016/s1875-2136(08)71801-1. [DOI] [PubMed] [Google Scholar]
- 10.Singh-Manoux A, Marmot M. High blood pressure was associated with cognitive function in middle-age in the Whitehall II Study. J Clin Epidemiol. 2005;58:1308–1315. doi: 10.1016/j.jclinepi.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Waldstein SR, Brown JR, Maier KJ, Katzel LI. Diagnosis of hypertension and high blood pressure levels negatively affect cognitive function in older adults. Ann Behav Med. 2005;29:174–180. doi: 10.1207/s15324796abm2903_3. [DOI] [PubMed] [Google Scholar]
- 12.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham Heart Study. Int J Obes Relat Metab Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 13.Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension–the Framingham Heart Study. Curr Alzheimer Res. 2007;4:111–116. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- 14.Waldstein SR, Katzel LI. Interactive relations of central versus total obesity and blood pressure to cognitive function. Int J Obes (Lond) 2006;30:201–207. doi: 10.1038/sj.ijo.0803114. [DOI] [PubMed] [Google Scholar]
- 15.Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 16.McGuinness B, Todd S, Passmore AP, Bullock R. Systematic review: blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. J Neurol Neurosurg Psychiatry. 2008;79:4–5. doi: 10.1136/jnnp.2007.118505. [DOI] [PubMed] [Google Scholar]
- 17.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, Lin PH, Svetkey LP, Stedman SW, Young DR. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 18.Shaw KA, Gennat HC, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006;(4):CD003817. doi: 10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angevaren M, Aufdemkampe G, Verhaar HJJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008;(3):CD005381. doi: 10.1002/14651858.CD005381.pub3. [DOI] [PubMed] [Google Scholar]
- 20.Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, Greenop KR, Almeida OP. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 21.Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y. Physical activity, diet, and risk of alzheimer disease. JAMA. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, Scarmeas N, Barberger-Gateau P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302:638–648. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blumenthal JA, Babyak MA, Hinderliter A, Watkins L, Craighead LW, Lin P, Caccia C, Johnson J, Waugh R, Sherwood A. Effects of the DASH Diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE Study. Arch Intern Med. 2010;170:126–135. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blumenthal JA, Rejeski WJ, Walsh-Riddle M, Emery CF, Miller H, Roark S, Ribisl PM, Morris PB, Brubaker P, Williams RS. Comparison of high- and low-intensity exercise training early after acute myocardial infarction. Am J Cardiol. 1988;61:26–30. doi: 10.1016/0002-9149(88)91298-2. [DOI] [PubMed] [Google Scholar]
- 25.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 26.Attix DK, Story TJ, Chelune GJ, Ball JD, Stutts ML, Hart RP, Barth JT. The prediction of change: normative neuropsychological trajectories. Clin Neuropsychol. 2009;23:21–38. doi: 10.1080/13854040801945078. [DOI] [PubMed] [Google Scholar]
- 27.Messinis L, Kosmidis MH, Tsakona I, Georgiou V, Aretouli E, Papathanasopoulos P. Ruff 2 and 7 Selective Attention Test: normative data, discriminant validity and test-retest reliability in Greek adults. Arch Clin Neuropsychol. 2007;22:773–785. doi: 10.1016/j.acn.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 29.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 30.Peila R, White LR, Masaki K, Petrovitch H, Launer LJ. Reducing the risk of dementia: efficacy of long-term treatment of hypertension. Stroke. 2006;37:1165–1170. doi: 10.1161/01.STR.0000217653.01615.93. [DOI] [PubMed] [Google Scholar]
- 31.Elias MF, Robbins MA, Elias PK. A 15-year longitudinal study of Halstead-Reitan neuropsychological test performance. J Gerontol B Psychol Sci Soc Sci. 1996;51:331–334. doi: 10.1093/geronb/51b.6.p331. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc. 1995;95:1103–1108. doi: 10.1016/S0002-8223(95)00300-2. [DOI] [PubMed] [Google Scholar]
- 33.Luchsinger JA, Mayeux R. Dietary factors and Alzheimer’s disease. Lancet Neurol. 2004;3:579–587. doi: 10.1016/S1474-4422(04)00878-6. [DOI] [PubMed] [Google Scholar]
- 34.Witte AV, Fobker M, Gellner R, Knecht S, Floel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A. 2009;106:1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reitan RM. Manual for Administration of Neuropsychological Test Batteries for Adults and Children. Tucson, AZ: Reitan Neuropsychological Laboratories, Inc; 1979. [Google Scholar]
- 36.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychiat. 1935;18:643–662. [Google Scholar]
- 37.Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 38.Lezak MD. Neuropsychological Assessment. 3. New York, NY: Oxford University Press; 1995. [Google Scholar]
- 39.Ruff RM, Niemann H, Allen CC. The Ruff 2 and 7 Selective Attention Test: a neuropsychological application. Percept Mot Skills. 1992;75:1311–1319. doi: 10.2466/pms.1992.75.3f.1311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.