Abstract
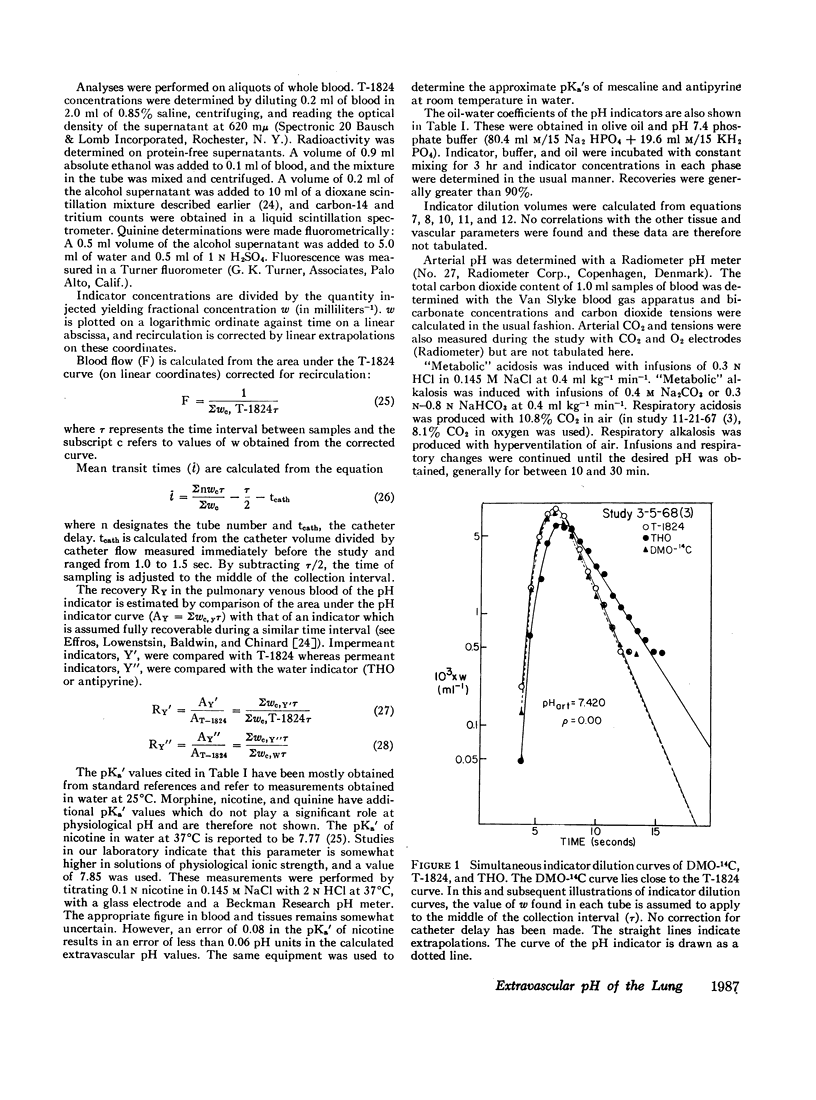
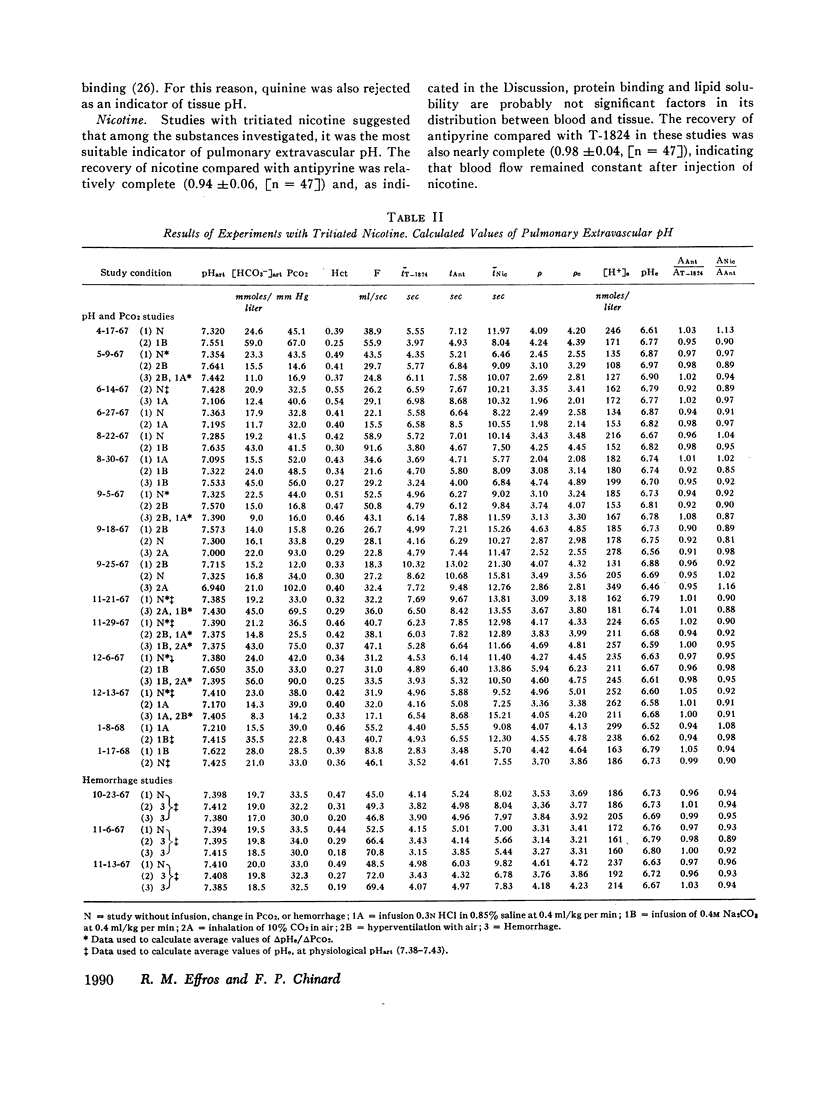
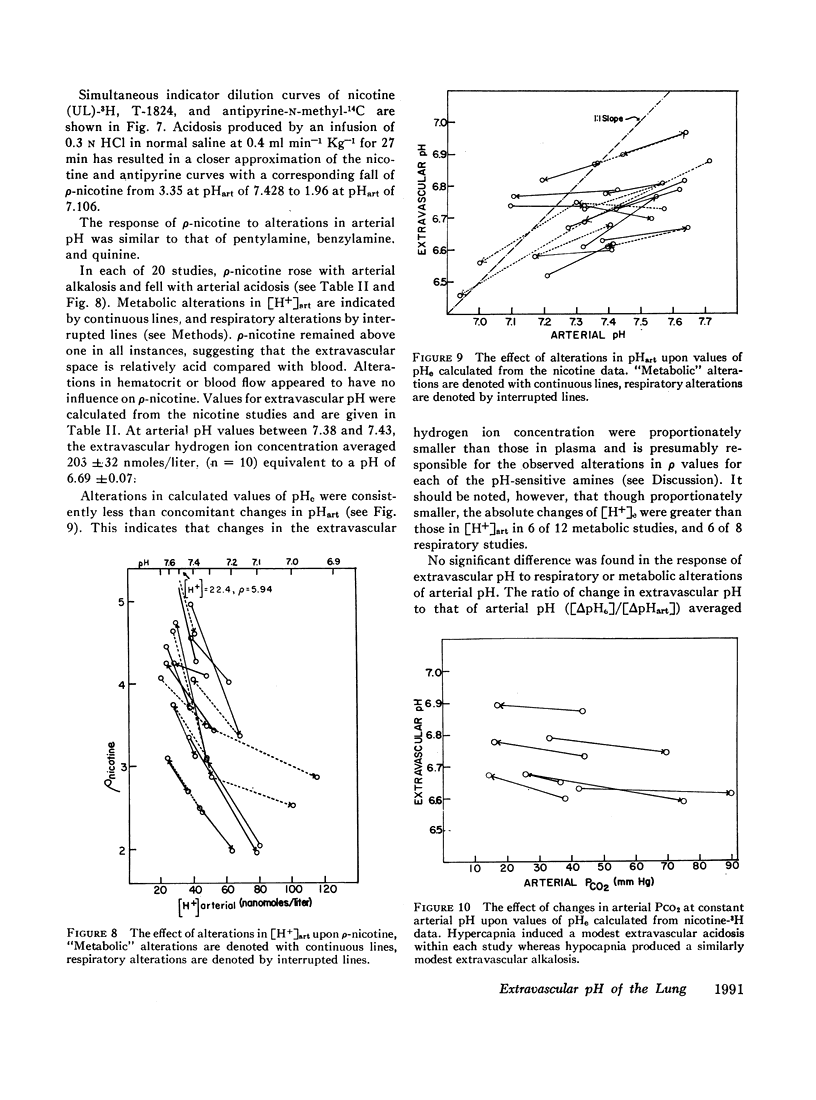
The partition of 5,5-dimethyloxazolidine-2,4-dione (DMO) and of 11 amines between the vascular and extravascular spaces of the lung has been determined by the multiple indicator dilution technique. Four amines (nicotine, pentylamine, quinine, and benzylamine) were found to have pH-sensitive tissue to blood concentration ratios. Of these, tritiated nicotine appears to be the nost satisfactory indicator of tissue pH and values for the pH of the pulmonary extravascular space (pHe) have been calculated from the nicotine data. At an arterial pH (pHart) between 7.38 and 7.43 pHe averaged 6.69 ±0.07.
Changes in pHe usually paralleled but were consistently less than concomitant changes in pHart. Alterations in PCO2 at constant pHart regularly produced relatively small, parallel changes in extravascular hydrogen ion concentrations. Local alterations in tissue pH due to PCO2 changes are apparently buffered quite rapidly and the pHe of the lung seems more closely linked to pHart than the cellular pH of other tissues.
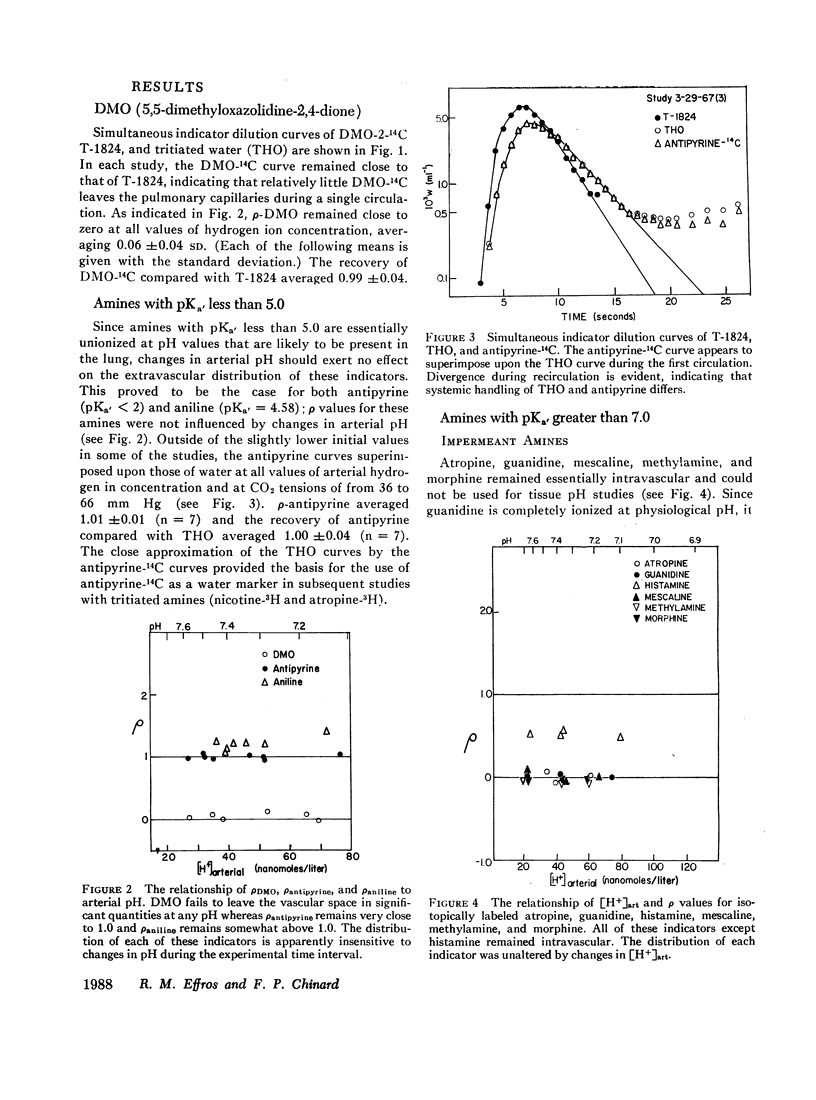
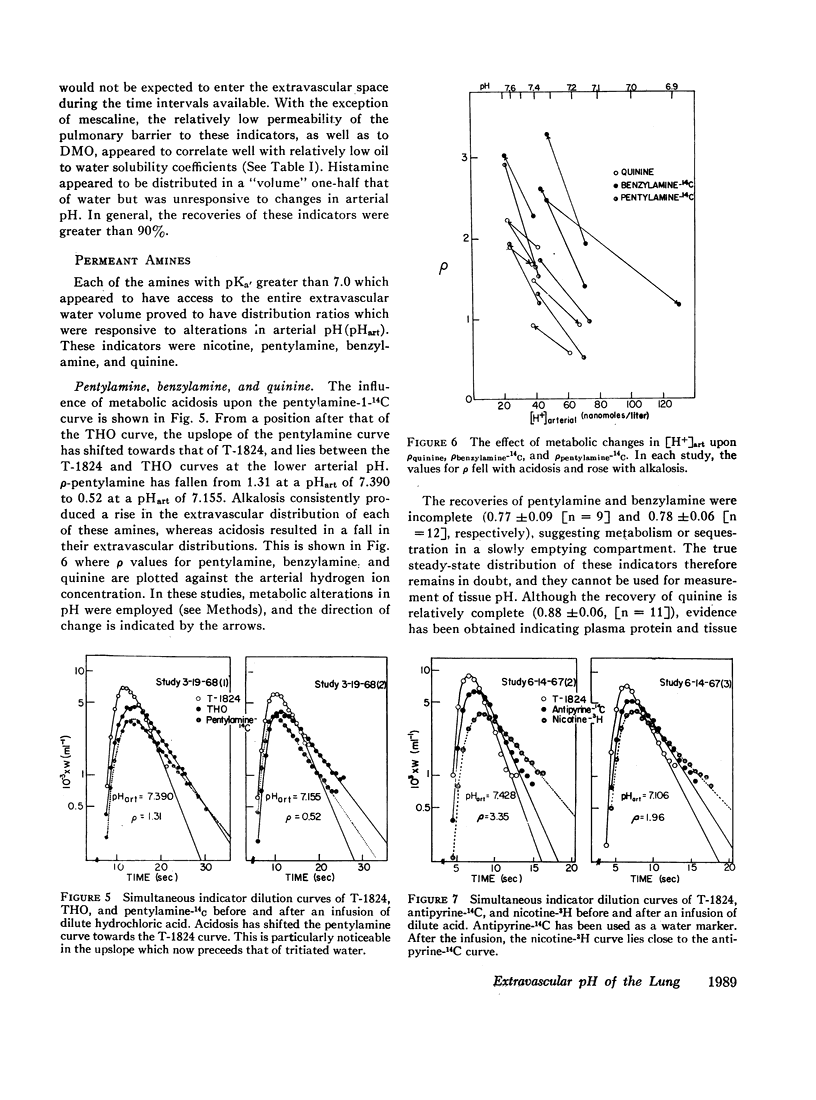
DMO, guanidine, methylamine, morphine, and atropine were confined to the vascular volume during the first circulation and could not be used to measure tissue pH. Histamine appeared to be bound to a pH-insensitive site. The extravascular distributions of antipyrine and aniline were unresponsive to alterations in arterial pH, presumably because they are essentially uncharged at pH levels found in the lung.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER S., ROY A., RELMAN A. S. INTRACELLULAR ACID-BASE REGULATION. I. THE RESPONSE OF MUSCLE CELLS TO CHANGES IN CO2 TENSION OR EXTRACELLULAR BICARBONATE CONCENTRATION. J Clin Invest. 1965 Jan;44:8–20. doi: 10.1172/JCI105129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADLER S., ROY A., RELMAN A. S. INTRACELLULAR ACID-BASE REGULATION. II. THE INTERACTION BETWEEN CO-2 TENSION AND EXTRACELLULAR BICARBONATE IN THE DETERMINATION OF MUSCLE CELL PH. J Clin Invest. 1965 Jan;44:21–30. doi: 10.1172/JCI105123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUMAN A., ROTHSCHILD M. A., YALOW R. S., BERSON S. A. Pulmonary circulation and transcapillary exchange of electrolytes. J Appl Physiol. 1957 Nov;11(3):353–361. doi: 10.1152/jappl.1957.11.3.353. [DOI] [PubMed] [Google Scholar]
- Beckett A. H., Triggs E. J. Buccal absorption of basic drugs and its application as an in vivo model of passive drug transfer through lipid membranes. J Pharm Pharmacol. 1967 Dec;19(Suppl):31S–41S. [PubMed] [Google Scholar]
- Butler T. C., Waddell W. J., Poole D. T. Intracellular pH based on the distribution of weak electrolytes. Fed Proc. 1967 Sep;26(5):1327–1332. [PubMed] [Google Scholar]
- CHINARD F. P., ENNS T., NOLAN M. F. Contributions of bicarbonate ion and of dissolved CO2 to expired CO2 in dogs. Am J Physiol. 1960 Jan;198:78–88. doi: 10.1152/ajplegacy.1960.198.1.78. [DOI] [PubMed] [Google Scholar]
- CHINARD F. P., ENNS T., NOLAN M. F. The permeability characteristics of the alveolar capillary barrier. Trans Assoc Am Physicians. 1962;75:253–262. [PubMed] [Google Scholar]
- CHINARD F. P., VOSBURGH G. J., ENNS T. Transcapillary exchange of water and of other substances in certain organs of the dog. Am J Physiol. 1955 Nov;183(2):221–234. doi: 10.1152/ajplegacy.1955.183.2.221. [DOI] [PubMed] [Google Scholar]
- Carter N. W., Rector F. C., Jr, Campion D. S., Seldin D. W. Measurement of intracellular pH with glass microelectrodes. Fed Proc. 1967 Sep;26(5):1322–1326. [PubMed] [Google Scholar]
- DUBOIS A. B., FENN W. O., BRITT A. G. CO2 dissociation curve of lung tissue. J Appl Physiol. 1952 Jul;5(1):13–16. doi: 10.1152/jappl.1952.5.1.13. [DOI] [PubMed] [Google Scholar]
- Effros R. M., Lowenstein J., Baldwin D. S., Chinard F. P. Vascular and extravascular volumes of the kidney of man. Circ Res. 1967 Feb;20(2):162–173. doi: 10.1161/01.res.20.2.162. [DOI] [PubMed] [Google Scholar]
- Hyde R. W., Puy R. J., Raub W. F., Forster R. E. Rate of disappearance of labeled carbon dioxide from the lungs of humans during breath holding: a method for studying the dynamics of pulmonary CO2 exchange. J Clin Invest. 1968 Jul;47(7):1535–1552. doi: 10.1172/JCI105846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIBLER R. F., O'NEILL R. P., ROBIN E. D. INTRACELLULAR ACID-BASE RELATIONS OF DOG BRAIN WITH REFERENCE TO THE BRAIN EXTRACELLULAR VOLUME. J Clin Invest. 1964 Mar;43:431–443. doi: 10.1172/JCI104928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORLOFF J., BERLINER R. W. The mechanism of the excretion of ammonia in the dog. J Clin Invest. 1956 Feb;35(2):223–235. doi: 10.1172/JCI103267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPAPORT E., KUIDA H., HAYNES F. W., DEXTER L. Pulmonary red cell and plasma volumes and pulmonary hematocrit in the normal dog. Am J Physiol. 1956 Apr;185(1):127–132. doi: 10.1152/ajplegacy.1956.185.1.127. [DOI] [PubMed] [Google Scholar]
- SACKNER M. A., FEISAL K. A., DUBOIS A. B. DETERMINATION OF TISSUE VOLUME AND CARBON DIOXIDE DISSOCIATION SLOPE OF THE LUNGS IN MAN. J Appl Physiol. 1964 May;19:374–380. doi: 10.1152/jappl.1964.19.3.374. [DOI] [PubMed] [Google Scholar]
- Struyvenberg A., Morrison R. B., Relman A. S. Acid-base behavior of separated canine renal tubule cells. Am J Physiol. 1968 May;214(5):1155–1162. doi: 10.1152/ajplegacy.1968.214.5.1155. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell W. J., Bates R. G. Intracellular pH. Physiol Rev. 1969 Apr;49(2):285–329. doi: 10.1152/physrev.1969.49.2.285. [DOI] [PubMed] [Google Scholar]
- Weiss G. B. Dependence of nicotine-C14 distribution and movements upon pH in frog sartorius muscle. J Pharmacol Exp Ther. 1968 Mar;160(1):135–147. [PubMed] [Google Scholar]


