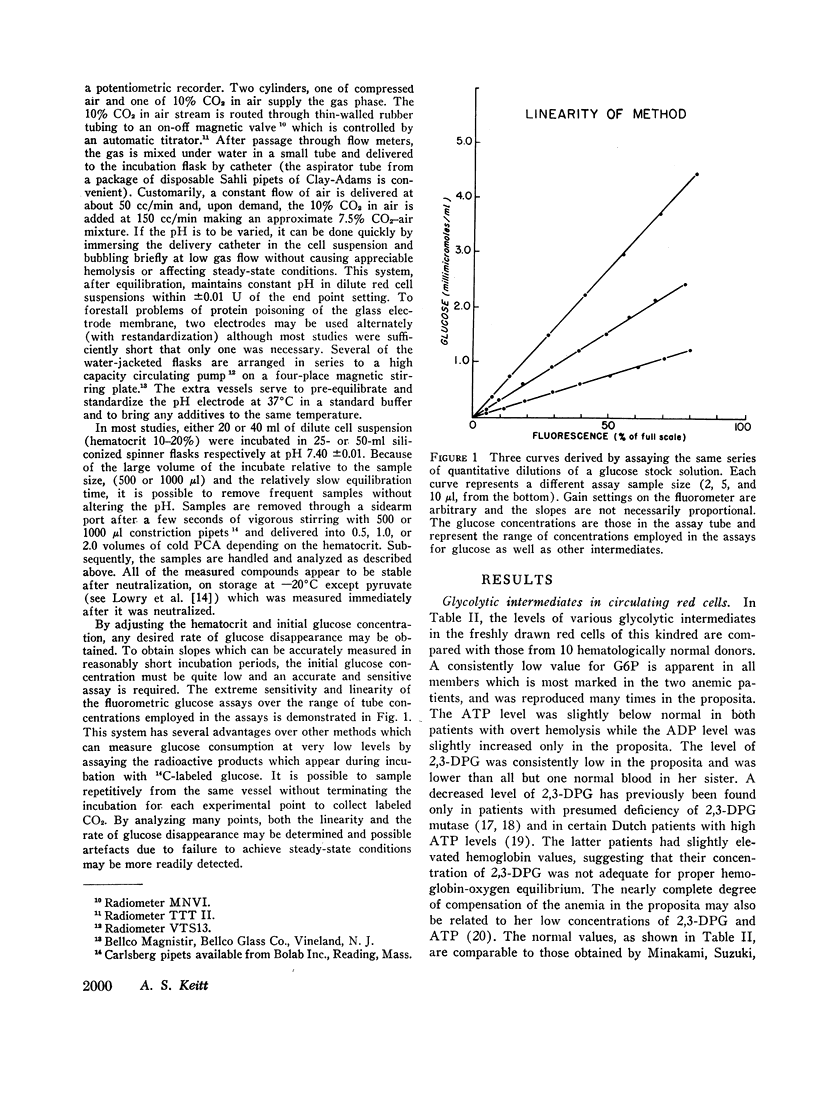
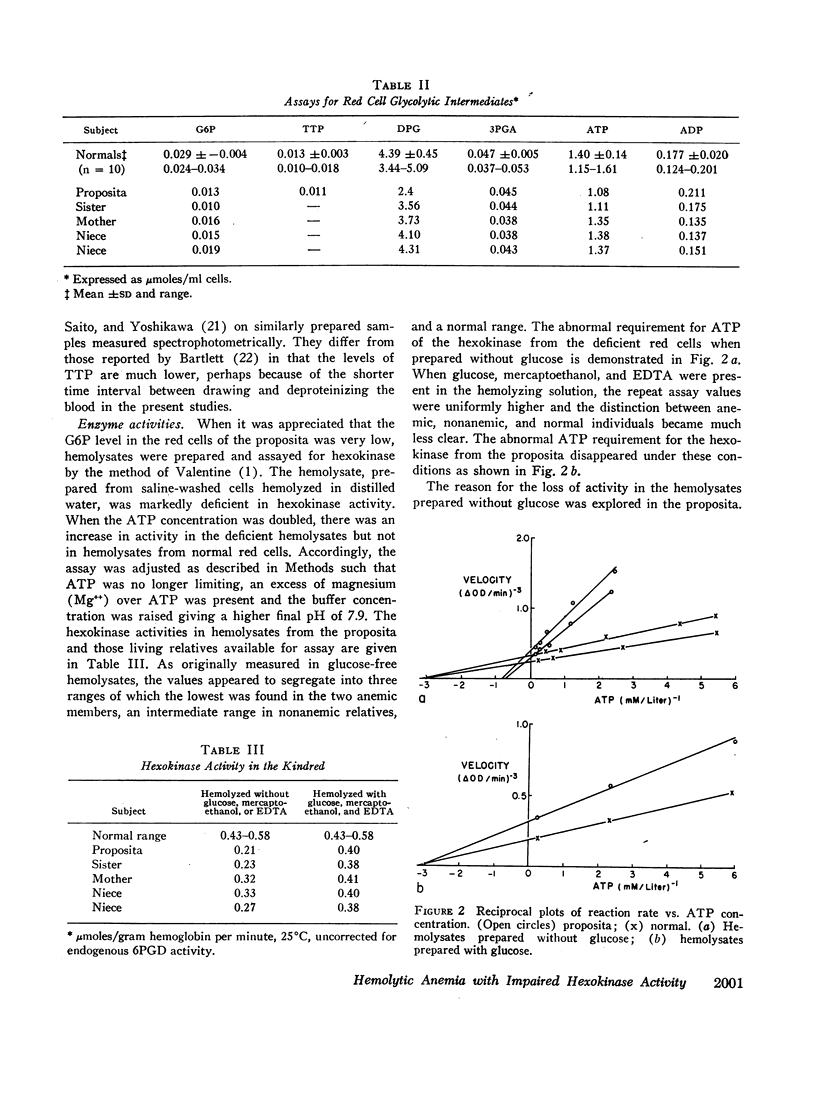
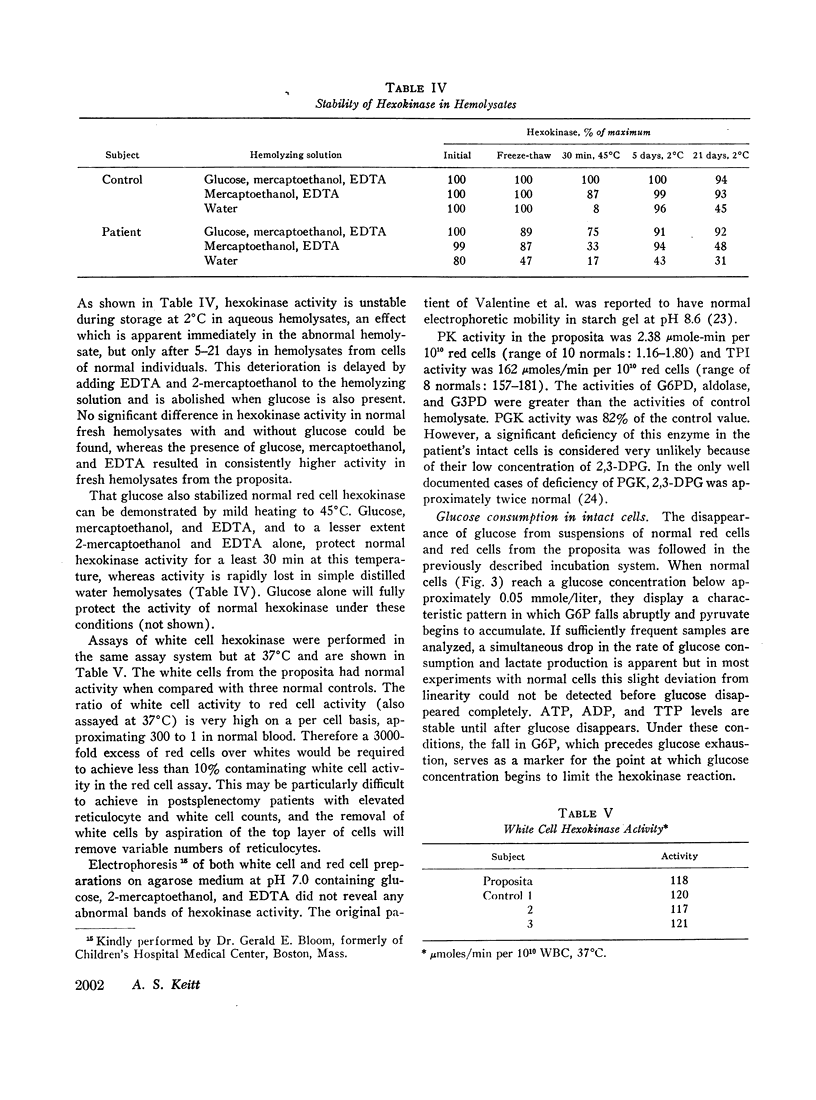
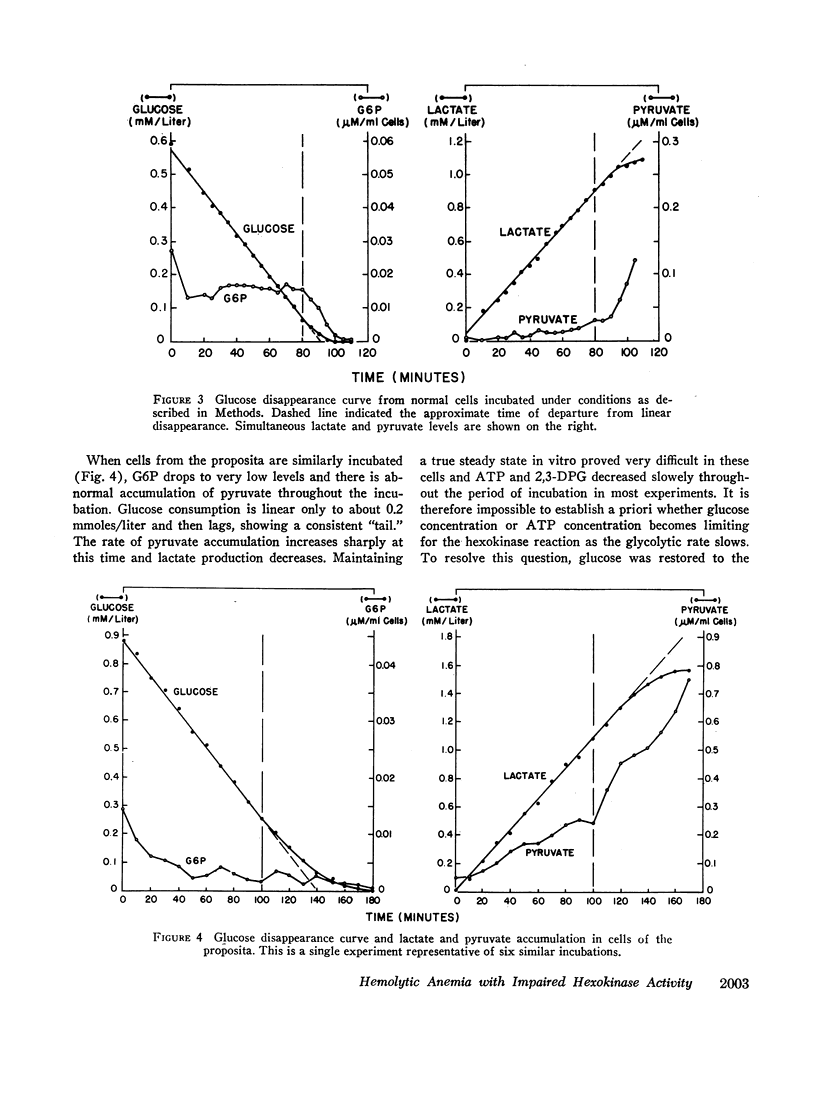
Abstract
Analyses of key glycolytic intermediates in freshly drawn red cells from six related individuals suggest that decreased hexokinase activity underlies the hemolytic process in the two members with overt hemolysis. Low red cell glucose 6-phosphate (G6P) was observed not only in the anemic patients but in the presumptive heterozygotes as well and served as a useful marker for the presence of the trait. Hexokinase activity was labile in distilled water hemolysates but was only slightly low when protected by glucose, mercaptoethanol, and ethylenediaminetetraacetate (EDTA). Normal red cell hexokinase was demonstrated to be dependent on glucose for maintenance of activity after heating to 45°C. The cells of the proposita are unable to utilize glucose efficiently at glucose concentrations lower than 0.2 mmole/liter whereas normal cells maintain linear glucose consumption to at least 0.05 mM glucose. These qualitative abnormalities could result from the presence of a mutant hexokinase with an abnormally reactive sulfhydryl group and altered substrate affinity in the red cells of this kindred.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP C. Maintenance of ATP level of incubated human red cells by controlling the pH. Transfusion. 1962 Nov-Dec;2:408–412. doi: 10.1111/j.1537-2995.1962.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Bartlett G. R. Phosphorus compounds in the human erythrocyte. Biochim Biophys Acta. 1968 Mar 11;156(2):221–230. doi: 10.1016/0304-4165(68)90251-1. [DOI] [PubMed] [Google Scholar]
- Bellingham A. J., Huehns E. R. Compensation in haemolytic anaemias caused by abnormal haemoglobins. Nature. 1968 Jun 8;218(5145):924–926. doi: 10.1038/218924a0. [DOI] [PubMed] [Google Scholar]
- Beutler E., Mathai C. K., Smith J. E. Biochemical variants of glucose-6-phosphate dehydrogenase giving rise to congenital nonspherocytic hemolytic disease. Blood. 1968 Feb;31(2):131–150. [PubMed] [Google Scholar]
- CHANCE B., HOLMES W., HIGGINS J., CONNELLY C. M. Localization of interaction sites in multi-component transfer systems: theorems derived from analogues. Nature. 1958 Nov 1;182(4644):1190–1193. doi: 10.1038/1821190a0. [DOI] [PubMed] [Google Scholar]
- CHAPMAN R. G., HENNESSEY M. A., WALTERSDORPH A. M., HUENNEKENS F. M., GABRIO B. W. Erythrocyte metabolism. V. Levels of glycolytic enzymes and regulation of glycolysis. J Clin Invest. 1962 Jun;41:1249–1256. doi: 10.1172/JCI104587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIMES A. J., MEISLER A., DACIE J. V. CONGENITAL HEINZ-BODY ANAEMIA. FURTHER EVIDENCE ON THE CAUSE OF HEINZ-BODY PRODUCTION IN RED CELLS. Br J Haematol. 1964 Jul;10:281–290. doi: 10.1111/j.1365-2141.1964.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- Holmes E. W., Jr, Malone J. I., Winegrad A. I., Oski F. A. Hexokinase isoenzymes in human erythrocytes: association of type II with fetal hemoglobin. Science. 1967 May 5;156(3775):646–648. doi: 10.1126/science.156.3775.646. [DOI] [PubMed] [Google Scholar]
- Jacob H. S., Brain M. C., Dacie J. V. Altered sulfhydryl reactivity of hemoglobins and red blood cell membranes in congenital Heinz body hemolytic anemia. J Clin Invest. 1968 Dec;47(12):2664–2677. doi: 10.1172/JCI105950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. S., Brain M. C., Dacie J. V., Carrell R. W., Lehmann H. Abnormal haem binding and globin SH group blockade in unstable haemoglobins. Nature. 1968 Jun 29;218(5148):1214–1217. doi: 10.1038/2181214a0. [DOI] [PubMed] [Google Scholar]
- Kaplan J. C., Beutler E. Hexokinase isoenzymes in human erythrocytes. Science. 1968 Jan 12;159(3811):215–216. doi: 10.1126/science.159.3811.215. [DOI] [PubMed] [Google Scholar]
- Keitt A. S. Pyruvate kinase deficiency and related disorders of red cell glycolysis. Am J Med. 1966 Nov;41(5):762–785. doi: 10.1016/0002-9343(66)90036-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Malone J. I., Winegrad A. I., Oski F. A., Holmes E. W., Jr Erythrocyte hexokinase isoenzyme patterns in hereditary hemoglobinopathies. N Engl J Med. 1968 Nov 14;279(20):1071–1077. doi: 10.1056/NEJM196811142792002. [DOI] [PubMed] [Google Scholar]
- Minakami S., Suzuki C., Saito T., Yoshikawa H. Studies on erythrocyte glycolysis. I. Determination of the glycolytic intermediates in human erythrocytes. J Biochem. 1965 Dec;58(6):543–550. doi: 10.1093/oxfordjournals.jbchem.a128240. [DOI] [PubMed] [Google Scholar]
- Nesbakken R., Eldjarn L. The inhibition of hexokinase by disulphides. Biochem J. 1963 Jun;87(3):526–532. doi: 10.1042/bj0870526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N., Baughan M. A., Miller D. R., Reed C. F., McIntyre O. R. An inherited molecular lesion of erythrocyte pyruvate kinase. Identification of a kinetically aberrant isozyme associated with premature hemolysis. J Clin Invest. 1968 Aug;47(8):1929–1946. doi: 10.1172/JCI105883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER A. S., VALENTINE W. N., HATTORI M., HEINS H. L., Jr HEREDITARY HEMOLYTIC ANEMIA WITH TRIOSEPHOSPHATE ISOMERASE DEFICIENCY. N Engl J Med. 1965 Feb 4;272:229–235. doi: 10.1056/NEJM196502042720503. [DOI] [PubMed] [Google Scholar]
- SCHRIER S. L. Studies of the metabolism of human erythrocyte membranes. J Clin Invest. 1963 Jun;42:756–766. doi: 10.1172/JCI104768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. G., Ueda S., Alperin J. B., Brimhall B., Jones R. T. Hemoglobin sabine beta 91 (f 7) leu to pro. An unstable variant causing severe anemia with inclusion bodies. N Engl J Med. 1969 Apr 3;280(14):739–745. doi: 10.1056/NEJM196904032801402. [DOI] [PubMed] [Google Scholar]
- Schrier S. L. Organization of enzymes in human erythrocyte membranes. Am J Physiol. 1966 Jan;210(1):139–145. doi: 10.1152/ajplegacy.1966.210.1.139. [DOI] [PubMed] [Google Scholar]
- Schröter W. Kongenitale nichtsphärocytäre hämolytische Anämie bei 2,3-Diphosphoglyceratmutase-Mangel der Erythrocyten im frühen Säuglingsalter. Klin Wochenschr. 1965 Nov 1;43(21):1147–1153. doi: 10.1007/BF01733168. [DOI] [PubMed] [Google Scholar]
- Schröter W., Tillmann W. Hexokinase isoenzymes in human erythrocytes of adults and newborns. Biochem Biophys Res Commun. 1968 Apr 5;31(1):92–97. doi: 10.1016/0006-291x(68)90035-1. [DOI] [PubMed] [Google Scholar]
- TANAKA K. R., VALENTINE W. N., MIWA S. Pyruvate kinase (PK) deficiency hereditary nonspherocytic hemolytic anemia. Blood. 1962 Mar;19:267–295. [PubMed] [Google Scholar]
- VESTERGAARD-BOGIND B. Automatic pH regulation of cell suspensions by a gasometric pH-stat. Scand J Clin Lab Invest. 1962;14:461–465. doi: 10.3109/00365516209051263. [DOI] [PubMed] [Google Scholar]
- Valentine W. N., Hsieh H. S., Paglia D. E., Anderson H. M., Baughan M. A., Jaffé E. R., Garson O. M. Hereditary hemolytic anemia associated with phosphoglycerate kinase deficiency in erythrocytes and leukocytes. A probable X-chromosome-linked syndrome. N Engl J Med. 1969 Mar 6;280(10):528–534. doi: 10.1056/NEJM196903062801003. [DOI] [PubMed] [Google Scholar]
- Valentine W. N., Oski F. A., Paglia D. E., Baughan M. A., Schneider A. S., Naiman J. L. Hereditary hemolytic anemia with hexokinase deficiency. Role of hexokinase in erythrocyte aging. N Engl J Med. 1967 Jan 5;276(1):1–11. doi: 10.1056/NEJM196701052760101. [DOI] [PubMed] [Google Scholar]


