Abstract
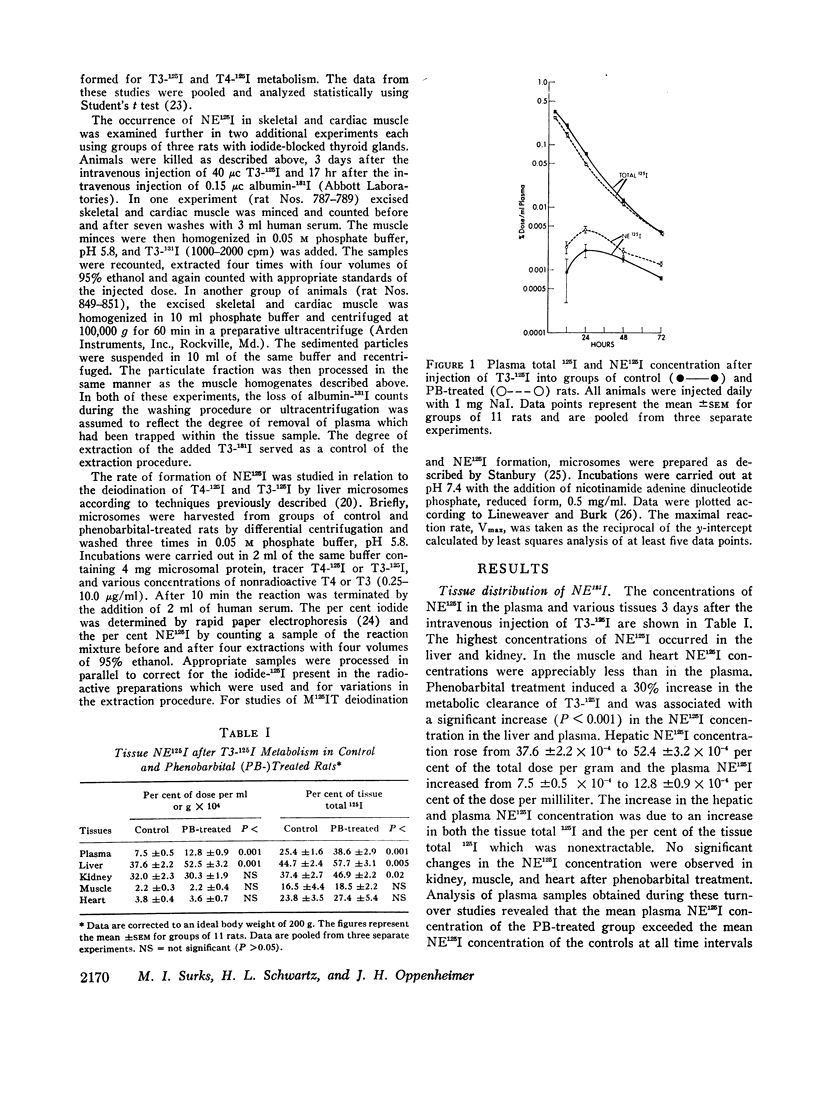
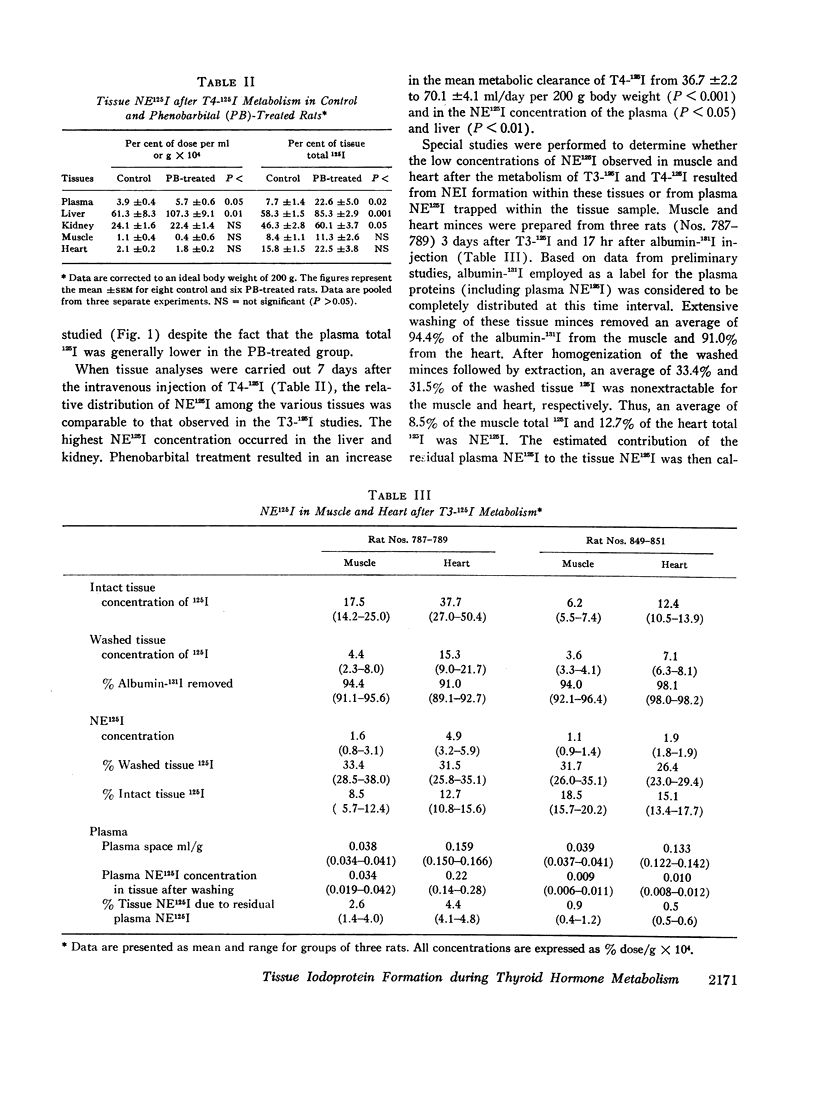
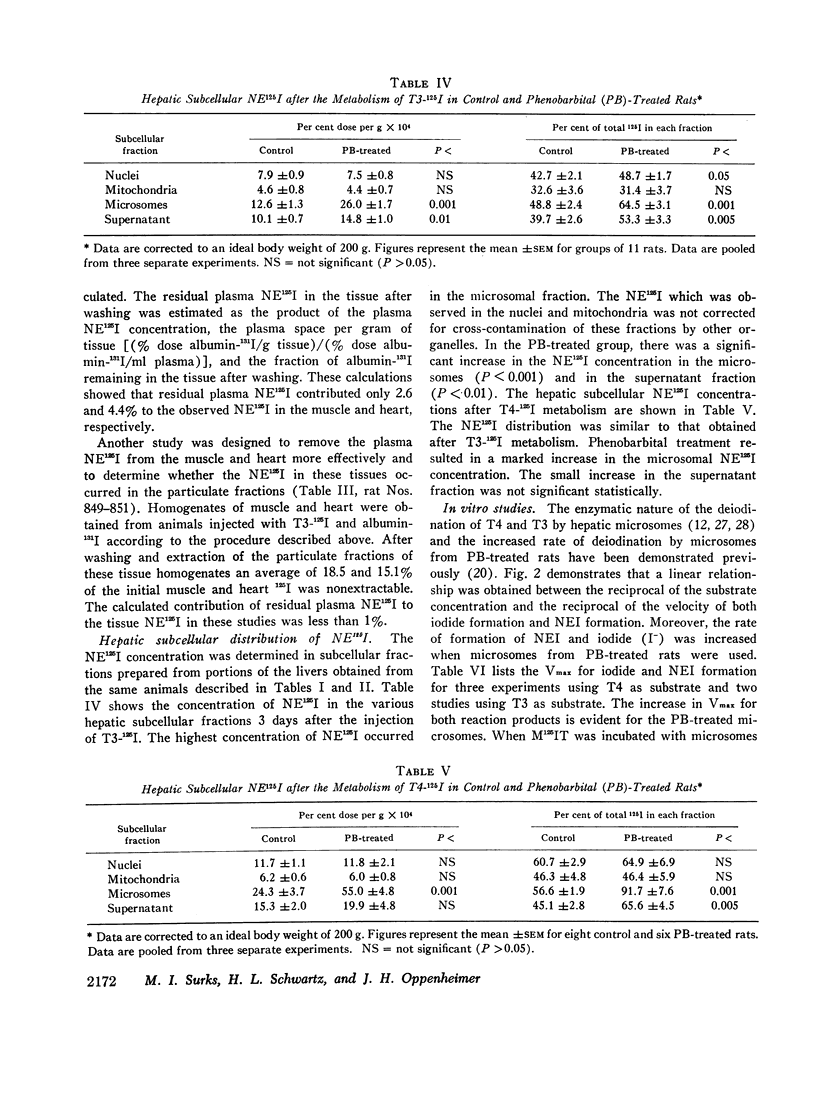
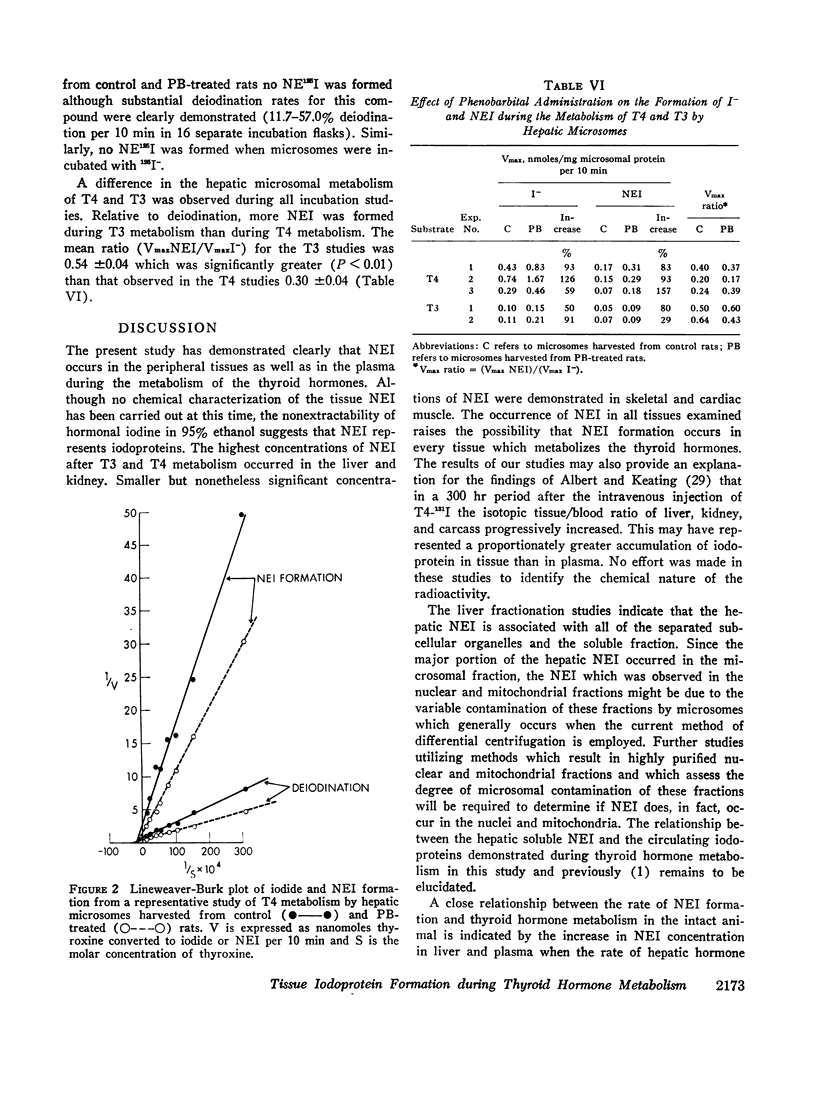
The formation of tissue iodoproteins during the peripheral metabolism of the thyroid hormones was examined by determining the concentration of nonethanol-extractable 125I (NE125I) in various tissues after the intravenous injection of 3,5,3′-triiodo-L-thyronine (T3-125I) and L-thyroxine-125I (T4-125I) in groups of rats with iodide-blocked thyroid glands. 3 days after T3-125I and 7 days after T4-125I injection the concentration of NE125I in the liver and kidney was 5-10 times greater than in plasma. Smaller but nonetheless significant concentrations of NE125I were demonstrated in skeletal and cardiac muscle. Hepatic subcellular fractionation studies revealed that the major portion of the liver NE125I was in the microsomal fraction. Lower concentrations of NE125I were present in the nuclear, mitochondrial, and soluble fractions. When similar studies were performed in groups of rats pretreated with phenobarbital, an increase in the metabolic clearance of T3-125I (30%) and T4-125I (100%) was observed along with a highly significant increase in the NE125I concentration of the liver and plasma. The increase in hepatic NE125I in these studies was primarily due to the microsomal component.
Incubation of hepatic microsomes with T3-125I and T4-125I showed that NEI formation as well as deiodination appeared to obey simple Michaelis-Menten kinetics. Moreover, the maximal rate of both deiodination and NEI formation was increased when microsomes harvested from phenobarbital-treated rats were employed.
These studies indicate that thyroid hormone metabolism results in the formation of structural and soluble tissue iodoproteins in addition to circulating iodoproteins. The rate of formation of these moieties in the liver and plasma appears to be related to the rate of hormone metabolism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERT A., KEATING F. R., Jr The role of the gastrointestinal tract, including the liver, in the metabolism of radiothyroxine. Endocrinology. 1952 Nov;51(5):427–443. doi: 10.1210/endo-51-5-427. [DOI] [PubMed] [Google Scholar]
- BERSON S. A., YALOW R. S. Radiochemical and radiobiological alterations of I 131-labeled proteins in solution. Ann N Y Acad Sci. 1957 Aug 30;70(1):56–68. doi: 10.1111/j.1749-6632.1957.tb35377.x. [DOI] [PubMed] [Google Scholar]
- Bernstein G., Artz S. A., Hasen J., Oppenheimer J. H. Hepatic accumulation of 125I-thyroxine in the rat: augmentation by phenobarbital and chlordane. Endocrinology. 1968 Feb;82(2):406–409. doi: 10.1210/endo-82-2-406. [DOI] [PubMed] [Google Scholar]
- Brown-Grant K. Further studies of the metabolism of thyroxine and 3,5,3'-triiodothyronine in the guinea-pig. J Physiol. 1967 Jul;191(1):167–176. doi: 10.1113/jphysiol.1967.sp008243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMERON C., FLETCHER K. An iodine compound associated with albumin in the plasma of thyrotoxic patients. Nature. 1959 Jan 10;183(4654):116–116. doi: 10.1038/183116a0. [DOI] [PubMed] [Google Scholar]
- FORD D. H., COREY K. R., GROSS J. The localization of thyroid hormones in the organs and tissues of the guinea pig: an autoradiographic and chromatographic study. Endocrinology. 1957 Oct;61(4):426–447. doi: 10.1210/endo-61-4-426. [DOI] [PubMed] [Google Scholar]
- GALTON V. A., INGBAR S. H. A photoactivated flavin-induced degradation of thyroxine and related phenols. Endocrinology. 1962 Feb;70:210–220. doi: 10.1210/endo-70-2-210. [DOI] [PubMed] [Google Scholar]
- GALTON V. A., INGBAR S. H. The mechanism of protein iodination during the metabolism of thyroid hormones by peripheral tissues. Endocrinology. 1961 Jul;69:30–38. doi: 10.1210/endo-69-1-30. [DOI] [PubMed] [Google Scholar]
- GLEASON G. I. Some notes on the exchange of iodine with thyroxine homologues. J Biol Chem. 1955 Apr;213(2):837–841. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACQUEMIN C., NUNEZ J., ROCHE J. [On the intermediate products and mechanism of deiodination of thyroid hormones]. Gen Comp Endocrinol. 1963 Jun;3:226–238. doi: 10.1016/0016-6480(63)90017-0. [DOI] [PubMed] [Google Scholar]
- LISSITZKY S., BENEVENT M. T., ROQUES M. [Enzymatic deiodination of thyroxin and its derivatives. II. Products formed and mechanism of the reaction]. Bull Soc Chim Biol (Paris) 1961;43:743–770. [PubMed] [Google Scholar]
- LISSITZKY S., ROQUES M., BENEVENT M. T. [Enzymatic deiodination of thyroxin and its derivatives. I. Purification and properties of thyroxin-deiodase from the rabbit muscle]. Bull Soc Chim Biol (Paris) 1961;43:727–742. [PubMed] [Google Scholar]
- NUNEZ J., RAPPAPORT L., JACQUEMIN C., ROCHE J. D'ESIODATION IN VIVO DE H-3- ET 3,5-I-125-THYROXINE. C R Seances Soc Biol Fil. 1964;158:12–17. [PubMed] [Google Scholar]
- Nakagawa S., Ruegamer W. R. Properties of a rat tissue iodothyronine deiodinase and its natural inhibitor. Biochemistry. 1967 May;6(5):1249–1261. doi: 10.1021/bi00857a005. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Bernstein G., Surks M. I. Increased thyroxine turnover and thyroidal function after stimulation of hepatocellular binding of thyroxine by phenobarbital. J Clin Invest. 1968 Jun;47(6):1399–1406. doi: 10.1172/JCI105831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLASKETT L. G. Studies on the degradation of thyroid hormones in vitro with compounds labelled in either ring. Biochem J. 1961 Mar;78:652–657. doi: 10.1042/bj0780652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinwein D., Rall J. E. Nonenzymatic deiodination of thyroid hormones by flavin mononucleotide and light. J Biol Chem. 1966 Apr 10;241(7):1636–1643. [PubMed] [Google Scholar]
- SCHNEIDER W. C., HOGEBOOM G. H. Intracellular distribution of succinoxidase and cytochrome oxidase activities in normal mouse liver and in mouse hepatoma. J Natl Cancer Inst. 1950 Feb;10(4):969–975. [PubMed] [Google Scholar]
- STANBURY J. B., JANSSEN M. A. The iodinated albuminlike component of the plasma of thyrotoxic patients. J Clin Endocrinol Metab. 1962 Oct;22:978–988. doi: 10.1210/jcem-22-10-978. [DOI] [PubMed] [Google Scholar]
- STANBURY J. B. The requirement of monoiodotyrosine deiodinase for triphosphopyridine nucleotide. J Biol Chem. 1957 Oct;228(2):801–811. [PubMed] [Google Scholar]
- Schwartz H. L., Bernstein G., Oppenheimer J. H. Effect of phenobarbital administration on the subcellular distribution of 125-I-thyroxine in rat liver: mportance of microsomal binding. Endocrinology. 1969 Feb;84(2):270–276. doi: 10.1210/endo-84-2-270. [DOI] [PubMed] [Google Scholar]
- Schwartz H. L., Kozyreff V., Surks M. I., Oppenheimer J. H. Increased deiodination of L-thyroxine and L-triiodothyronine by liver microsomes from rats treated with phenobarbital. Nature. 1969 Mar 29;221(5187):1262–1263. doi: 10.1038/2211262a0. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Oppenheimer J. H. Formation of iodoprotein during the peripheral metabolism of 3,5,3'-triiodo-L-thyronine-125I in the euthyroid man and rat. J Clin Invest. 1969 Apr;48(4):685–695. doi: 10.1172/JCI106026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TATA J. R. The partial purification and properties of thyroxine dehalogenase. Biochem J. 1960 Nov;77:214–226. doi: 10.1042/bj0770214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TATA J. R. Transiodination of proteins during enzymic de-iodination of thyroxine. Nature. 1960 Sep 17;187:1025–1026. doi: 10.1038/1871025a0. [DOI] [PubMed] [Google Scholar]
- WYNN J., GIBBS R., ROYSTER B. Thyroxine degradation. I. Study of optimal reaction conditions of a rat liver thyroxine-degrading system. J Biol Chem. 1962 Jun;237:1892–1897. [PubMed] [Google Scholar]
- WYNN J., GIBBS R. Thyroxine degradation. II. Products of thyroxine degradation by rat liver microsomes. J Biol Chem. 1962 Nov;237:3499–3505. [PubMed] [Google Scholar]


