Abstract
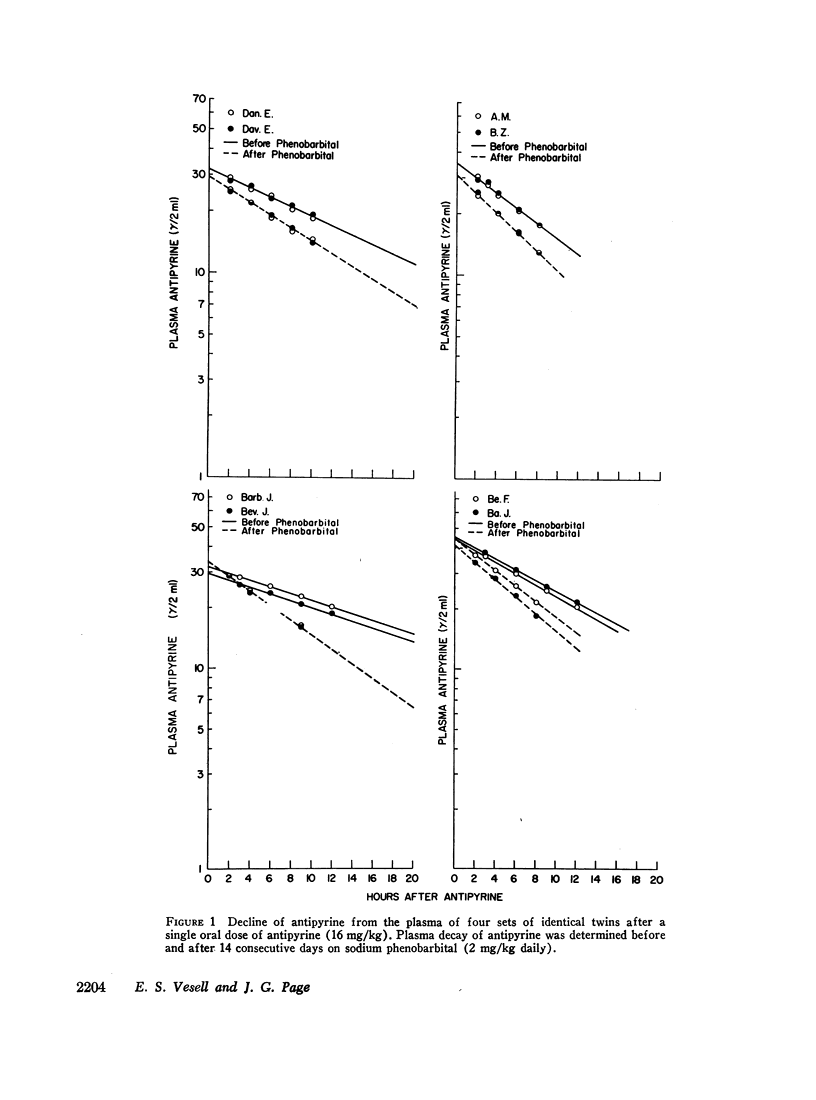
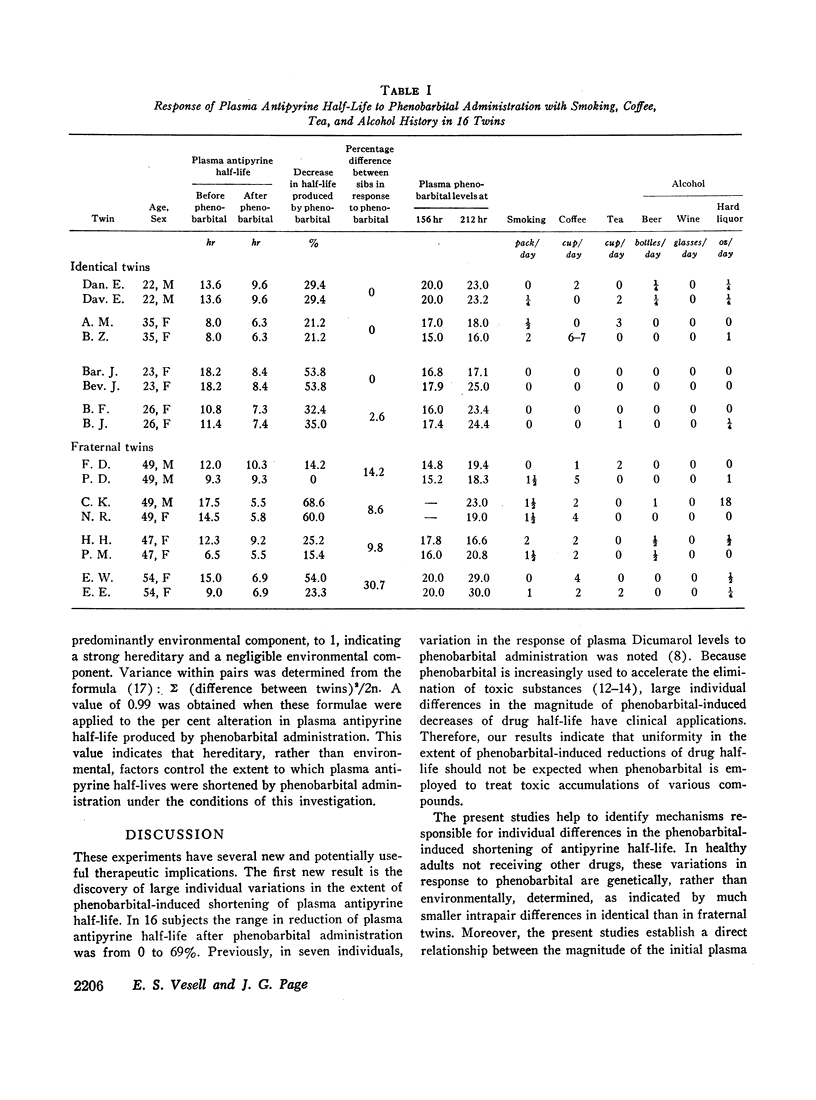
The mean half-life of antipyrine in the plasma of four sets of identical and four sets of fraternal twins after a single oral dose of 16 mg/kg of antipyrine was 12.7 ±SD 3.3 hr. After 2 wk on sodium phenobarbital (2 mg/kg daily) the half-life of antipyrine in the plasma of these twins was reduced to 8.0 ±SD 1.5 hr. Shortening of the plasma antipyrine half-life occurred in all but one of these 16 normal, adult volunteers, but there was considerable variation in the extent of reduction which ranged from 0 to 69%. Phenobarbital administration decreased individual variations in antipyrine metabolism as indicated by the smaller standard deviation of the plasma antipyrine half-lives after phenobarbital than observed initially and by the narrowed range of variation in plasma antipyrine half-lives from 2.8-fold initially to 1.8-fold after phenobarbital. These results suggest that some inducing agents may be used to minimize individual variations in drug metabolism where such variations create therapeutic problems by exposing patients who slowly metabolize certain drugs to toxicity and other patients who rapidly metabolize some drugs to undertreatment.
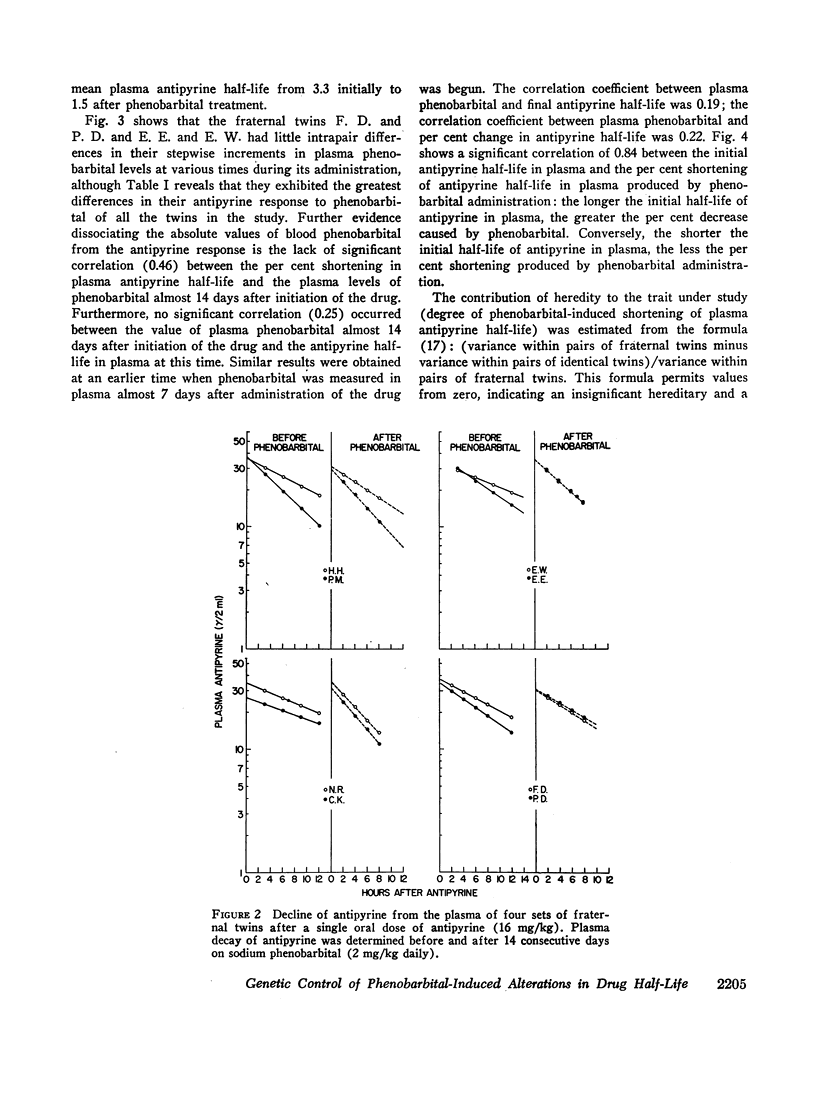
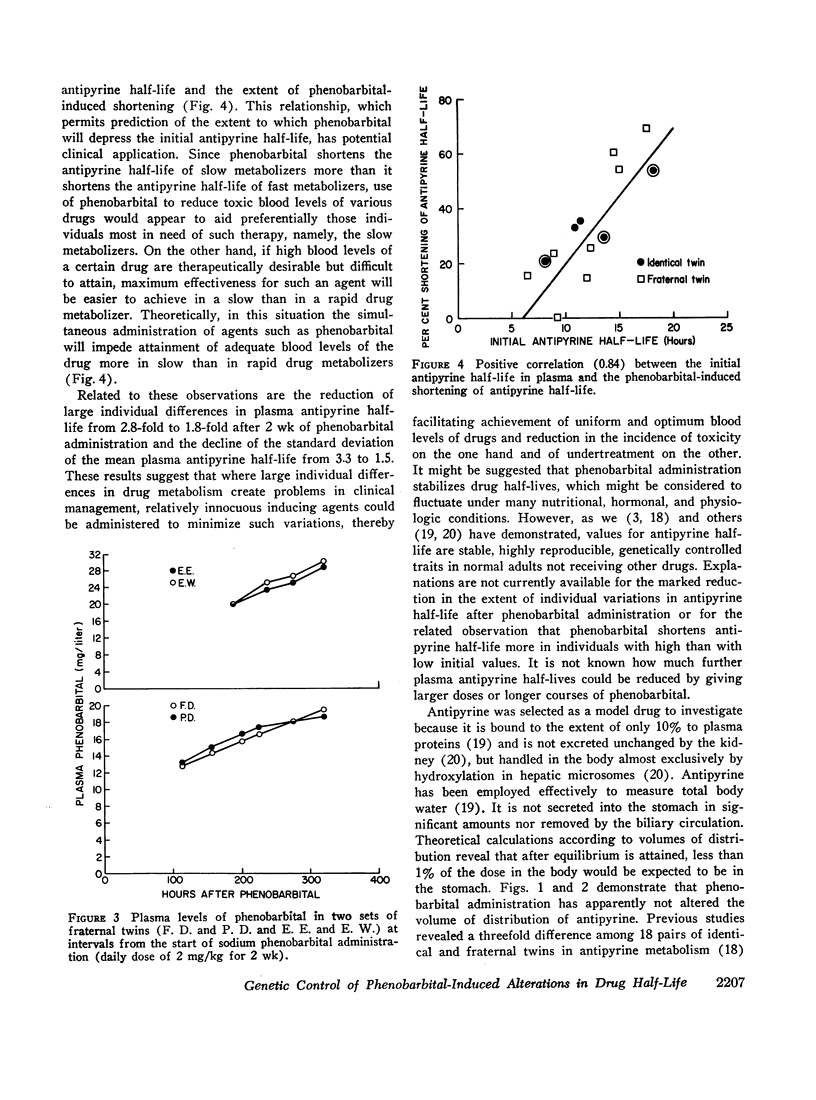
During the course of phenobarbital administration blood levels were determined. Phenobarbital blood levels correlated neither with the final values for plasma antipyrine half-lives nor with the per cent reduction in plasma antipyrine half-life produced by phenobarbital treatment. There was a direct relationship between initial antipyrine half-lives and the per cent shortening of antipyrine half-life produced by phenobarbital administration: the shorter the initial antipyrine half-life, the less the reduction caused by phenobarbital treatment. Larger intrapair variances in fraternal than in identical twins indicate genetic, rather than environmental, control of phenobarbital-induced alterations in plasma antipyrine half-life.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODIE B. B., AXELROD J. The fate of antipyrine in man. J Pharmacol Exp Ther. 1950 Jan;98(1):97–104. [PubMed] [Google Scholar]
- BUTLER T. C., MAHAFFEE C., WADDELL W. J. Phenobarbital: studies of elimination, accumulation, tolerance, and dosage schedules. J Pharmacol Exp Ther. 1954 Aug;111(4):425–435. [PubMed] [Google Scholar]
- CRAM R. L., JUCHAU M. R., FOUTS J. R. DIFFERENCES IN HEPATIC DRUG METABOLISM IN VARIOUS RABBIT STRAINS BEFORE AND AFTER PRETREATMENT WITH PHENOBARBITAL. Proc Soc Exp Biol Med. 1965 Apr;118:872–875. doi: 10.3181/00379727-118-29994. [DOI] [PubMed] [Google Scholar]
- CUCINELL S. A., CONNEY A. H., SANSUR M., BURNS J. J. DRUG INTERACTIONS IN MAN. I. LOWERING EFFECT OF PHENOBARBITAL ON PLASMA LEVELS OF BISHYDROXYCOUMARIN (DICUMAROL) AND DIPHENYLHYDANTOIN (DILANTIN). Clin Pharmacol Ther. 1965 Jul-Aug;6:420–429. doi: 10.1002/cpt196564420. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Drug metabolism and therapeutics. N Engl J Med. 1969 Mar 20;280(12):653–660. doi: 10.1056/NEJM196903202801210. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Crigler J. F., Jr, Gold N. I. Effect of sodium phenobarbital on bilirubin metabolism in an infant with congenital, nonhemolytic, unconjugated hyperbilirubinemia, and kernicterus. J Clin Invest. 1969 Jan;48(1):42–55. doi: 10.1172/JCI105973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayton P. G., Cucinell S. A., Weiss M., Perel J. M. Dose-dependence of drug plasma level decline in dogs. J Pharmacol Exp Ther. 1967 Nov;158(2):305–316. [PubMed] [Google Scholar]
- Hunninghake D. B., Azarnoff D. L. Drug interactions with warfarin. Arch Intern Med. 1968 Apr;121(4):349–352. [PubMed] [Google Scholar]
- Mitoma C., Sorich T. J., 2nd, Neubauer S. E. The effect of caffeine on drug metabolism. Life Sci. 1968 Feb 1;7(3):145–151. doi: 10.1016/0024-3205(68)90328-7. [DOI] [PubMed] [Google Scholar]
- Page J. G., Vesell E. S. Hepatic drug metabolism in ten strains of Norway rat before and after pretreatment with phenobarbital. Proc Soc Exp Biol Med. 1969 May;131(1):256–261. doi: 10.3181/00379727-131-33853. [DOI] [PubMed] [Google Scholar]
- Ramboer C., Thompson R. P., Williams R. Controlled trials of phenobarbitone therapy of neonatal jaundice. Lancet. 1969 May 10;1(7602):966–968. doi: 10.1016/s0140-6736(69)91862-5. [DOI] [PubMed] [Google Scholar]
- Rubin E., Lieber C. S. Alcohol, other drugs, and the liver. Ann Intern Med. 1968 Nov;69(5):1063–1067. doi: 10.7326/0003-4819-69-5-1063. [DOI] [PubMed] [Google Scholar]
- Vesell E. S. Factors altering the responsiveness of mice to hexobarbital. Pharmacology. 1968;1(2):81–97. doi: 10.1159/000135949. [DOI] [PubMed] [Google Scholar]
- Vesell E. S., Page J. G. Genetic control of dicumarol levels in man. J Clin Invest. 1968 Dec;47(12):2657–2663. doi: 10.1172/JCI105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. The distribution and excretion of phenobarbital. J Clin Invest. 1957 Aug;36(8):1217–1226. doi: 10.1172/JCI103518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel D. G., Broadie L. L. Stimulatory effect of nicotine on the metabolism of meprobamate. Toxicol Appl Pharmacol. 1966 May;8(3):455–459. doi: 10.1016/0041-008x(66)90055-x. [DOI] [PubMed] [Google Scholar]
- Yaffe S. J., Levy G., Matsuzawa T., Baliah T. Enhancement of glucuronide-conjugating capacity in a hyperbilirubinemic infant due to apparent enzyme induction by phenobarbital. N Engl J Med. 1966 Dec 29;275(26):1461–1466. doi: 10.1056/NEJM196612292752602. [DOI] [PubMed] [Google Scholar]



