Abstract
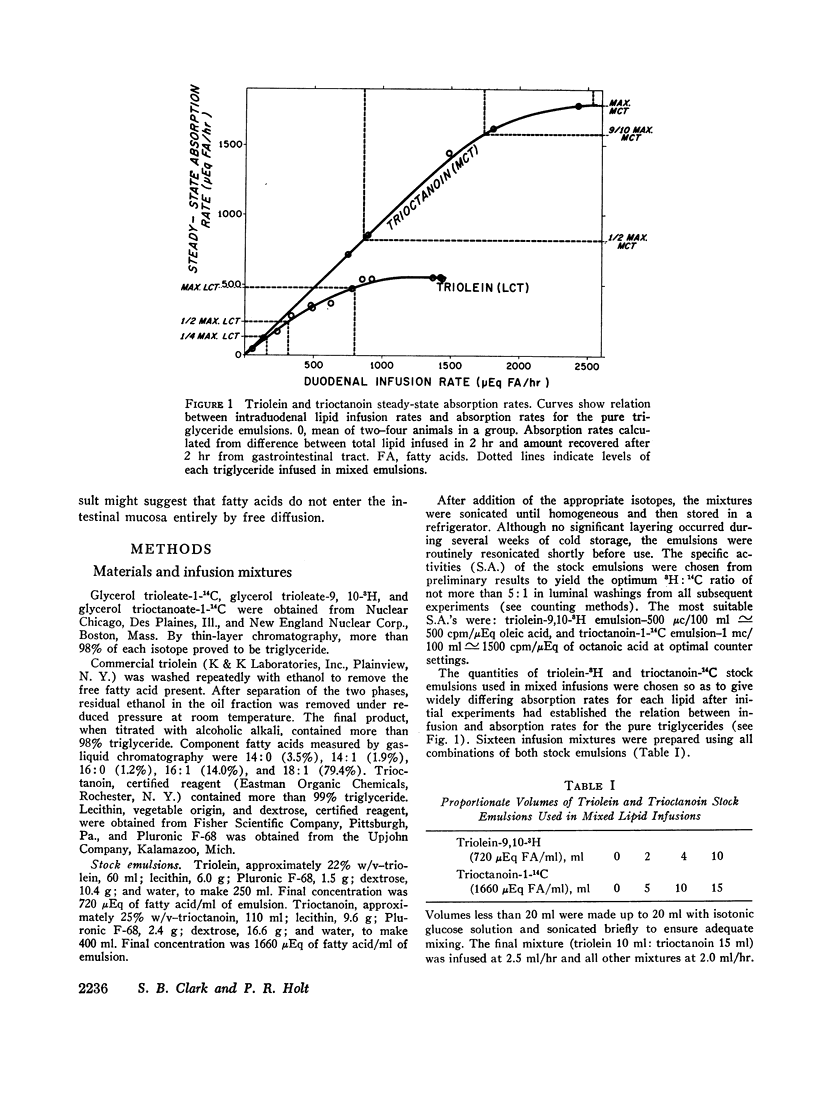
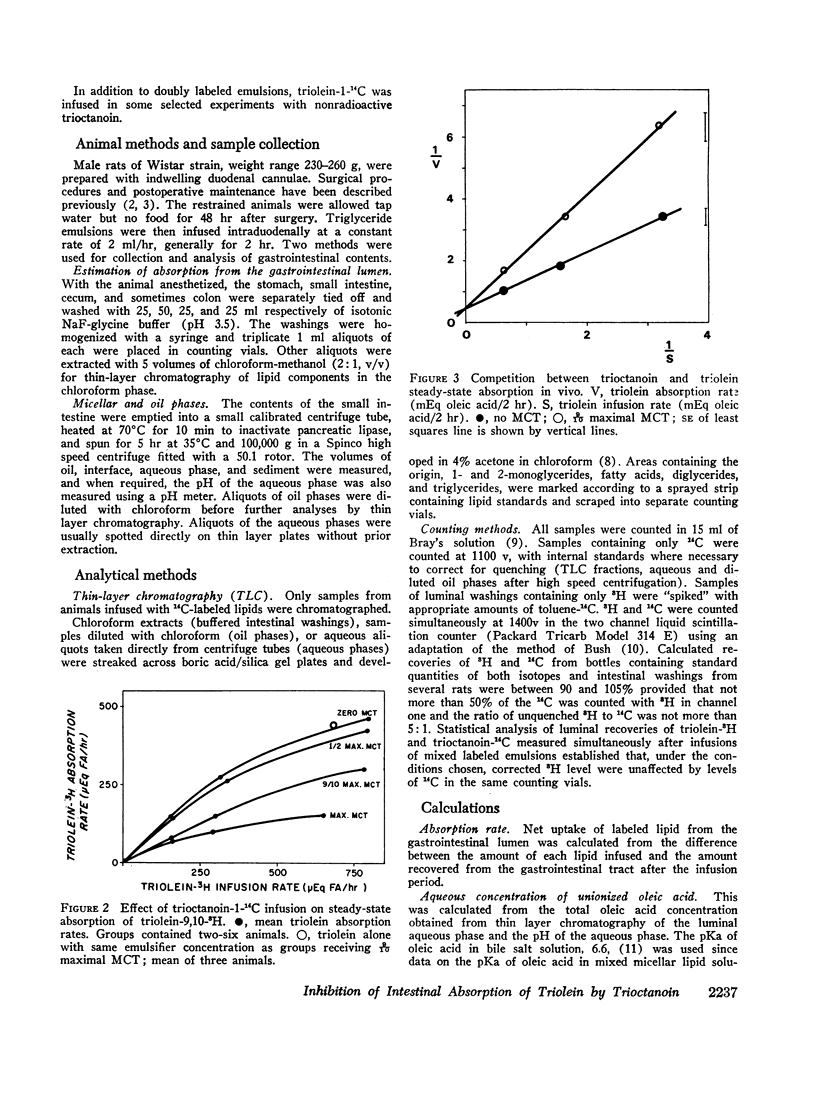
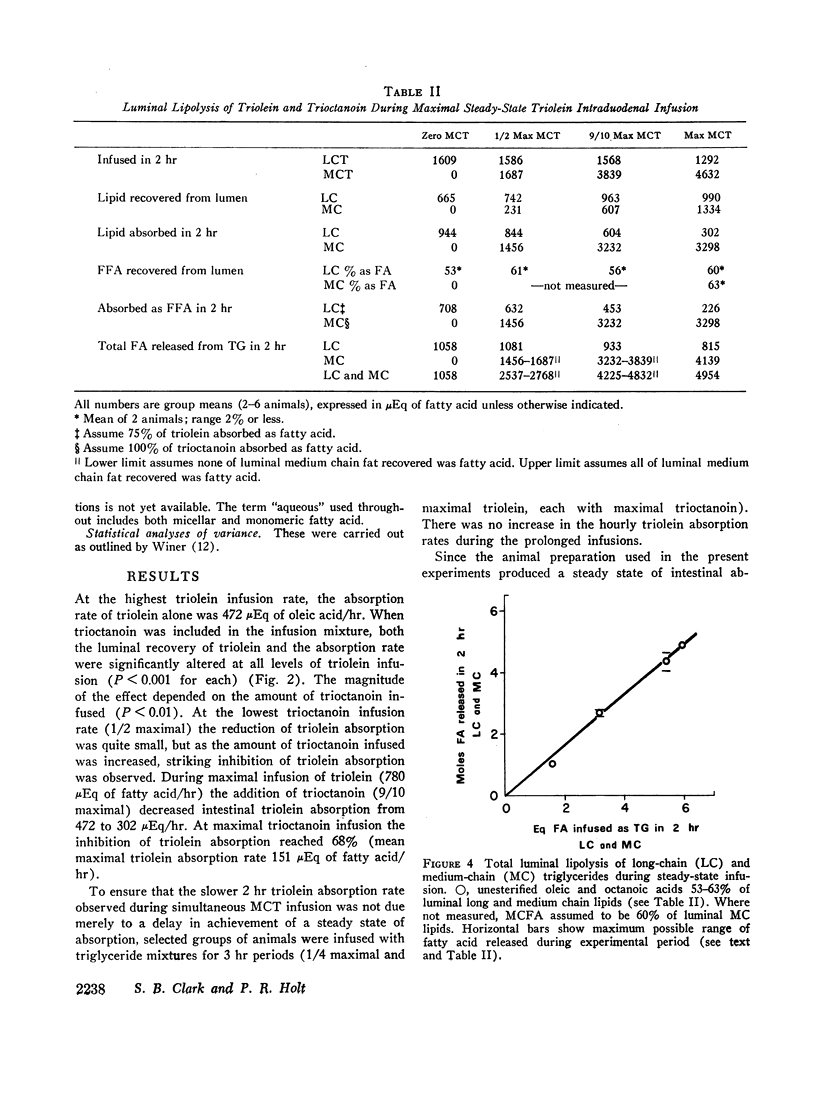
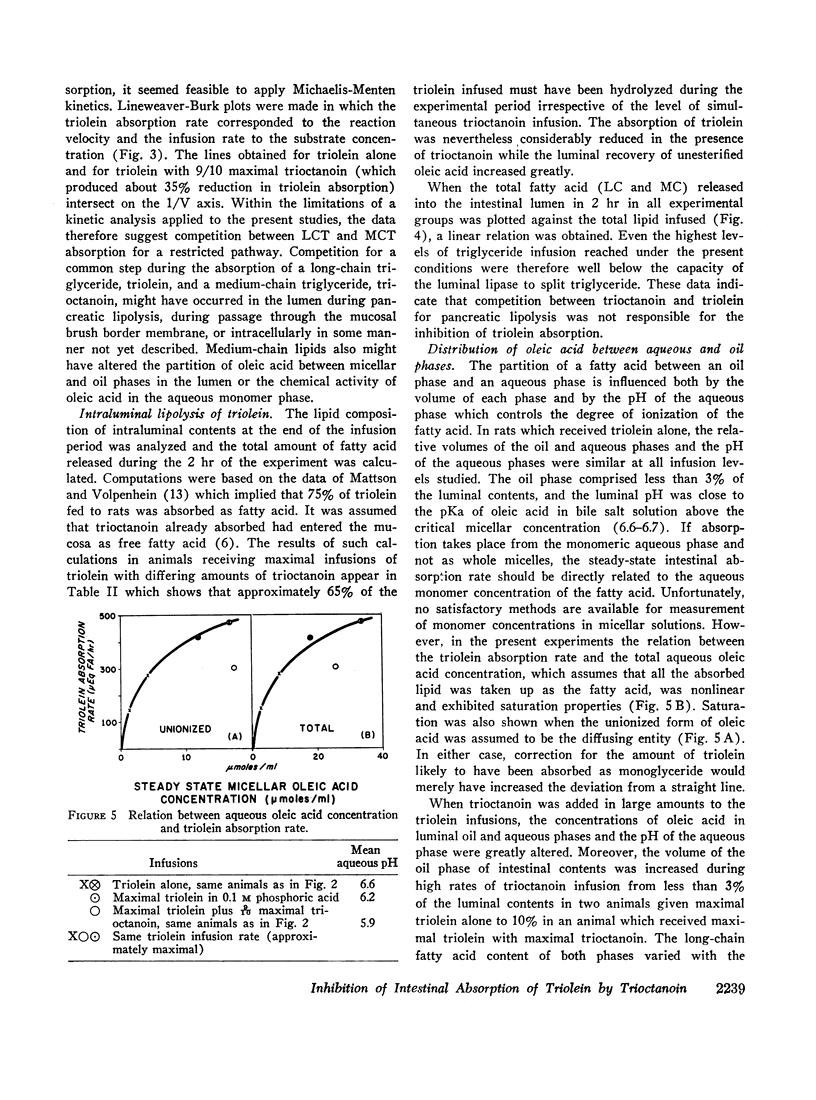
Maximal steady-state intestinal absorption rates in unanesthetized rats for triolein, a long-chain triglyceride, and for trioctanoin, a medium-chain triglyceride, are known to differ. Both these lipids are hydrolyzed in the intestinal lumen but the products of hydrolysis are metabolized differently by the mucosal cell. Intraduodenal infusion of trioctanoin was found to reduce steady-state triolein absorption. Luminal lipolysis was shown not to be rate-controlling. High rates of trioctanoin infusion significantly lowered the pH of the luminal aqueous phase and altered the partition of oleic acid between aqueous and oil phases. Two possible mechanisms for the inhibition of triolein uptake are considered. In the intestinal lumen medium chain lipids might have lowered the activity of oleic acid monomers in the aqueous phase and reduced passive diffusion into mucosal cells. Alternatively, competition between long and medium chain fatty acids for some common receptor during transport into the intestinal mucosal cell may have occurred.
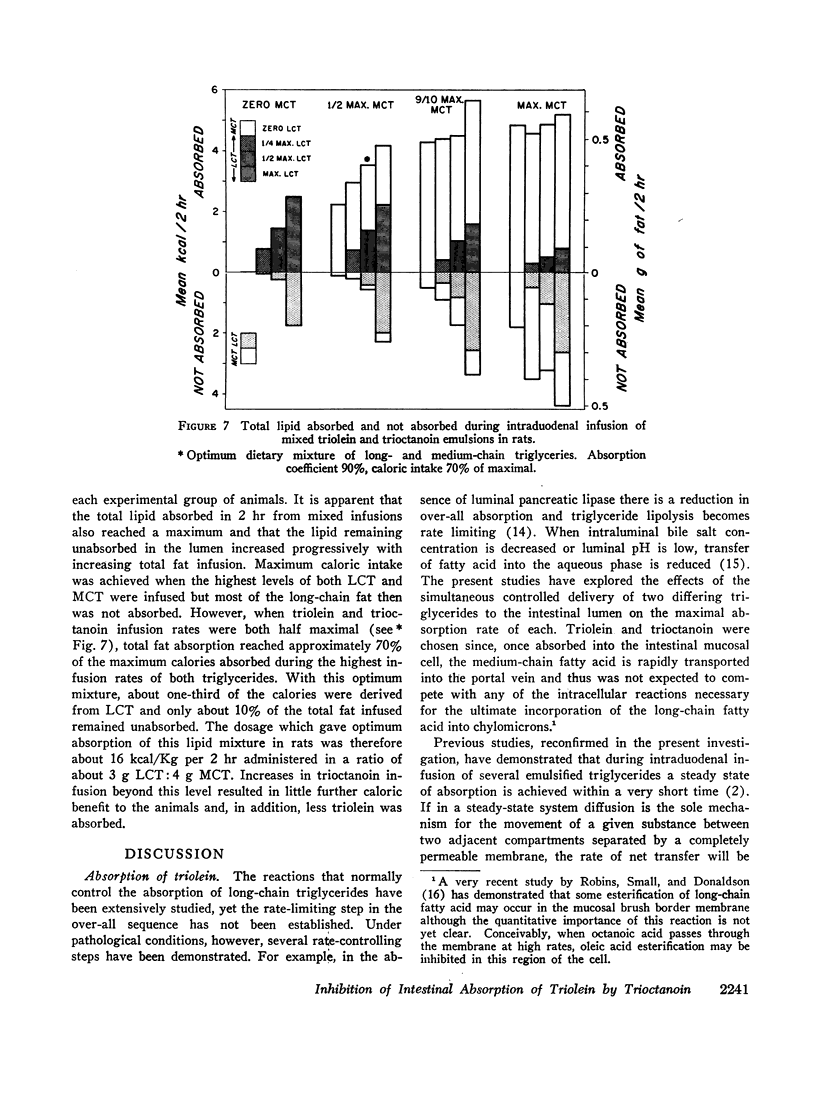
Despite significant inhibition of triolein absorption by high levels of trioctanoin, the maximum number of calories absorbed from mixtures of triglycerides exceeded the maxima from either glyceride alone. The optimum proportion of triolein to trioctanoin in lipid infusion mixtures was about 3:4 by weight and the optimum dosages about half maximal for each triglyceride, which represented a caloric intake of 4 kcal/rat per 2 hr. The absorption coefficient for this lipid mixture was about 90%. It is suggested that in patients who have a limited intestinal absorptive capacity dietary fat intake might be doubled with a caloric supplement of medium-chain triglycerides without increase in steatorrhea of long-chain fat.
Full text
PDF
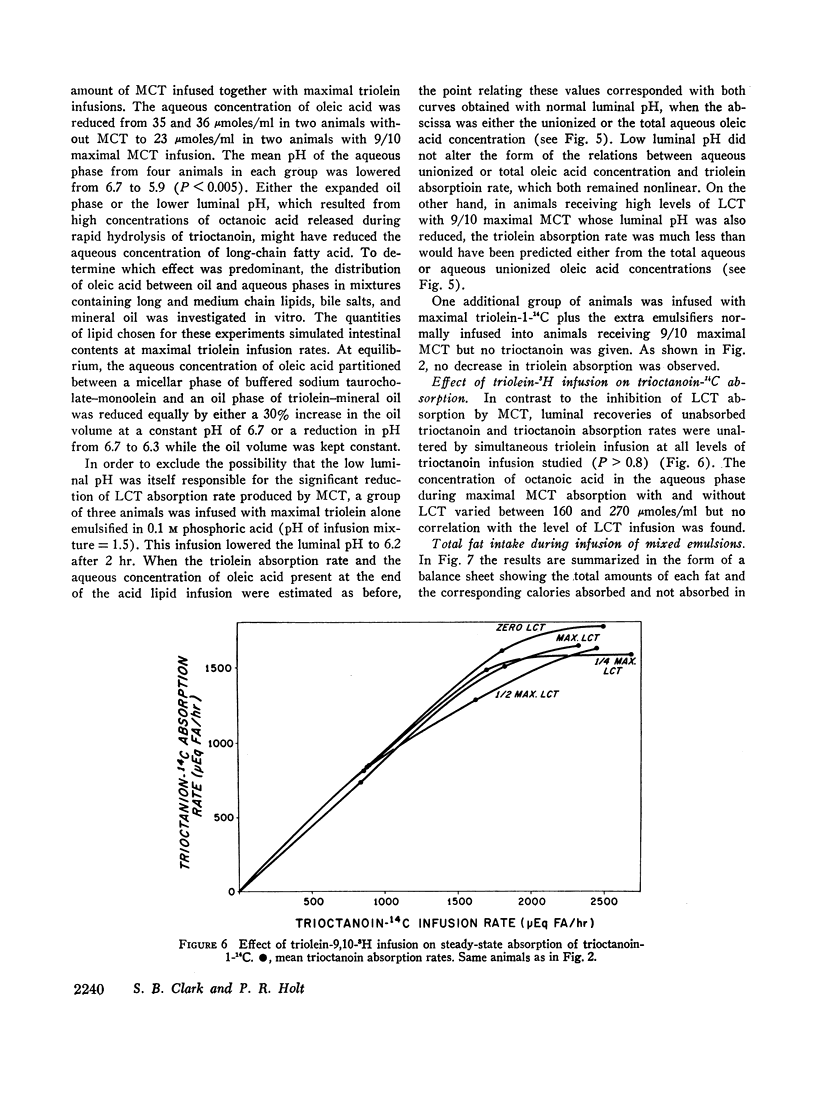


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. M. Intestinal malabsorption in childhood. Arch Dis Child. 1966 Dec;41(220):571–596. doi: 10.1136/adc.41.220.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNETT S. INTESTINAL ABSORPTIVE CAPACITY AND SITE OF ABSORPTION OF FAT UNDER STEADY STATE CONDITIONS IN THE UNANAESTHETIZED RAT. Q J Exp Physiol Cogn Med Sci. 1964 Apr;49:210–218. doi: 10.1113/expphysiol.1964.sp001721. [DOI] [PubMed] [Google Scholar]
- BENNETT S., SIMMONDS W. J. Absorptive capacity and intestinal motility in unanaesthetized rats during intraduodenal infusion of fat. Q J Exp Physiol Cogn Med Sci. 1962 Jan;47:32–38. doi: 10.1113/expphysiol.1962.sp001572. [DOI] [PubMed] [Google Scholar]
- Barry R. J., Jackson M. J., Smyth D. H. Transfer of propionate by rat small intestine in vitro. J Physiol. 1966 Jan;182(1):150–163. doi: 10.1113/jphysiol.1966.sp007815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. B., Holt P. R. Rate-limiting steps in steady-state intestinal absorption of trioctanoin-1-14C. Effect of biliary and pancreatic flow diversion. J Clin Invest. 1968 Mar;47(3):612–623. doi: 10.1172/JCI105757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESNUELLE P., SAVARY P. SPECIFICITIES OF LIPASES. J Lipid Res. 1963 Oct;4:369–384. [PubMed] [Google Scholar]
- FERNANDES J., VAN DE KAMER J. H., WEIJERS H. A. The absorption of fats studied in a child with chylothorax. J Clin Invest. 1955 Jul;34(7 Pt 1):1026–1036. doi: 10.1172/JCI103152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERNANDES J., van de KAMER J. H., WEIJERS H. A. Differences in absorption of the various fatty acids studied in children with steatorrhea. J Clin Invest. 1962 Mar;41:488–494. doi: 10.1172/JCI104502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFMANN A. F., BORGSTROEM B. THE INTRALUMINAL PHASE OF FAT DIGESTION IN MAN: THE LIPID CONTENT OF THE MICELLAR AND OIL PHASES OF INTESTINAL CONTENT OBTAINED DURING FAT DIGESTION AND ABSORPTION. J Clin Invest. 1964 Feb;43:247–257. doi: 10.1172/JCI104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFMANN A. F., BORGSTROM B. Hydrolysis of long-chain monoglycerides in micellar solution by pancreatic lipase. Biochim Biophys Acta. 1963 Jun 18;70:317–331. doi: 10.1016/0006-3002(63)90755-8. [DOI] [PubMed] [Google Scholar]
- Hofmann A. F. A physicochemical approach to the intraluminal phase of fat absorption. Gastroenterology. 1966 Jan;50(1):56–64. [PubMed] [Google Scholar]
- Hogben C. A. Fat absorption: a transport problem. Gastroenterology. 1966 Jan;50(1):51–55. [PubMed] [Google Scholar]
- Hyun S. A., Vahouny V., Treadwell C. R. Mechanism of stimulation of cholesterol absorption by 2-ethyl-n-caproic acid in vivo. Biochim Biophys Acta. 1967 Apr 4;137(2):306–314. doi: 10.1016/0005-2760(67)90106-3. [DOI] [PubMed] [Google Scholar]
- Lyon I. Studies on transmural potentials in vitro in relation to intestinal absorption. V. Kinetic characteristics of lipid interactions with rat gut. Biochim Biophys Acta. 1968 Aug;163(1):75–84. doi: 10.1016/0005-2736(68)90034-5. [DOI] [PubMed] [Google Scholar]
- MATTSON F. H., VOLPENHEIN R. A. THE DIGESTION AND ABSORPTION OF TRIGLYCERIDES. J Biol Chem. 1964 Sep;239:2772–2777. [PubMed] [Google Scholar]
- PORTE D., Jr, ENTENMAN C. FATTY ACID METABOLISM IN SEGMENTS OF RAT INTESTINE. Am J Physiol. 1965 Apr;208:607–614. doi: 10.1152/ajplegacy.1965.208.4.607. [DOI] [PubMed] [Google Scholar]
- Vahouny G. V., Nelson J., Treadwell C. R. Inhibition of triglyceride synthesis in everted intestinal sacs. Proc Soc Exp Biol Med. 1968 Jun;128(2):495–500. doi: 10.3181/00379727-128-33049. [DOI] [PubMed] [Google Scholar]


