Abstract
We describe here mutants of Mycoplasma pulmonis that were obtained by using a minitransposon, Tn4001TF1, which actively transposes but is then unable to undergo subsequent excision events. Using Tn4001TF1, we disrupted 39 genes previously thought to be essential for growth. Thus, the number of genes required for growth has been overestimated. This study also revealed evidence of gene duplications in M. pulmonis and identified chromosome segregation proteins that are dispensable in mycoplasmas but essential in Bacillus subtilis.
Introduction
Because of their small genomes, mycoplasmas are commonly chosen as model organisms for the study of the minimal gene set needed to support the growth of free-living bacteria and are ideal for dissecting the role of individual genes in pathogenesis. The most comprehensive work has been performed with Mycoplasma genitalium. Using transposon mutagenesis, 387 of the 480 protein-coding regions and all of the 37 genes coding for RNA species were identified as essential for growth of M. genitalium (Glass et al., 2006). Because of the lack of gene duplication and the absence of redundant pathways, it should be expected that the 580-kb genome of M. genitalium would have more essential genes than a bacterium with a large genome such as Bacillus subtilis, a Gram-positive bacterium to which the mycoplasmas are phylogenetically related. Indeed, only 271 of the 4,100 genes of B. subtilis are thought to be essential (Kobayashi et al., 2003).
Mycoplasma pulmonis is a pathogen of rats and mice and the etiological agent of murine respiratory mycoplasmosis (Lindsey & Cassell, 1973). In an earlier study of a transposon library of M. pulmonis, 321 of the 782 protein-coding regions were identified as dispensable for growth (French et al., 2008). The criteria used to consider a gene to be inactivated were that at least 10% of the coding region from the annotated 5′ start site or at least 15% of the coding region from the 3′ end would be truncated. The size of the library was large enough to conclude that nonessential genes greater than 1 kb were inactivated.
The previous studies relied on Tn4001T, which transposes actively in the mycoplasmal genome. A more recent study in Mycoplasma arthritidis made use of the Tn4001TF1 minitransposon, derived from Tn4001 (Dybvig et al., 2008). The minitransposon readily transposed into the genome, but once inserted, was unable to undergo subsequent transposition events. This minitransposon was applied to M. pulmonis in the current study. The use of the minitransposon generated a superior library with inactivation of 39 genes that were previously thought to be essential.
Materials and methods
Library construction
M. pulmonis strain CT (Davis et al., 1986) was propagated in mycoplasma broth (MB) and mycoplasma agar (MA) as described (Dybvig et al., 2000; Simmons & Dybvig, 2003). CT was transformed with plasmid pTF85, carrying minitransposon Tn4001TF1 (Dybvig et al., 2008), by using 36% polyethylene glycol and selecting for resistance to tetracycline as described (Dybvig et al., 2000). Individual colonies were picked, grown in 1 ml MB and stored at −80°C as described (French et al., 2008).
Mapping of transposon insertion sites
The genomic location of the transposon was determined for each library member by DNA sequence analysis of an inverse PCR product containing the junction between the transposon and the adjacent mycoplasmal DNA by using primers and reaction conditions as described (French et al., 2008; Teachman et al., 2002). The sequence of the junction was compared to the complete genome sequence for identification of the transposon insertion site (Chambaud et al., 2001). For each transformant that disrupted a gene in the current library that had not been disrupted in the previous library, the genomic position of the transposon was confirmed by performing two sets of PCR amplifications, analyzed on agarose gels stained with ethidium bromide, as described (French et al., 2008). The first set used a transposon-specific primer paired with a gene-specific primer. The presence of a PCR product of the predicted size indicated that the transposon was at the expected location provided that the same PCR product was absent when using the parental wild-type strain as template. For the second PCR amplification, two gene-specific primers were used that would flank the site of the transposon. If the expected product was obtained with wild-type DNA as template but no product was obtained with the transformant DNA as template, it was concluded that the gene was disrupted and that the transformant lacked a second, intact copy of the gene.
For some transformants, the PCR amplifications confirmed the location of the transposon but also detected the presence of an intact copy of the gene. In these cases, the transformant culture was subcloned and the two PCR reactions performed again on each subclone. Prior to subcloning, cell aggregates were disrupted by sonicating in a sonifier (model 250/450; Branson, Danbury, CT) at a power level of 5 and duty cycle of 10% for 20 sec. These conditions maximally increased the CFU of the cultures. No gene was considered to be disrupted unless the PCR data indicated that at least one subclone had the transposon at the expected site with no intact copy of the gene.
Gene duplication
Rarely, the PCR data indicated that all subclones of a transformant had an intact copy of the gene that was disrupted by the transposon. The presence of both a disrupted and an intact copy of the gene suggested gene duplication. In these cases, the identity of the PCR products was confirmed by performing another PCR amplification. The products from the first amplification that had used primers that flanked the insertion site of the transposon were used as template, and an internal set of primers were used for amplification. In cases where there was doubt about the results, the PCR products were also sequenced.
Results and discussion
Disrupted genes
A total of 1210 different minitransposon insertion sites were mapped. Thus, the library is smaller than the original Tn4001T library for which 1856 different insertion sites were mapped (French et al., 2008). Combined, the libraries provide excellent coverage with on average a transposon insertion site every 300 bp in the 960-kb genome of M. pulmonis.
Using the same criteria for considering a gene to be inactivated as described above for the previous library, 84 genes were inactivated by the minitransposon but not by Tn4001T in the original library (see Table S1) with 39 of these genes previously identified as essential for growth (French et al., 2008). For each of the 84 genes, PCR analyses confirmed the location of the transposon and demonstrated the absence of an intact copy of the gene. The 321 genes inactivated in the original library and the 84 additional genes inactivated in the minitransposon library bring the total number of inactivated genes in M. pulmonis to 405. None of the genes coding for RNA species were disrupted in the transposon libraries.
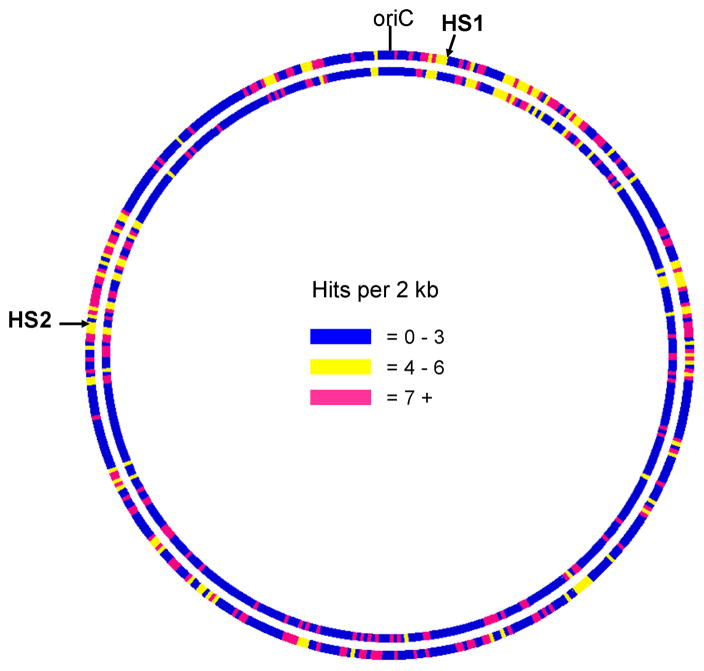
The 1.4-kb NADH oxidase gene (MYPU_0230) was disrupted in the minitransposon library. In the original library transposon insertions mapped to this gene in 27 transformants, but in each case additional PCR analyses failed to confirm the position of the transposon in MYPU_0230 (French et al., 2008). Because the minitransposon inactivated genes thought to be essential such as MYPU_0230, the distribution of the transposon insertion sites was examined for both libraries. The distribution found to be highly similar (Fig. 1). Most of the differences may be due to random chance, with the exception of two hot spots for transposon insertion that were identified in the original library as HS1 and HS2 (French et al., 2008). In the minitransposon library, the density of transposon insertion sites within HS1 and HS2 was not higher than for other regions and hence the distribution of transposon insertions may be more uniform.
Fig. 1.
Map illustrating the density of transposon insertion sites in the original Tn4001T library (outer circle) and the Tn4001TF1 minitransposon library (inner circle). The genome was divided into 480 blocks of 2 kb each with the number of transposon insertion sites color coded as indicated. oriC refers to the chromosomal origin of replication. HS1 and HS2 refer to hot spots for transposon insertion in the Tn4001T library that are absent in the Tn4001TF1 library.
Because there were no substantial differences in the distribution of transposon insertion sites in the libraries, alternative explanations for the inactivation of what were previously thought to be essential genes were considered. One possibility was that some nonessential genes are required for optimal growth and mutants with these genes disrupted were lost from the original library due to transposon excision, which is known to occur precisely at a high frequency (Krause et al., 1997; Mahairas et al., 1989). Growth curves were performed and the doubling times calculated as described (Dybvig et al., 1989). The wild-type parent and a transformant that contained the minitransposon at an intergenic site had doubling times of 2.0 hours with a standard deviation (SD) of 0.1 hours. The minitransposon mutant with a disruption in the NADH oxidase gene had a doubling time of 3.2 hours (SD = 0.1 hours). With a reduction in growth rate by 50%, ample opportunity exists for revertants to eventually dominate a culture. Tn4001 excision is often precise (Mahairas et al., 1989) and occurs at a high frequency in M. pulmonis (Dybvig et al., 2000). Thus, reversion due to loss of the transposon would be commonplace when using Tn4001T but not when using the minitransposon. Orthologs of 18 of the 84 genes knocked out in the minitransposon library but not the original library were identified previously (Glass et al., 2006) as being essential in M. genitalium (Table 1). These 18 genes lack any obvious paralog in M. pulmonis that might have compensated for the gene loss. Many of these 18 genes may be similarly nonessential in M. genitalium but not inactivated previously due to the reversions that occur when using an active transposon.
Table 1.
Genes disrupted by Tn4001TF1 but reportedly essential in M. genitalium or B. subtilis
| Gene | Product | Gene length (bp) | Mg ortholog | Essential gene in* |
|
|---|---|---|---|---|---|
| Mg | Bs | ||||
| 230 | NADH oxidase | 1434 | MG275 | Yes | |
| 250 | Lipoprotein, nuclease | 768 | MG186 | Yes | |
| 870 | DNA-binding protein Hu | 378 | MG353 | Yes | |
| 1060 | Protoporphirogen oxidase HemK | 690 | MG259 | Yes | |
| 1150 | Segregation and condensation protein A ScpA | 747 | MG213 | No | Yes |
| 2370 | Phosphate acetyltransferase Oligopeptide ABC transporter permease protein |
954 | MG299 | Yes | |
| 2840 | OppC Oligopeptide ABC transporter ATP-binding protein |
1152 | MG078 | Yes | |
| 2850 | OppD | 1101 | MG079 | Yes | |
| 2980 | Pseudouridylate synthase, D | 888 | MG209 | Yes | |
| 3010 | Conserved hypothetical protein | 921 | MG265 | Yes | |
| 3140 | Deoxyribose-phosphate adolase DeoC | 687 | MG050 | Yes | |
| 3220 | Adenine phosphoribosyltransferase | 510 | MG276 | Yes | |
| 3640 | Glucose permease | 1806 | MG069 | Yes | |
| 3800 | GTP-binding protein | 879 | MG387 | Yes | |
| 5060 | Methionine sulfoxide reductase B | 435 | MG448 | Yes | |
| 5110 | Transketolase | 1845 | MG066 | No | Yes |
| 6210 | Endonuclease IV | 831 | MG235 | Yes | |
| 6990 | ATP synthase beta chain | 1404 | MG399 | Yes | |
| 7140 | Chromosome segregation ATPase SMC | 2937 | MG298 | No | Yes |
| 7260 | Leucine aminopeptidase | 1362 | MG391 | Yes | |
| 7620 | Pyruvate dehydrogenase E2 component | 945 | MG272 | Yes | |
Essential genes in Mg (M. genitalium) and Bs (B. subtilis) as reported by Glass et al., 2006 and Kobayashi et al., 2003.
The essential genes of mycoplasmas have been compared often to those of Bacillus subtilis because of their phylogenetic relationship (Dybvig et al., 2008; French et al., 2008; Glass et al., 2006). Three of the M. pulmonis genes knocked out by the minitransposon have essential orthologs in B. subtilis (Table 1). Interestingly, orthologs of these three genes are nonessential in M. genitalium. The tkt gene coding for transketolase is essential in B. subtilis for growth in minimal medium when using glucose as the sole carbon source (Kobayashi et al., 2003) but is nonessential when alternative carbon sources and aromatic amino acids are available (Sasajima & Yoneda, 1974; Sasajima & Kumada, 1981). The finding that tkt (MYPU_5110) is nonessential in mycoplasmas is not surprising because of the rich medium required for growth. The other two genes that are essential for growth of B. subtilis but not the mycoplasmas coded for SMC (MYPU_ 7140 gene product) and the segregation and condensation protein ScpA (MYPU_ 1150 gene product). These proteins co-localize in B. subtilis and are required for growth at temperatures above 23°C and for normal chromosome segregation (Mascarenhas et al., 2002). The M. pulmonis mutants used in this study were grown at 37°C, the optimal growth temperature for this organism. Perhaps the processes of chromosome segregation and cell division differ in mycoplasmas from those of other bacteria because of the lack of a cell wall, rendering the SMC and ScpA proteins dispensable under normal growth conditions.
Gene duplication
PCR analysis of minitransposon mutants provided evidence for gene duplication. For some mutants, the PCR amplifications performed to verify that a gene was disrupted yielded a product confirming that the transposon disrupted the gene but also yielded a second product indicative of an intact copy of the gene. The discrepancy could be resolved usually by subcloning the mutant. In most cases, when individual subclones were analyzed by PCR, at least one subclone had the gene disrupted with no intact copy present. Thus, the gene was mutable. In a few cases, the PCR analyses indicated that all subclones, five were analyzed, had both a disrupted and an intact copy of the gene (Table 2). The duplications were not necessary to maintain viability due to the inactivation of essential genes. The genes disrupted in transformants JS003 and JS170 are not essential because other transformants in the library had the same genes inactivated without an intact copy being present, and transformant JS620 has the transposon inserted into an intergenic region with apparent duplication. Little is known about the frequency and size of duplications in mycoplasmal genomes, but several examples of duplicated sequences have previously been described in M. pulmonis (Bhugra & Dybvig, 1993; Dybvig et al., 1998; Dybvig et al., 2007; Shen et al., 2000). Duplication events may result from DNA replication errors or involve gene transfer between cells of M. pulmonis (Teachman et al., 2002).
Table 2.
Transformants containing possible gene duplication
| Transformant | Nucleotide position | Gene | Product | Gene length (bp) | Gene inactivated in other transformants of M. pulmonis |
|---|---|---|---|---|---|
| JS003 | 187277 | MYPU_1780 | tRNA/rRNA methyltransferase | 687 | Yes |
| JS620 | 642673 | Intergenic | NA | NA | NA |
| JS170 | 927936 | MYPU_7570 | Hypothetical protein | 294 | Yes |
Concluding remarks
These results underscore the important consideration that past studies have inferred the essentiality of a mycoplasmal gene based on the use of elements that transpose actively in the genome and thus have overestimated the minimal gene set. The use of minitransposons that are stable once inserted into the genome give a more accurate appraisal of gene essentiality.
Supplementary Material
Acknowledgments
This work was supported by NIH grant AI63909.
References
- Bhugra B, Dybvig K. Identification and characterization of IS1138, a transposable element from Mycoplasma pulmonis that belongs to the IS3 family. Mol Microbiol. 1993;7:577–584. doi: 10.1111/j.1365-2958.1993.tb01148.x. [DOI] [PubMed] [Google Scholar]
- Chambaud I, Heilig R, Ferris S, et al. The complete genome of the murine respiratory pathogen Mycoplasma pulmonis. Nucleic Acids Res. 2001;29:2145–2153. doi: 10.1093/nar/29.10.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JK, Thorp RB, Parker RF, White H, Dziedzic D, D’Arcy J, Cassell GH. Development of an aerosol model of murine respiratory mycoplasmosis in mice. Infect Immun. 1986;54:194–201. doi: 10.1128/iai.54.1.194-201.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybvig K, Sitaraman R, French CT. A family of phase-variable restriction enzymes with differing specificities generated by high-frequency gene rearrangements. Proc Natl Acad Sci USA. 1998;95:13923–13928. doi: 10.1073/pnas.95.23.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybvig K, French CT, Voelker LL. Construction and use of derivatives of transposon Tn4001 that function in Mycoplasma pulmonis and Mycoplasma arthritidis. J Bacteriol. 2000;182:4343–4347. doi: 10.1128/jb.182.15.4343-4347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybvig K, Simecka JW, Watson HL, Cassell GH. High-frequency variation in Mycoplasma pulmonis colony size. J Bacteriol. 1989;171:5165–5168. doi: 10.1128/jb.171.9.5165-5168.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybvig K, Cao Z, French CT, Yu H. Evidence for type III restriction and modification systems in Mycoplasma pulmonis. J Bacteriol. 2007;189:2197–2202. doi: 10.1128/JB.01669-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybvig K, Zuhua C, Lao P, Jordan DS, French CT, Tu A-HT, Loraine AE. Genome of Mycoplasma arthritidis. Infect Immun. 2008;76:4000–4008. doi: 10.1128/IAI.00516-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French CT, Lao P, Loraine AE, Matthews BT, Yu H, Dybvig K. Large-scale transposon mutagenesis of Mycoplasma pulmonis. Mol Microbiol. 2008;69:67–76. doi: 10.1111/j.1365-2958.2008.06262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JI, Assas-Garcia N, Alperovich N, Yooseph S, Lewis MR, Maruf M, Hutchinson CA, III, Smith HO, Venter JC. Essential genes of a minimal bacterium. Proc Nat Acad Sci USA. 2006;103:425–430. doi: 10.1073/pnas.0510013103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Ehrlich SD, Albertini A, et al. Essential Bacillus subtilis genes. Proc Nat Acad Sci USA. 2003;100:4678–4683. doi: 10.1073/pnas.0730515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DC, Proft T, Hedreyda CT, Hilbert H, Plagens H, Herrmann R. Transposon mutagenesis reinforces the correlation between Mycoplasma pneumoniae cytoskeletal protein HMW2 and cytadherence. J Bacteriol. 1997;179:2668–2677. doi: 10.1128/jb.179.8.2668-2677.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey JR, Cassell GH. Experimental Mycoplasma pulmonis infection in pathogen-free mice. Models for studying mycoplasmosis of the respiratory tract. Amer J Pathol. 1973;72:63–90. [PMC free article] [PubMed] [Google Scholar]
- Mahairas GG, Lyon BR, Skurray RA, Pattee PA. Genetic analysis of Staphylococcus aureus with Tn4001. J Bacteriol. 1989;171:3968–3972. doi: 10.1128/jb.171.7.3968-3972.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas J, Soppa J, Strunnikov AV, Graumann PL. Cell cycle-dependent localization of two novel prokaryotic chromosome segregation and condensation proteins in Bacillus subtilis that interact with SMC protein. EMBO J. 2002;21:3108–3118. doi: 10.1093/emboj/cdf314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasajima K, Yoneda M. D-Sedoheptulose 7-phosphate: D-glyceraldehyde 3-phosphate glycoaldehydetransferase and D-ribulose 5-phosphate 3-epimerase mutants of a Bacillus species. Agric Biol Chem. 1974;38:1305–1310. [Google Scholar]
- Sasajima K, Kumada T. Change in the regulation of enzyme synthesis under catabolite repression in Bacillus subtilis pleiotrophic mutant lacking transketolase. Agric Biol Chem. 1981;45:2005–2012. [Google Scholar]
- Shen X, Yu H, Gumulak J, French CT, Zou N, Dybvig K. Gene rearrangements in the vsa locus of Mycoplasma pulmonis. J Bacteriol. 2000;182:2900–2908. doi: 10.1128/jb.182.10.2900-2908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WL, Dybvig K. The Vsa proteins modulate susceptibility of Mycoplasma pulmonis to complement killing, hemadsorption, and adherence to polystyrene. Infect Immun. 2003;71:5733–5738. doi: 10.1128/IAI.71.10.5733-5738.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachman AM, French CT, Yu H, Simmons WL, Dybvig K. Gene transfer in Mycoplasma pulmonis. J Bacteriol. 2002;184:947–951. doi: 10.1128/jb.184.4.947-951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.