Abstract
Our understanding of the development of the retina and visual pathways has seen enormous advances during the past twenty-five years. New imaging technologies, coupled with advances in molecular biology, have permitted a fuller appreciation of the histotypical events associated with proliferation, fate determination, migration, differentiation, pathway navigation, target innervation, synaptogenesis and cell death, and in many instances, in understanding the genetic, molecular, cellular and activity-dependent mechanisms underlying those developmental changes. The present review considers those advances associated with the lineal relationships between retinal nerve cells, the production of retinal nerve cell diversity, the migration, patterning and differentiation of different types of retinal nerve cells, the determinants of the decussation pattern at the optic chiasm, the formation of the retinotopic map, and the establishment of ocular domains within the thalamus.
Keywords: proliferation, fate determination, migration, differentiation, stratification, mosaic, coverage, axonal outgrowth, decussation, retinotopic map, binocular segregation
Introduction
In 1986, when Vision Research published its 25th Anniversary Issue, there was no chapter dedicated to “developmental visual anatomy”, being the summary descriptor provided by the editors for the present chapter. The closest coverage was provided within a chapter on visual development, focusing upon the acquisition of visual function, the consequences of early visual deprivation or restricted visual exposure, and on the associated plasticity within visual cortex (Teller & Movshon, 1986). It is interesting to re-visit that historical overview now, twenty-five years later, to appreciate the excitement within the field during those golden years of visual neurophysiology. Three pioneers in our understanding of the development of the visual system received the Nobel Prize in Physiology or Medicine during that era, in 1981, Roger Sperry, David Hubel and Torsten Wiesel, and the contributions of two of them feature prominently within that article. As acknowledged by the authors, “In 1960, the neurobiology of visual development was dominated by the work of Roger Sperry”. But rather than this being the prelude to a tribute, Sperry is taken to task for his preoccupation with the hard-wiring of the visual pathway, and his impact for the era under review was largely dismissed: ‘Sperry’s relentless emphasis on the independence of neural development from neural function in the developing animal was to have a short life after 1961" (p. 1486, original italics).
Since that anniversary issue in 1986, the past twenty-five years have witnessed unprecedented experimental as well as conceptual advances in our understanding of the development of the retina and sub-cortical visual pathways, much of it occurring well before the onset of visual function. Many of these advances vindicate a hard-wiring perspective such as Sperry’s, relying upon cell-signaling interactions independent of neural transmission, while others show that neural function long before the onset of photo-transduction plays a critical role in the formation of neural circuitry. The phenomenal scientific pace of the past twenty-five years has been made possible largely by new technologies that continue to expand the front of developmental neurobiology in general. The experimental advances have been a consequence of the revolution in molecular biology and by the availability of new imaging technologies, permitting genetic dissection of the molecular factors and cellular interactions underlying retinal and optic pathway development, and the visualization of single neurons or populations of cells as they pass through the cell cycle, express transcription factors and the downstream genes they regulate, migrate to their specific layers, differentiate their characteristic morphologies, navigate an axonal trajectory to central visual structures, establish and refine their synaptic connections, and undergo programmed cell death. The present review will not consider in detail those technical advances themselves; the reader is directed to another recent colorful review providing ample coverage of “this ever-expanding toolbox” (Mason, 2009). The consequent experimental results have led to new conceptual insights, altering the ways in which we think about retinal development and target innervation, and the present focus will be upon these changes in our understanding.
One should not fault the myopia of the former review too much; without a doubt, we simply could not appreciate the full nature of the neurobiological issues at play twenty-five years ago1. Visual cortex was where the action was, and electrophysiology was the tool of choice for understanding the mechanics underlying visual function. We now know so much more about the pre-visual development of the retina and sub-cortical visual pathways, from a decidedly cellular and molecular biological perspective, that I will restrict the present coverage accordingly, and unashamedly, as vision will hardly be mentioned.
By comparison with the other chapters in this special issue of Vision Research, the purview of the present chapter is vast, encompassing advances not only in our understanding of the various components establishing the complex architecture and connectivity of the neural retina, but also of the visual pathways and their innervation of target visual structures. Any such review of strides taken over a defined period of time must to some extent be idiosyncratic (as in that former paper), but I believe these issues largely summarize the major conceptual and experimental advances during the past twenty-five years. I have chosen to highlight eight issues, briefly recapitulating these advances and sacrificing much detail due to space limitations. Each of these topics has been reviewed in far greater detail elsewhere, and doubtless researchers working on development of the visual system will find reason enough to feel frustrated by the brevity of the present effort. Rather, my intended audience has been that collection of vision researchers that digest the literature on retinal and pathway development with only modest fervor, to give them a synopsis of the major advances during this era, as well as current students and post-docs working within this field of developmental neurobiology that may not appreciate the degree to which this field has advanced. The latter group need only compare the coverage of the developing retina and visual pathway provided by textbooks then in use (e.g. Jacobson, 1978; Purves & Lichtman, 1985) with that provided more recently (Sanes, Reh, & Harris, 2006) to appreciate the remarkable evolution in our understanding of these developmental processes. The former textbooks reflect the strong foundations of the field drawn from experimental embryology and neurophysiology but now seem sadly deficient in providing much account of the histotypical interactions between cells or of the genes expressed and molecular signals they set in motion that participate in these events.
Development of the retina
Twenty-five years ago, while we had some appreciation that an early eye field was derived from the neural plate and was critical for the development of the retina, we had no knowledge of the transcriptional control of this process by a handful of early eye-field genes that are now understood to command a downstream cascade of genes critical for assembling the mature retina (Zuber & Harris, 2006). As the eye cup emerges and expands in size, the factors modulating cell cycle kinetics have been dissected with increasing detail, including the molecular mechanisms driving interkinetic nuclear translocation, the intracellular and extracellular determinants of cell-cycle exit, and the factors that coordinate the wave of neurogenesis progressing from its site of initiation (Agathocleous & Harris, 2009; Baye & Link, 2007; Del Bene, Wehman, Link, & Baier, 2008; Dyer & Cepko, 2001; Levine & Green, 2004; Martins & Pearson, 2008; Norden, Young, Link, & Harris, 2009). The present coverage will begin with the emerging neural retina at the outset of neurogenesis, considering advances in our understanding of the lineage relationships between retinal neurons, the determination of neuronal cell-types and the production of species-specific retinal architecture, the control of neuronal positioning, and the determinants of morphological differentiation.
1. What are the lineal relationships between the different cell types of the retina?
Retinal progenitors were understood to expand the pool of post-mitotic precursor cells that would ultimately adopt various cellular fates, but there was no firm understanding of whether dedicated progenitors yielded particular types of cell, or if progenitors were multi-potent. While birth-dating studies had already shown that each type of retinal nerve cell was born in a distinct window during retinal neurogenesis (Carter-Dawson & LaVail, 1979; Dräger, 1985; Hinds & Hinds, 1979; Sidman, 1961; Young, 1985), these provided no insight into the clonal relationships between the cells of the retina. In the late 1980s, two different approaches were employed to label single retinal progenitor cells in order to identify their progeny at subsequent stages of maturity. One was to inject single cells with cytoplasmic tracers that would remain detectable within progeny despite progressive dilution following repeated cell divisions (Holt, Bertsch, Ellis, & Harris, 1988; Wetts & Fraser, 1988). The other was to use replication-deficient retroviruses encoding reporter genes to infect single cells, therein bypassing the problem of progressive dilution with repeated mitoses (Turner & Cepko, 1987; Turner, Snyder, & Cepko, 1990). Both approaches yielded comparable findings, that retinal progenitor cells were in fact multi-potent, producing clones of cells that included a variety of retinal neuronal types as well as Müller glia. They lacked, however, any retinal astrocytes, handily accounted for, at roughly the same time, by the demonstration that astrocytes are immigrants to the neural retina, being derived from a distinct progenitor cell in the optic stalk and migrating into the inner retina during the period of retinal neurogenesis (Ling & Stone, 1988; Stone & Dreher, 1987; Watanabe & Raff, 1988).
The retinal clones arising from such multi-potent progenitors were striking in their heterogeneity, particularly within the mouse retina, some containing relatively few cells while others containing in excess of 100 cells, averaging around 50 per clone (Turner et al., 1990). Not surprisingly, when progenitors were labeled at earlier stages during the neurogenetic period, clones were typically larger and included more cell types, specifically, those known to be generated at such earlier stages (Fekete, Perez-Miguelsanz, Ryder, & Cepko, 1994; Turner et al., 1990). Much of the variability at a given age had been interpreted to reflect the lineage-independence of fate assignments–that the micro-environment of the developing retina was largely responsible for ascribing a specific fate to a given precursor after it had left the cell cycle. This was challenged by other labeling strategies that marked retinal progenitors far earlier during development, suggesting a basic consistency to clonal organization in the mouse retina (Reese, Necessary, Tam, Faulkner-Jones, & Tan, 1999; Williams & Goldowitz, 1992a; 1992b). The retinas of such chimeric mice showed clones that maintained some approximation to a constant ratio of rods, bipolar cells, Muller glia and amacrine cells. The estimated number of such clonal units was far in excess of the number of cells making up the sparsest retinal cell types, consistent with some heterogeneity between retinal clones lacking certain minority cell types, but these results suggested some of the previously documented variability in clonal consistency in the rodent retina as being simply a consequence of tapping into the lineage tree at variable times and/or branches. More recently, other researchers have reported conspicuous variability in retinal clones when identified from the outset of neurogenesis, in Xenopus (Wong & Rapaport, 2009). Some of this discrepancy with the data from mouse may ultimately prove to be explained by a relative constancy in the clonal amplification of later-generated and predominant cell types typical of nocturnal rodents. Still others have found a surprising degree of reproducibility in the clonal constituency arising from progenitors expressing the transcription factor Ath5 (Poggi, Vitorino, Masai, & Harris, 2005), suggesting a strongly cell-intrinsic constraint on cellular fate.
The fact that birth-dating studies described, for nearly all species examined, gradients of neurogenesis for each type of cell that showed substantial spatio-temporal overlap (Harman & Beazley, 1987; Harman, Sanderson, & Beazley, 1992; Rapaport, Fletcher, LaVail, & Rakic, 1992; Rapaport, Wong, Wood, Yasumura, & LaVail, 2004; Reese & Colello, 1992; Sidman, 1961; Walsh & Polley, 1985; Young, 1985) was regarded as further evidence of a multi-potentiality amongst retinal progenitor cells that was not lineage-restricted. Nevertheless, certain trends were becoming increasingly apparent across species, in particular, that there was a conserved temporal ordering to these neurogenetic windows for the different cell types. Retinal ganglion cells were the first cell type to be produced, followed by horizontal cells and cones, while amacrine cells, rods, bipolar cells and Muller glia were produced progressively later (see Rapaport, 2006, for review). Given the temporal overlap in the neurogenetic windows for individual cell types in a patch of retina, it remained unclear whether single progenitors yielded cell types in a strict order, or if local variations in microenvironmental signals that changed over time were solely responsible for determining the fates of neighboring cells born at the same time.
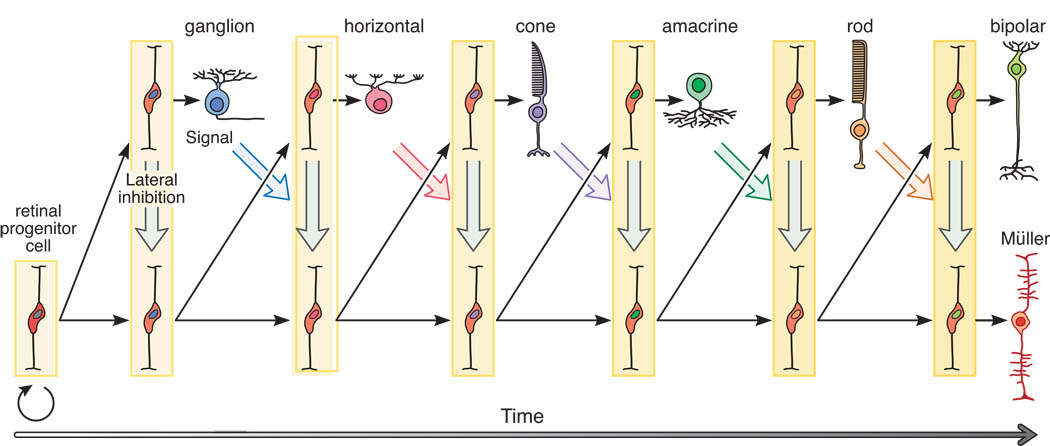
This issue was only very recently resolved in Xenopus, by identifying the constituents of single labeled clones marked at the earliest stages of eye-cup formation that had also been exposed to bromodeoxyuridine (Brdu) at some variable stage for the remainder of neurogenesis. The lack of Brdu, therefore, was a reliable indicator that a given cell in the clone had been born prior to all of the other Brdu+ cells within the clone. What was critically observed in this study was that there was a directionality in the order of cell production: once production of a particular cell type had commenced within a clone, the subsequently generated progeny within that clone virtually never included any members of those types that had already commenced neurogenesis earlier (Wong & Rapaport, 2009). Retinal progenitors, it would appear, are multi-potent, but their multi-potentiality declines as development proceeds. Not every progenitor will yield every type of retinal cell, but for those cell types that it does yield, they are produced in a precise ordering (figure 1). Unidirectional changes in competence apparently occur cell-autonomously, accounting for the fact that a progenitor might very well yield a cell type for which its immediate neighbor is no longer competent for production.
Figure 1.
Retinal progenitor cells are multipotent, competent to produce all types of retinal cell. This competence is progressively restricted, ensuring a unidirectional sequence of cell production, but not all progenitors at the outset of neurogenesis will produce each type of cell. At the left, early symmetrical divisions expand the population of progenitors. As their numbers increase, so do associated signals derived from them, eventually allowing proneural gene expression and Notch-Delta mediated lateral inhibition (vertical grey arrows) to permit only a subset of progenitors to leave the cell cycle (defining the onset of neurogenesis) and to differentiate. Newborn neurons provide signals (diagonal colored arrows) that restrict production of the same cell types, directing later-generated cells to subsequent fates. With the acquisition of additional cell types, so the micro-environment changes progressively (saturating vertical rectangles), and these extrinsic signals, coupled with intrinsic changes, alter the transcription factors expressed by progenitors as a function of time (colored nuclei). Thus changing cellular competence, intercellular signaling between progenitors, and secreted signaling by differentiating cells interact to yield variability in clonal constituency. The schematic implies a stem-like lineage tree, but by the insertion of additional divisions at various locations along this time-line, progenitors within this lineage tree may yield terminal divisions producing two postmitotic daughters (e.g. a ganglion cell and a cone photoreceptor, or a pair of horizontal cells, or a later-generated neuron and Muller glial cell, as shown at the far right), or daughters that both divide again, thereby amplifying the population of progenitors that produce later-generated cell types (e.g. rod photoreceptors and bipolar cells). (Modified from Agathocleous & Harris,2009).
2. How is the diversity of retinal cell types established?
This cell-autonomous behavior is only presumed to restrict the potentiality of retinal progenitors, not to specify the precise constituents that are produced within any particular clone. Twenty-five years ago, the role of environmental signals that impinge upon dividing progenitor cells and post-mitotic precursors was being investigated primarily using dissociated cell and retinal explant culture assays, but there was relatively little appreciation for the role of cell-intrinsic determinants that are now understood to participate in this process, permitting, repressing or directing cell fate decisions (but see Reh, 1991). The role of environmental signals, including those produced by newly differentiating cells of a given type that inhibit production of the same cell type, have been amply reviewed (Adler, 2000; Agathocleous & Harris, 2006; Lamba, Nelson, Karl, & Reh, 2008; Levine, Fuhrmann, & Reh, 2000; Lillien, 1998; Reichenbach et al., 1998). Some of those earlier studies described the fate changes produced in vitro when cultured cells developed in the presence of a particular type of cell (Waid & McLoon, 1998; Watanabe & Raff, 1990; 1992), while a few others sought to ablate a hypothesized cellular source of a signaling molecule in vivo and examine changes in the production of subsequently generated neurons (Reh & Tully, 1986; Tyler, Carney, & Cameron, 2005). More refined in vivo manipulations of gene expression in single labeled progenitors have subsequently shown how clonal constituency can be altered dramatically by upregulating ligand or receptor expression (e.g. Dorsky, Chang, Rapaport, & Harris, 1997; Lillien, 1995). For instance, Notch-Delta signaling between retinal progenitors has been shown to regulate proneural gene expression (see Ohsawa & Kageyama, 2008, for review). Such lateral inhibitory interactions between an initially equi-potential population ensure that not all progenitors start producing post-mitotic precursors at the same time (vertical grey arrows in figure 1). As a minority come to experience less Notch receptor activation, activating their own proneural genes leading to the commitment and differentiation of early-generated cell types (e.g. a retinal ganglion cell), so these early-generated neurons produce signals within the local environment that influence cell fate choice. It is a simple matter to envision progressively later-generated cell types, as they get added to this evolving microenvironment and begin to differentiate, creating additional signals that further impinge upon progenitors and precursors, signaling or constraining fate assignments or providing differentiation signals (colored arrows in figure 1). Curiously, manipulations that eliminate a type of retinal neuron show greatest effects upon the subsequent production of like-type cells, and relatively little impact upon other later-generated cells (Mu, Fu, Sun, Liang, Maeda, Frishman & Klien, 2005; Reh & Tully, 1986; Tyler et al., 2005; Waid & McLoon, 1998; Williams, Cusato, Raven, & Reese, 2001).
Not all progenitor cells or newly post-mitotic neurons in the local micro-environment may be competent to respond to these signals, however, and it is this field of developmental neuroscience that has witnessed explosive growth in the past two decades due to the revolution in molecular biology yielding fresh insight into the control of neuronal fate and differentiation, and how such mechanisms relate to the control upon proliferation (Agathocleous & Harris, 2009; Livesey & Cepko, 2001; Trimarchi, Stadler, & Cepko, 2008). For instance, the role played by individual transcription factor genes can now be assessed by determining whether proliferating cells or postmitotic precursors express them (Jusuf & Harris, 2009; Poggi et al., 2005), and by examining the retinal phenotype or the cellular morphogenesis when such genes (singly or in combination) are knocked out or overexpressed (Akagi, Inoue, Miyoshi, Bessho, Takahashi, Lee, Guillemot & Kageyama, 2004; Badea, Cahill, Ecker, Hattar, & Nathans, 2009; Feng, Xie, Joshi, Yang, Shibasaki, Chow & Gan, 2006; Oh , Khan, Novelli, Khanna, Strettoi & Swaroop, 2007); by examining the clonal phenotype arising from infected or electroporated progenitor cells in which gene function is altered (Dyer, Livesey, Cepko, & Oliver, 2003; Matsuda & Cepko, 2007); by defining the downstream genetic consequences following gene deletion (Mu & Klein, 2008; Yoshida, Mears, Friedman, Carter, He, Oh, Jing, Farjo, Fleury, Barlow, Hero & Swaroop , 2004); by identifying the gene expression profile of single cells (Cherry, Trimarchi, Stadler, & Cepko, 2009; Trimarchi, Stadler, Roska, Billings, Sun, Bartch & Cepko, 2007; Trimarchi et al., 2008); and by defining the upstream regulatory mechanisms controlling gene expression itself (Cherry et al., 2009; Hsiau, Diaconu, Myers, Lee, Cepko & Corbo, 2007; Kim, Matsuda, & Cepko, 2008; Trimarchi et al., 2007; 2008; Willardsen, Suli, Pan, Marsh-Armstrong, Chien, El-Hodiri, Brown, Moore & Vetter, 2009).
A variety of transcription factors, mostly of the basic helix-loop-helix (bHLH) and homeodomain families, have been shown to be expressed by subsets of retinal progenitor cells or their post-mitotic progeny, the retinal precursor cells, at particular periods during retinal development, and to be critical for the acquisition of particular retinal fates or for their subsequent differentiation into particular types of a given cell (Ohsawa & Kageyama, 2008). Indeed, the simple inactivation or mis-expression of many of these genes can lead to striking alterations in the cellular composition of the retina, in some cases, specific for single types of cell, due to adoption of an alternative fate, or to a failure to differentiate correctly, often followed by cell death. For example, one of the earliest expressing of these genes is Math5, critical for the production of retinal ganglion cells, as when it is knocked out, the population of retinal ganglion cells is dramatically reduced (Brown, Patel, Brzezinski, & Glaser, 2001; Kay, Finger-Baier, Roeser, Staub, & Baier, 2001). As lineage-mapping studies (i.e. studies identifying the descendants of progenitors that had activated a particular gene) have shown that horizontal cells, amacrine cells and photoreceptors also descend from the population of progenitor cells that had expressed Math5 during early development in the mouse retina (Yang, Ding, Pan, Deng, & Gan, 2003), this transcription factor was said to bestow competence to produce the ganglion cell fate rather than being a final fate-determining gene for ganglion cells (i.e. it is necessary, but not sufficient). Math5 is subsequently downregulated in those cells fated to become retinal ganglion cells, when Brn3b and Isl1 are then activated, each of which is critical for the differentiation of retinal ganglion cells (Mu, Fu, Sun, Beremand, Thomas & Klein, 2005; Pan, Deng, Xie, & Gan, 2008). Math5 mutants show reduced Brn3b expression, and Brn3b knock-out mice show reductions in the size of the ganglion cell population (Gan, Wang, Huang, & Klein, 1999), as do Isl1-conditional knock-out mice (Elshatory, Everhart, Deng, Xie, Barlow & Gan, 2007). Different Brn3 family members have recently been shown to participate in generating the diversity of retinal ganglion cell types (Badea et al., 2009), while Isl1 also plays a role in the differentiation of other retinal cell types (see below). Exactly what controls ganglion cell fate specification downstream of Math5 is unclear, although recent studies reinterpreting the role of Brn3b suggests it acts to specify ganglion cell fate rather than control differentiation (Qiu, Jiang, & Xiang, 2008).
Another transcription factor gene, Foxn4, plays a role in progenitor cells in the production of amacrine and horizontal cell fates, as its knock-out leads to a reduction in the former and a complete loss of the latter (Li , Mo, Yang, Price, Shen & Xiang 2004). Downstream of Foxn4 lies Ptf1a, turning on only after terminal division (Jusuf & Harris, 2009), though showing a comparable phenotype when knocked out (Fujitani, Fujitani, Luo, Qiu, Burlison, Long, Kawaguchi, Edlund, MacDonald, Furukawa, Fujikado, Magnuson, Xiang & Wright, 2006). Prox1, in turn, is downstream of Pt1fa, being expressed in differentiating horizontal and amacrine cells, though is critical for acquisition of the horizontal fate alone (Dyer et al., 2003). Amacrine cell fates, by contrast, are determined when Pt1fa is co-expressed in precursors along with two other competence factors, NeuroD and Math3, acting through Barhl2 to drive the differentiation of glycinergic amacrine cells (Mo, Li, Yang, & Xiang, 2004), BhlhB5 for GABAergic subtypes (Feng et al., 2006), and Isl1 for cholinergic amacrine cells (Elshatory et al., 2007).
Otx2 plays a role in directing cells toward a photoreceptor fate, as its knock-out leads to their loss (along with bipolar cells), while virally-mediated expression in progenitor cells promotes a photoreceptor fate (Nishida, Furukawa, Koike, Tano, Aizawa, Matsuo & Furukawa, 2003). Otx2 acts through the cone-rod homeobox gene Crx, also critical for the differentiation of photoreceptors (Furukawa, Morrow, & Cepko, 1997). Downstream of Crx, the ligand-activated transcription factor Rorβ acts as a switch, directing photoreceptors to become rods, as its knock-out leads to this population of photoreceptors differentiating as cones (Jia, Oh, Ng, Srinivas, Brooks, Swaroop & Forrest, 2009). The rod-directive actions of Rorβ are mediated through the downstream activation of the transcription factor Nrl, since its knock-out also yields this population of photoreceptors to differentiate as cones (Mears, Kondo, Swain, Takada, Bush, Saunders, Sieving & Swaroop, 2001), while the forced expression of Nrl in the entire Crx-expressing population leads to an outer nuclear layer composed exclusively of rods (Oh, Khan, Novelli, Khanna, Strettoi & Swaroop, 2007). Nrl in turn acts through Nr2e3 to produce rod photoreceptors, for knock-out of the latter also yields a cone-like phenotype in cells that would have otherwise been rods (Corbo & Cepko, 2005; Haider, Naggert, & Nishina, 2001). In all three of these knock-outs, containing surplus cones at the expense of the rod photoreceptors, the former differentiate as UV-cones evidenced by their opsin expression phenotype. The establishment of M-cones requires the expression and activation of the thyroid hormone-activated transcription factor Trβ2, requiring NeuroD upstream of it for its expression, as knocking out either yields a lack of M cones (Liu, Etter, Hayes, Jones, Nelson, Hartman, Hartman, Forrest & Reh, 2008; Ng, Hurley, Dierks, Srinivas, Saltó, Vennström, Reh & Forrest, 2001; Roberts, Srinivas, Forrest, Morreale de Escobar, & Reh, 2006).
Bipolar cell production requires the presence of the bHLH transcription factors Mash1 and Math3, for in the absence of both (but not either one alone), they fail to form altogether, becoming Müller glia instead (Tomita, Moriyoshi, Nakanishi, Guillemot, & Kageyama, 2000). Bipolar cells are also dependent upon the transcription factor gene, Chx10, for in its absence, these cells fail to form (Burmeister, Novak, Liang, Basu, Ploder, Hawes, Vidgen, Hoover, Goldman, Kalnins, Roderick, Taylor, Hankin & McInnes, 1996). Chx10 is normally expressed in all retinal progenitors early, ultimately being retained only in bipolar cells, and is believed to play a repressive role upon other proneural genes like Ath5 leading to the production of other cell-types (Vitorino, Jusuf, Maurus, Kimura, Higashijima & Harris, 2009). Bipolar cells also require Isl1 expression, for its conditional knock-out yields a drastically reduced bipolar cell population consequent to cell death during the first postnatal week (Elshatory et al., 2007). Twelve different bipolar cell sub-types are present in the mouse retina (Wässle, Puller, Müller, & Haverkamp, 2009), and transcription factors critical for the differentiation and/or survival of some of these have been identified, including Vsx1 and Irx5 for multiple OFF-cone bipolar cell subtypes (Cheng, Chow, Lebel, Sakuma, Cheung, Thanabalasingham, Zhang, Bruneau, Birch, Hui, McInnes & Cheng, 2005; Chow, Volgyi, Szilard, Ng, McKerlie, Bloomfield, Birch & McInnes, 2004), Bhlhb5 for the type 2 OFF-cone bipolar cell (Feng, Xie, Joshi, Yang, Shibasaki, Chow & Gan, 2006), and Bhlhb4 for rod bipolar cells (Bramblett, Pennesi, Wu, & Tsai, 2004).
Müller glial cell production arises from terminal divisions following the genesis of all neuronal cell types, yielding two daughters, one differentiating as a bipolar cell or rod photoreceptor stipulated by the relevant proneural genes then present. The other daughter cell however, is inhibited from cell cycle re-entry, despite heightened Notch activation, due to higher levels of the cell cycle inhibitor p27/Kip1 in these later progenitors, differentiating as Müller glia instead (Dorsky, Rapaport, & Harris, 1995; Ohnuma, Philpott, Wang, Holt, & Harris, 1999). Down-regulating Notch activity, like the knock-out of either Hes1 or Hes5 (being downstream effectors of Notch activation that suppress proneural gene activity), all yield reduced Müller glial production, while mis-expression of these promote glial features (Furukawa, Mukherjee, Bao, Morrow, & Cepko, 2000; Hojo et al., 2000; Nelson, Gumuscu, Hartman, & Reh, 2006). Sox9, expressed initially in all retinal progenitors but ultimately turned off in all but the last-generated Müller glial cells, also participates in Müller glial specification, since knocking out Sox9 function reduces Müller glial cells (Poché, Furuta, Chaboissier, Schedl, & Behringer, 2008), while Notch activity itself regulates transcription of Sox9 (Muto, Iida, Satoh, & Watanabe, 2009). Indeed, Notch signaling later during retinal development may more actively promote the Müller glial fate, as well as repress particular types of neuronal fates, rather than simply repressing proneural gene expression (Jadhav, Mason, & Cepko, 2006; Yaron, Farhy, Marquardt, Applebury, & Ashery-Padan, 2006; see Jadhav, Roesch, & Cepko, 2009, for review).
The above summary, while showing the critical roles of various genes in fate specification and differentiation (if ignoring much of the nuances of interpretation, the documented species differences and other contradictions present within the literature, as well as the detailed understanding of the regulatory actions shown for some of these factors and the full spectrum of their effects), is undoubtedly simplifying the complex nature of the downstream gene regulatory events and combinatorial coding that yields the richness of retinal cell diversity (Agathocleous & Harris, 2006; Mu & Klein, 2008; Trimarchi et al., 2008). Furthermore, how those genes influencing fate specification are regulated within an increasingly heterogeneous population of progenitor cells, either through cell-intrinsic changes associated with subsequent cell divisions, or by way of extrinsic signals within the evolving microenvironment, are relatively under-explored. In a few cases, the signaling molecules and their receptors through which they modulate retinal fates have been shown to engage the above transcription factors, for instance, GDF11 and FGF controlling the window of retinal cell competence for ganglion cell production by modulating Math5 (Kim, Wu, Lander, Lyons, Matzuk & Calof, 2005; Willardsen et al., 2009). The extent to which the population of progenitors is heterogeneous at the very outset of neurogenesis is also debated, as is the understanding of how such variation later on is established, and whether it is due to a reproducible program or reflects a stochastic component in the establishment of cellular biases (Agathocleous & Harris, 2006). The role of symmetric versus asymmetric divisions as a function of developmental time and in response to extracellular signals also remains to be understood (Cayouette, Poggi, & Harris, 2006). Likewise, studies are only beginning to dissect the determinants of the temporal window of neurogenesis, clarifying the nature of the signaling events that triggers its onset and spread (Agathocleous, Iordanova, Willardsen, Xue, Vetter, Harris & Moore, 2009; Hufnagela, Lea, Riesenberga, & Brown, 2010) and those that ensure an eventual cessation of divisions (Ohnuma, Philpott, Wang, Holt & Harris, 1999). But by conceptualizing fate specification in light of the above cell-intrinsic and environmental determinants, we can readily envision variations upon these themes that should lead to the characteristic differences between retinal organization in different species.
Vertebrate retinas conform to a basic organizational plan. Species differences are due primarily to differences in the ratios of the various constituent cell types, these differences being most apparent when comparing related nocturnal versus diurnal species. How those variations might come about during development is easy to imagine by modulating gene expression or function underlying the above intrinsic and extrinsic factors that confer retinal cell fate or direct differentiation (Reichenbach & Robinson, 1995; Reichenbach et al., 1998). That nature might do this through allelic variants of the above genes is suggested by considering the variation in retinal cell number in different strains of mice. Multiple retinal cell classes exhibit conspicuous differences in cell number that are independent of variation in retinal area between different mouse strains, for example, retinal ganglion cells (Williams, Strom, Rice, & Goldowitz, 1996), horizontal cells (Williams, Strom, Zhou, & Yan, 1998) and dopaminergic amacrine cells (Whitney, Raven, Ciobanu, Williams, & Reese, 2009). The genetic dissection of such variation in traits has blossomed in recent years, made possible by multiple resources. The genomes of inbred strains have been sequenced, making possible the identification of single nucleotide polymorphisms that underlie such genetic variation. There are a wide variety of mouse strain resources that can aid in this approach, including recombinant inbred strains (Williams, Gu, Qi, & Lu, 2001) and chromosome substitution strains (Singer, Hill, Burrage, Olszens, Song, Justice, O'Brien, Conti, Witte, Lander & Nadeau, 2004). There is as well an expanding gene expression database for eye and retinal transcripts derived from these same mouse strains (Geisert, Lu, Freeman-Anderson, Templeton, Nassr, Wang, Gu, Jiao & Williams, 2009), which can aid in the identification of candidate genes residing at such genomic loci where quantitative traits have been mapped.
For example, by using recombinant inbred mouse strains derived from two inbred laboratory strains for which the genomes have each been sequenced, such natural variation in cell number, which shows as much as a two-fold difference for the population of horizontal cells and a nearly four-fold difference for dopaminergic amacrine cells, can be mapped to discrete loci within the mouse genome, where allelic variants responsible for some of this natural variation must be present (Reese, Raven, Whitney, Williams, Elshatory & Gan, 2008; Whitney, Raven, Ciobanu, Williams & Reese 2009; Williams, Strom, & Goldowitz, 1998). While one could envision that some of this natural variation might be caused by polymorphisms in genes that modulate cell proliferation or cell cycle kinetics, affecting multiple populations of retinal neurons, to date, the variations in the populations of distinct retinal cell types between different strains of mice are not correlated (Whitney, Raven, & Reese, 2007), and the quantitative trait loci identified for each of these traits differ, suggesting that they are each modulated (within these strains, at least) independently, by affecting genes that influence fate assignment or differentiation (Reese et al., 2008), by modulating genes controlling cell survival (Whitney et al., 2009), or by some combination of these.
Recently, however, it has been suggested that alterations in cell-cycle exit decisions (i.e. the onset and termination of the neurogenetic period within the developing retina) may be a simple yet sufficient means of yielding substantial inter-species differences in retinal organization. The retinas of nocturnal versus diurnal species of new world monkey differ in the relative sizes of their rod and their cone photoreceptor populations, respectively. As the neurogenetic window for cone photoreceptor production precedes that for the rods in all species examined, perhaps the species differences arise from simply delaying cell-cycle exit relative to the temporal variation in environmental and cell-intrinsic factors described in the preceding section that impact fate assignments. The greater size of the rod photoreceptor population in the nocturnal monkey, Aotus azarae, correlates with another later-generated (and functionally related) cell type, the rod bipolar cells, while the size of the cone photoreceptor population is absolutely smaller, as is another early-generated retinal cell type, the retinal ganglion cell population, when compared with the diurnal monkey, Cebus apella. Consistent with the hypothesis that these differences in four types of retinal cell reflect a delay in cell-cycle exit decisions, the nocturnal species was shown to exhibit a protracted neurogenetic period, a shorter cell cycle at comparable maturational ages, and variation in cell-cycle associated genes or proteins that are in line with a delayed neurogenetic window (Dyer, Martins, da Silva Filho, Muniz, Silveira, Cepko & Finlay, 2009). Forcing single progenitors to withdraw prematurely from the cell cycle validates the expectation that later-generated cell types within identified clones are reduced in frequency (Dorsky et al., 1997; Dyer et al., 2003), so it will be interesting to witness the application of conditional transgenic strategies in the developing mouse retina to modulate the period of cell cycle withdrawal upon retinal architecture and the underlying populations of retinal neurons.
3. How do newborn neuroblasts migrate to their laminar destinations?
Retinal progenitors undergo mitosis at the ventricular surface, and for those divisions yielding a daughter cell that withdraws from the cell cycle, many of those neuroblastic precursors must then migrate to their future laminar destinations. Unlike the developing neocortex, where newborn neuroblasts migrate conspicuous distances at progressively later stages and benefit from a radial glial scaffold that guides them through an expanding white matter forming beneath the cortical plate, neuroblast migration in the retina was considered a simpler feat, played out over a shorter distance amongst a mixed population of progenitor and precursor cells that themselves should provide some degree of radial support for their migration. Early studies on the migratory behavior of newborn neuroblasts were largely speculative, based on Golgi-staining and electron microscopy studies at progressively earlier stages, from which inferences were drawn about the transitional stages of different types of neuron (Hinds & Hinds, 1974; 1979; 1983; Morest, 1970). They suggested that rod and cone precursors maintain an attachment at the ventricular surface following their final mitotic division, subsequently extending a basally directed radial process through which the nucleus translocates to achieve an eventual somal positioning within the outer (or sometimes winding up within the inner) nuclear layer (Spira, Hudy, & Hannah, 1984). For the other cell types, however, the means by which they migrated were harder to interpret in the absence of distinctive markers that also labeled the entirety of the cell. Antibodies that labeled the plasma membrane of distinct cell types provided one means of better inferring the migratory behavior of different cell classes, as have fluorescent reporter genes driven by cell-specific promoters. Imaging the latter in vivo has shown in greater detail the progressive changes in morphology in conjunction with neuroblast movement. These studies have suggested two distinct modes by which inner retinal neurons arrive at their final laminar positions:
The earliest born cells, the retinal ganglion cells, extend a leading (basally-directed) process through which the nucleus subsequently translocates (McLoon & Barnes, 1989). This radial process gives rise to the developing axon of the ganglion cell, extending across the inner retinal surface even before the nucleus has arrived at the future ganglion cell stratum itself (figure 2a–c). Ganglion cell differentiation may proceed to such an extent that the axon has navigated a course into the optic nerve well before somal translocation has been completed, evidenced by the fact that bipolar-shaped neurons at intermediate locations in the retinal neuroepithelium could be labeled from the developing optic nerve (Dunlop, 1990; Snow & Robson, 1994). The behavior of the trailing end of the cell is less clear: some studies provide evidence for a remaining attachment at the ventricular surface until translocation is completed (McLoon & Barnes, 1989), while other more recent in vivo imaging studies suggest that the cell relinquishes its apical attachment before translocation is completed (Poggi et al., 2005).
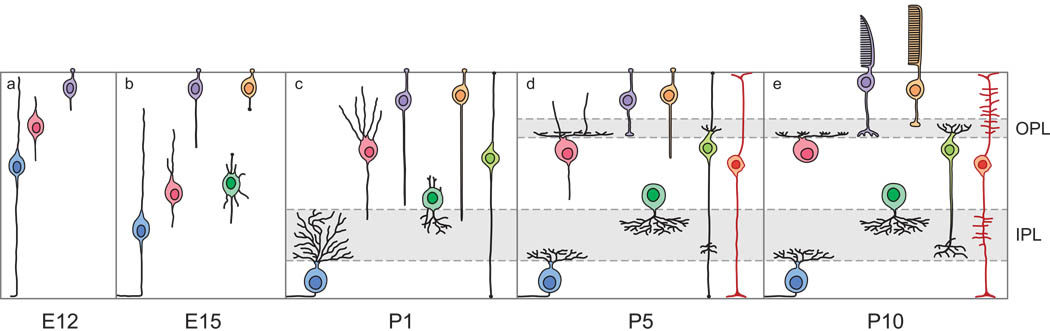
Figure 2.
Retinal precursors initiate a cell-type specific differentiation program, controlling their migratory behavior and their morphogenesis. Shown are five different developmental time-points, conveying these migratory and morphological distinctions as the different cell-types achieve their laminar positioning and adult morphologies. Cell type conventions as in figure 1. (Modified from Mumm & Lohmann, 2006).
Such nuclear translocation may be a common means for retinal neuroblasts to achieve their final positioning. Even the cone photoreceptor cells exhibit such nuclear translocation (figure 2b–d), despite their attachment to the future outer limiting membrane and their eventual somal positioning adjacent to it (Poggi et al., 2005; Rich, Zhan, & Blanks, 1997). For these cells, like the rods, they extend a basally directed process, but one that does not extend to the inner retinal surface, particularly at later developing stages (Johnson, Williams, Cusato, & Reese, 1999). Bipolar cells also undergo a similar nuclear translocation, at the latest stages of retinal development, when the thickness of the developing retina is greatest. For this cell type, both the leading and trailing processes attach to their respective retinal margins (the inner and outer limiting membranes) as the nucleus translocates to the future inner nuclear layer (figure 2c–e). Subsequently, the inner and outer processes detach as axonal terminations form in the inner plexiform layer and after dendritic processes begin to emerge within the outer plexiform layer, respectively (Morgan, Dhingra, Vardi, & Wong, 2006).
Amacrine cells, by contrast, show a striking difference in their migratory behavior, undergoing active cellular migration without obvious leading or trailing processes directed to, or attached at, the inner retinal surface or ventricular surface, respectively (figure 2a–c). These cells had been described in Golgi preparations as having far more complex morphologies than the simpler bipolar shape associated with cells undergoing nuclear translocation (Prada, Puelles, Genis-Gálvez, & Ramirez, 1987), and more recent in vivo imaging studies have shown these cells to extend undirected processes that may participate in sensing the local environment during migration (Godinho, Mumm, Williams, Schroeter, Koerber, Park, Leach & Wong, 2005). Still other amacrine cells apparently retain a simple bipolar morphology as they migrate, though lacking attachments at either surface of the retina, but how this difference might relate to ultimate bipolar cell subtype is unclear (Prada et al., 1987). This behavior is more reminiscent of that for migrating horizontal cells, which adopt a generally bipolar morphology (figure 2a–b) lacking inner or outer attachments as they migrate away from the ventricular surface (Huckfeldt, Schubert, Morgan, Godinho, Di Cristo, Huang & Wong, 2009; Schnitzer & Rusoff, 1984).
The horizontal cells have attracted recent attention because they exhibit a unique aspect to their migratory behavior, overshooting the horizontal cell stratum (figure 2b) to coalesce within the future amacrine cell layer before migrating back to their final destination (Edqvist & Hallböök, 2004; Edqvist, Lek, Boije, Lindbäck, & Hallböök, 2008). Two further features of this process are worth nothing here: First, the apically directed migration, at least in the mouse retina, requires the transcription factor gene Lim1, for when it is conditionally knocked out, horizontal cells remain positioned at deeper locations within the inner nuclear layer amongst the amacrine cells, differentiating dendritic arbors within the inner plexiform layer (Poché, Kwan, Raven, Furuta, Reese & Behringer, 2007). Second, in the chick retina, the horizontal cells apparently re-enter the cell cycle and divide within these deeper locations before migrating apically, back to the horizontal cell layer (Boije, Edqvist, & Hallböök, 2009), consistent with other recent lineage-mapping studies suggesting that some progenitors divide to yield two horizontal cells of the same subtype (Rompani & Cepko, 2008). In the zebrafish, such divisions producing pairs of horizontal cells have been imaged in vivo to take place in the horizontal cell layer itself (Godinho, Williams, Claassen, Provost, Leach, Kamermans & Wong, 2007), after these cells have migrated back from the inner retina (Jusuf & Harris, 2009). This unique propensity to re-enter the cell cycle after migration and expression of horizontal cell-specific genes has been related to other recent demonstrations that mature horizontal cells can re-enter the cell cycle and give rise to retinoblastoma (Ajioka, Martins, Bayazitov, Donovan, Johnson, Frase, Cicero, Boyd, Zakharenko & Dyer, 2007).
4. How is cellular positioning upon the retinal surface determined?
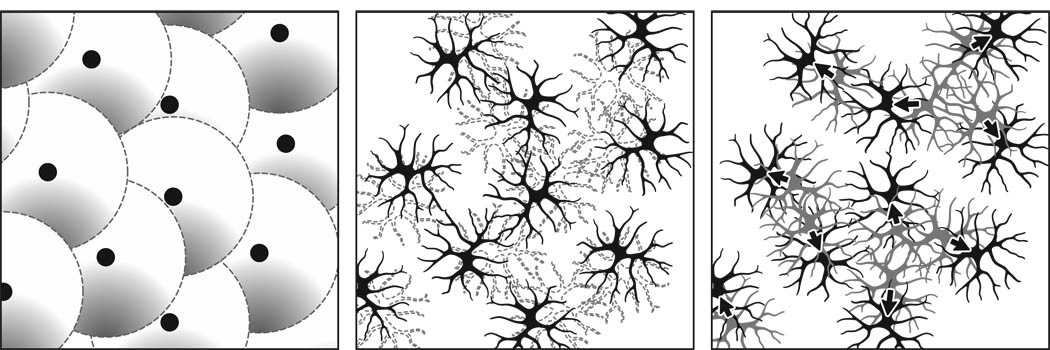
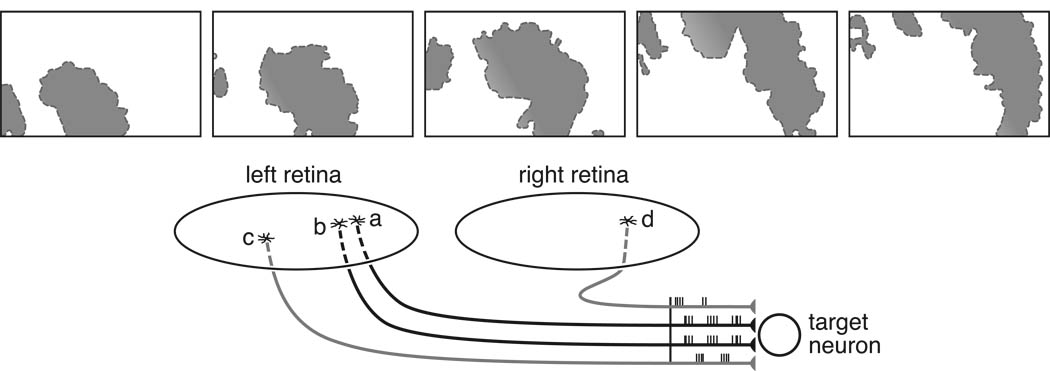
Retinal nerve cell classes are distributed as regular retinal arrays, commonly referred to as retinal mosaics (Wässle & Riemann, 1978), and the patterning of such arrays was generally assumed to reflect a periodicity in the fate-determining events responsible for producing those nerve cell classes (e.g. McCabe, Gunther, & Reh, 1999), as is the case for the patterned distribution of photoreceptor types in the eye of Drosophila (Ready, Hanson, & Benzer, 1976). While such periodic fate determination events (outlined above) undoubtedly contribute to the grain of such mosaics (figure 3a), we now know from experimental studies that the final patterning of a mosaic is refined by cell death (figure 3b) and by cellular movements in the plane of the retina (figure 3c), at least for certain types of retinal nerve cell. Similar conclusions have been drawn from modeling studies seeking to simulate the role of fate determination events, cell death and tangential dispersion, either singly or in combination (Eglen & Willshaw, 2002; Eglen, 2006).
Figure 3.
The regularity of a retinal mosaic may be the product of distinct developmental events acting at different stages, dependent upon cell type. Periodic fate determining events may establish a regular pattern amongst a population of retinal precursors forming a retinal mosaic (a). At later stages during differentiation, naturally occurring cell death may eliminate closely positioned neighbors (dotted profiles in b), because of limited trophic support from afferents or targets. Mutual repulsion may also drive homotypic cells apart (arrows in c). (Modified from Reese, 2008b).
Interfering with naturally occurring cell death, for instance, yields mosaics of cholinergic amacrine cells and dopaminergic amacrine cells in which the minimal intercellular spacing between homotypic neighbors is reduced (as expected, given the increase in cell density). In addition, however, the resultant regularity of the mosaic is also degraded, suggesting that cell death serves to eliminate the closest near neighbors, thereby enhancing patterning (Raven, Eglen, Ohab, & Reese, 2003; Resta, Novelli, Di Virgilio, & Galli-Resta, 2005). Where the mosaics can also be identified reliably prior to naturally occurring cell death, they have been shown to be less ordered relative to later stages following cell death (Galli-Resta & Novelli, 2000). Yet in other mosaics that do not appear to be modulated by naturally occurring cell death (e.g. the horizontal cells), mosaic order increases at later developmental stages when cell number remains stable (Raven, Stagg, Nassar, & Reese, 2005b). Such experiments suggest that nerve cells move in the plane of the retina to space themselves apart, and are supported by other studies employing clonal boundary analysis, revealing such progressive tangential dispersion of particular types of retinal nerve cells (Reese, Harvey, & Tan, 1995; Reese et al., 1999). Very recently, live imaging of labeled horizontal cells has shown such tangential movements, providing the most direct evidence that these movements contribute to the improvement in mosaic patterning (Huckfeldt et al., 2009). Those studies implicate early differentiating dendritic interactions in defining a domain within which the soma of a cell will occupy a central location; subsequent events may then regulate further dendritic growth and overlap (Huckfeldt et al., 2009; Reese, Raven, & Stagg, 2005), followed, in some cases, by additional passive displacements that in turn may degrade an initially ordered mosaic (e.g. Whitney, Raven, Keeley, & Reese, 2008).
5. How plastic is the acquisition of cellular morphology in the retina?
The recognition that visual cortical cells were conspicuously influenced by the visual environment during the early post-natal period, but that cells at earlier stages of the visual pathway appeared immune to these same effects (Daw & Wyatt, 1974; Wiesel & Hubel, 1963), led to the general presumption that sub-cortical structures lacked the capacity for plasticity, likely to be hard-wired during earlier development. The acquisition of cellular morphology within the retina was thought to be driven largely by cell-intrinsic developmental programs, and that the associated connectivity of individual cell-types required only appropriate process recognition to yield stratification and synaptogenesis within the plexiform layers, giving rise to the physiological properties of these cells. The past 25 years have witnessed a dramatic revision of this perspective, at least for certain types of retinal cells, following various studies showing that some cell-types undergo substantial morphological restructuring during early development, and that the mature properties of these cells' morphologies are critically dependent upon the presence of neighboring cells or upon the signals those cells transmit. Indeed, some of that plasticity has been shown to depend critically upon the visual environment itself. The following section will parse such “developmental plasticity” effects into those that regulate stratification, those that regulate dendritic overlap, and those regulate dendritic patterning
Regulation of stratification
Most mature retinal cell-types distribute their processes to restricted sub-strata within the plexiform layers. During development, however, some of these cell-types undergo a progressive restructuring of these axons or dendrites, rather than establishing their mature morphology through a steady, progressive transformation from a simple neuroblastic morphology. Some of this transformation is associated with the establishment of cellular positioning, either with respect to depth within the retina, or relative to homotypic neighbors, as discussed above; in other cases, such changes in morphology appear to take place independent of cellular migration or dispersion. For instance, after horizontal cells have migrated to their future position in the emerging inner nuclear layer, they exhibit an elaborate stellate dendritic morphology (figure 2) prior to stratifying within the outer plexiform layer (Huckfeldt et al., 2009; Reese et al., 2005). Curiously, the sole source of afferent innervation to their dendrites, the pedicles of the cone photoreceptors, stratifies within the outer plexiform layer ahead of this dendritic stratification by the horizontal cells, but the former are not required for the latter to reorganize, shown in coneless transgenic mice (Reese et al., 2005) or in transgenic mice in which all cones have been re-specified to acquire a rod fate (Raven, Oh, Swaroop, & Reese, 2007). The photoreceptors themselves have been shown to overshoot the developing outer plexiform layer in advance of its formation (figure 2) (Pow & Hendrickson, 2000), and in the ferret's retina, they extend as far as the inner plexiform layer before eventually retracting to the outer plexiform layer (Johnson et al., 1999). That this transient morphology might play some developmental role in the inner plexiform layer is suggested by the fact that these processes do not simply extend to the inner limiting membrane, but rather stratify in one of two sub-strata in the inner plexiform layer coincident with the processes of cholinergic amacrine cells (Johnson et al., 1999). They clearly recognize the latter to achieve this stratification, since ablation of the cholinergic amacrine cells disrupts this transient photoreceptor stratification (Johnson, Raven, & Reese, 2001).
The guidance for such stratification is likely mediated by cell-surface molecules that confer specificity (Honjo, Tanihara, Suzuki, Tanaka, Honda & Takeichi, 2000), and recent studies have identified candidates that mediate such heterotypic recognition in the inner plexiform layer (Yamagata & Sanes, 2008). One might expect that the earliest differentiating retinal cell-types in the inner retina should yield such laminar cues for other later-differentiating cells. It was therefore somewhat surprising that early ablation of the entire population of retinal ganglion cells (Williams et al., 2001) or of the cholinergic amacrine cells (Reese, Raven, Giannotti, & Johnson, 2001), two of the earliest generated cell-types in the inner retina (Reese & Colello, 1992) has, to date, yielded no conspicuous effect upon the organization of the inner plexiform layer. Unlike the cholinergic amacrine cells though, which exhibit a clear stratification nearly as early as these cells differentiate their processes within the inner plexiform layer (Reese et al., 2001; Stacy & Wong, 2003), at least some retinal ganglion cell types undergo a conspicuous reorganization of their dendritic fields within the inner plexiform layer, from an initially diffuse dendritic arbor to one that eventually stratifies within one, or two, discrete sub-strata in the inner (ON) or outer (OFF) portions of the inner plexiform layer (figure 2). Just how diffuse or non-specific that early morphology is has been contested (Bodnarenko, Jeyarasasingam, & Chalupa, 1995; Bodnarenko, Yeung, Thomas, & McCarthy, 1999; Coombs, Van Der List, & Chalupa, 2007; Lohmann & Wong, 2001; Maslim, Webster, & Stone, 1986; Stacy & Wong, 2003), but this developmental change appears to underlie a well-documented reduction in the proportion of retinal ganglion cells that respond to both the onset and offset of light (ON-OFF responses) (Tian & Copenhagen, 2001; 2003; Wang, Liets, & Chalupa, 2001). Furthermore, this morphological change is modulated by visual activity, as light deprivation reduces the proportion of mono-stratified dendritic fields and the proportion of ganglion cells showing only ON or OFF responses (see Tian, 2008, for review). Exactly how this effect is mediated is unclear: initial studies suggested that pharmacological blockade of ON-bipolar cell activation within the outer plexiform layer could mimic this effect of light deprivation (Bodnarenko & Chalupa, 1993; Bodnarenko et al., 1995) yet more recent studies silencing the output of these same ON-bipolar cells fails to disrupt stratification in the inner plexiform layer (Kerschensteiner, Morgan, Parker, Lewis, & Wong, 2009). Likewise, completely eliminating the cone afferents within the outer plexiform layer does not alter the dendritic stratification of either the horizontal cells (Reese et al., 2005) or the Type 7 cone bipolar cells (Keeley & Reese, 2010b), both normally post-synaptic to the cones.
Regulation of dendritic overlap
For post-receptoral retinal mosaics, each exhibits a characteristic degree of dendritic overlap for a given type of cell. Some cell-types extend their dendrites to the tips of homotypic dendritic fields, producing a tiling of the retinal surface (having a coverage factor of 1, indicating that every locus on the retina is subserved by the dendritic arbor of only a single cell of this type), while other cell-types overlap one another conspicuously, yielding a ten-fold or greater increase in coverage factor (see Reese, 2008a, for review). These type-specific differences are presumed to enable each cell type to mediate its unique functions within the retinal circuitry, and are thought to arise during development by homotypic interactions that constrain dendritic overlap. 25 years ago, a handful of experimental studies showed how the dendritic morphology of various retinal ganglion cell classes was influenced by interactions with neighboring ganglion cells, for instance, by biasing dendritic growth to one side of the field when neighboring cells on that same side were ablated (Eysel, Peichl, & Wässle, 1985; Hitchcock, 1989; Linden & Perry, 1982; Perry & Linden, 1982; Perry & Maffei, 1988), or by either increasing or decreasing the entire dendritic field following depletion or crowding of homotypic neighbors, respectively (Kirby & Chalupa, 1986; Leventhal, Schall, & Ault, 1988; Troilo, Xiong, Crowley, & Finlay, 1996). These early studies spawned the view that dendritic growth should be controlled by homotypic interactions, a view that was reinforced by subsequent imaging of filled pairs of cells during development–contacts were frequently present across overlapping dendritic arbors of homotypic neighbors (Lohmann & Wong, 2001). The fact that dendritic field size was found to increase as a function of eccentricity for most retinal cell classes, and that homotypic density often declines as a function of retinal eccentricity to yield a constant dendritic coverage (Wässle, Peichl, & Boycott, 1978), was regarded as further evidence that cell types other than ganglion cells also exhibited homotypic regulation of dendritic field size.
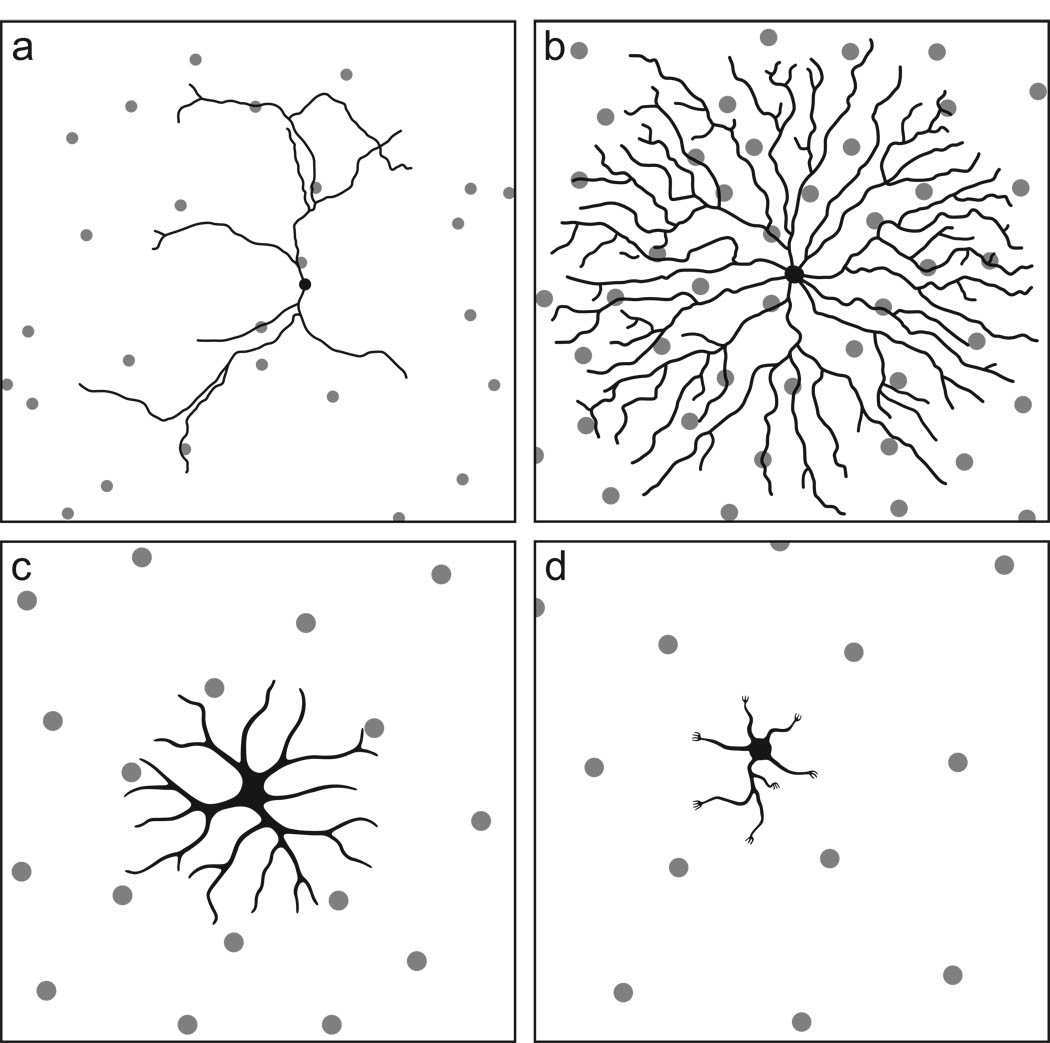
Recently this view has been tested directly for select types of retinal nerve cells in the mouse retina. For example, genetic depletion of homotypic neighbors has failed to show any evidence for such regulation of dendritic growth for either the melanopsin-positive retinal ganglion cells, or for the alpha-like neurofilament-rich (SMI-32) retinal ganglion cells (Lin, Wang, & Masland, 2004), two ganglion cell classes that achieve a coverage factor of between 1 and 2. Dopaminergic amacrine cells, having a greater dendritic overlap, on the order of 7 (figure 4a), also do not appear to regulate their dendritic morphology in relation to homotypic neighbors, although they may be susceptible to some heterotypic influence upon their dendritic field size (Keeley & Reese, 2010a). And cholinergic amacrine cells, perhaps the best-understood of all retinal cell-types requiring dendritic overlap to achieve their functionality (Zhou & Lee, 2008), exhibit a coverage factor around 30 in the mouse retina (figure 4b) but do not modulate their growth when the homotypic population is partially ablated during early postnatal development (Farajian, Raven, Cusato, & Reese, 2004; see also Keeley, Whitney, Raven, & Reese, 2007). Horizontal cells, by contrast, with a coverage factor of about 6 (figure 4c), show a conspicuous (two-fold) modulation of dendritic field area that is inversely related to homotypic cell density, evidenced by comparing strains that vary in horizontal cell number (Reese et al., 2005), or through genetic reduction in the normal population of horizontal cells (Poché, Raven, Kwan, Furuta, Behringer & Reese, 2008). Bipolars cells, having a dendritic coverage factor around 1 (figure 4d), achieve a tiling characteristic of some retinal ganglion cells (Wässle et al., 2009). Preliminary data suggest that this cell type is sensitive to homotypic neighbors, for genetic manipulations that increase or decrease the density of Type 7 cone bipolars yield correspondingly smaller versus larger dendritic field areas (Sammy Lee, unpub. obs.). For those cell types that do not evidence homotypic regulation of dendritic field growth in such manipulation studies, but have been shown to vary their dendritic field size as a function of retinal eccentricity, they presumably achieve such variation in dendritic growth through passive elongation driven by retinal expansion (Reese, 2008b), but other heterotypic signals may regulate their growth (Keeley & Reese, 2010a; Mehta & Sernagor, 2006).
Figure 4.
The morphology of four different retinal cell types in relation to their retinal mosaics. a. Dopaminergic amacrine cells differentiate a dendritic arbor that is indifferent to the presence of other dendrites arising from the same cell as well as from those of homotypic neighbors. b. Cholinergic amacrine cells differentiate a dendritic arbor in which dendrites avoid one another, yet overlaps those of numerous homotypic neighbors. c. Horizontal cell somata constrain further dendritic outgrowth from their homotypic neighbors. d. Bipolar cell dendritic arbors achieve a tiling of the retinal surface, colonizing pedicles within their dendritic fields while also sharing those at the dendritic boundary with adjacent cells. The panels are not drawn to the same scale, intending only to portray the relationship between morphology and homotypic density. (Modified from Reese, 2008b).
The signaling between homotypic neighbors that regulate such interactions are relatively unexplored, but it is interesting to consider features of these cells’ morphologies from this perspective: the dendritic fields of cone bipolar cells do not overlap, but they may share single pedicles (Wässle et al., 2009), suggesting that they colonize a field of pedicles by restraining further growth when confronting the dendritic field of a like-type cell, i.e. a simple contact-mediated inhibition of growth may be sufficient to define the approximate field area (figure 4d). Horizontal cells, on the other hand, extend dendritic fields that overlie one another more extensively, sharing pedicles with multiple neighboring cells throughout the dendritic field. The radius of their dendritic fields approximates the average near-neighbor distance (figure 4c), suggesting a cessation of dendritic growth triggered by interaction with neighboring somas, perhaps mediated by some threshold level of cell-surface molecule achieved on the soma itself, or a factor secreted from the soma locally.
There has been recent excitement about a prospective role played by the Down syndrome cell adhesion molecule, Dscam, in regulating homotypic dendritic repulsion, particularly as it is expressed in restricted populations of retinal neurons in the mouse retina (Fuerst, Koizumi, Masland, & Burgess, 2008; Fuerst, Bruce, Tian, Elstrott, Feller, Erskine, Singer & Burgess, 2009). In Drosophila, Dscam has thousands of alternatively spliced isoforms, giving single homotypic neighbors their own unique identity (Hattori, Chen, Matthews, Salwinski, Sabatti, Grueber & Zipursky, 2009). The neurites of these cells consequently engage in “self-avoidance”, but freely overlap the dendritic fields of homotypic neighbors. If, however, these cells are engineered to express only one of these thousands of isoforms, then their processes exhibit both self-avoidance as well as homotypic avoidance with those from neighboring like-type cells (see Millard & Zipursky, 2008, for review). Curiously, in the mouse retina, while different cell types express Dscam, the mouse has only a single Dscam isoform, but rather than its mediating avoidance or repulsion between homotypic neighbors, it appears to mask an adhesive interaction: dopaminergic amacrine cells extend their dendrites in the wild-type mouse retina that appear oblivious to other dendrites from the same cell (e.g. figure 4a) or to dendrites from like-type cells in the vicinity (Keeley & Reese, 2010a), yet in the Dscam-mutant retina, the processes of these cells fasciculate in a manner never observed in the wild-type condition (Fuerst et al., 2008). Dscam, then, may provide a “non-stick” coating, preventing fasciculation that is itself specific to different cell-types (Fuerst & Burgess, 2009).
Interestingly, it is the cholinergic amacrine cell that differentiates a dendritic arbor that is most like the behavior expected by a genetic mechanism such as that above in Drosophila mediating self-repulsion yet permitting overlap with like-type neighbors. Cholinergic amacrine cells differentiate a dendritic arbor that approximates a space-filling function, dividing as a function of distance from the soma but rarely crossing over neighboring dendrites from the same cell. These cells establish such an immense degree of dendritic overlap with homotypic neighbors (figure 4b) that their growth would appear indifferent to them, while at the same time minimizing proximity to neighboring processes from the same soma. Models of morphogenesis that implement space-filling algorithms generate dendritic fields similar to those of real cholinergic amacrine cells (Sugimura, Shimono, Uemura, & Mochizuki, 2007), but the underlying signals generating this behavior, and how homotypic neighbors might be immune to cross-talk, remain to be determined.
Regulation of dendritic patterning
Nearly 25 years ago, a number of studies examined the branching pattern of individual, intracellularly-labeled, retinal ganglion cells during early development. Many of these studies observed that these cells were often excessively branched before relinquishing many short processes to yield the more typical branch pattern observed in maturity (Dann, Buhl, & Peichl, 1987, 1988; Ramoa, Campbell, & Shatz, 1987, 1988). More recently, the dynamics of dendritic branching have been studied by labeling single cells in living retinas and examining the extension and retraction of processes during early development as they investigate the local environment. This motility of the processes of immature retinal ganglion cells has been shown to be influenced by synaptic transmission (Wong & Wong, 2000; 2001), as may be expected since such cells form contacts with heterotypic neighbors with which they ultimately secure synaptic contacts (Stacy and Wong 2003); such early communication may then participate in the stabilization of some branches and the elimination of others (see Wong & Ghosh, 2002, for review). Doubtless cell-intrinsic programs contribute to the distinctive dendritic branching patterns that discriminate different types of retinal ganglion cell from the earliest stages, and that may participate in their recapitulation in vitro (Montague & Friedlander, 1991), but the fine patterning within the dendritic field seems likely to depend upon afferent innervation and the activity those afferents convey. One of the most dramatic examples of afferent modulation of dendritic patterning comes not from a retinal ganglion cell though, but rather, the horizontal cell: these cells establish periodic clusters of dendritic terminal endings at each cone pedicle in the outer plexiform layer. In transgenic mice lacking all cones, their dendritic fields are largely barren of higher-order dendritic branches and clustered terminal endings, while in “conefull” mice (in which all of the rods are re-specified to become cones), their fields have hypertrophied, filling the entire field-area with branches that occupy the full depth of the outer plexiform layer (Raven et al., 2007; Reese et al., 2005). Dark-rearing does not reproduce the horizontal cell phenotype observed in the coneless mouse, but the Cacna1f-mutant mouse does, disrupting all synaptic transmission in the outer plexiform layer due to defective calcium channel assembly in the photoreceptor terminals (Raven et al., 2008). This developmental plasticity of the horizontal cell is all the more striking in the absence of similar changes in the other primary target of the cone pedicles in the outer plexiform layer, the cone bipolar cells (Keeley & Reese, 2010b). When considered in conjunction with the horizontal cell's exceptional capacity for remodeling in retinal degeneration (Lewis & Fisher, 2006) and its ability to re-enter the cell cycle and contribute to retinoblastoma (Ajioka et al., 2007), the plasticity of the horizontal cell appears to be unique amongst retinal nerve cells (Poché & Reese, 2009).
Development of the optic pathway
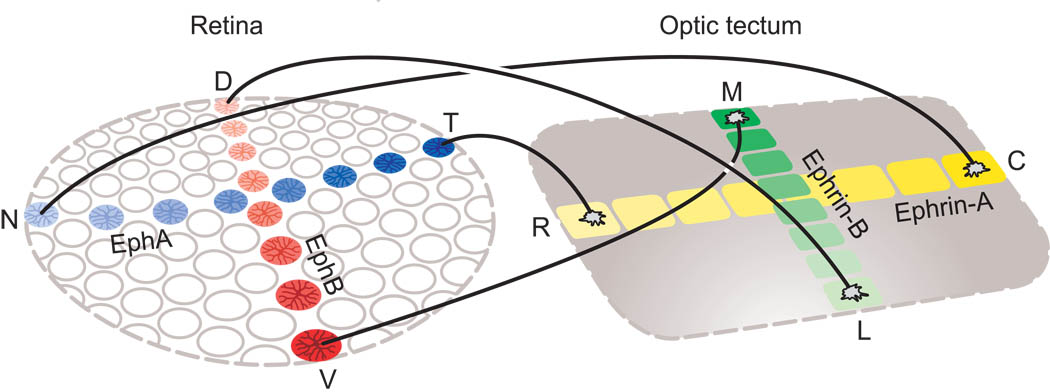
Over the past two decades, a multitude of factors have been shown to play a role in guiding optic axons to their target nuclei within the brain. Receding gradients of chondroitin sulfate proteoglycans (CSPGs) direct axonal growth radially toward the optic nerve head (Brittis, Canning, & Silver, 1992); cell-surface molecules promote fasciculation of later-arriving axons (Brittis & Silver, 1995; Ott, Bastmeyer, & Stuermer, 1998); Netrin signaling contributes to fiber entry into the optic stalk (Deiner, Kennedy, Fazeli, Serafini, Tessier-Lavigne & Sretavan, 1997); Slit proteins influence the positioning of optic axons as they arrive at the base of the brain (Plump, Erskine, Sabatier, Brose, Epstein, Goodman, Mason & Tessier-Lavigne, 2002); CSPGs contribute to the chronotopic reordering of optic axons as they enter the optic tract (Leung, Taylor, & Chan, 2003); and Slit and Semaphorin proteins provide directional signals further caudally within the optic tract (Atkinson-Leadbeater, Bertolesi, Hehr, Webber, Cechmanek & McFarlane, 2010). These and other factors that influence optic axonal growth and directionality have been reviewed elsewhere recently (Erskine & Thompson, 2008; Inatani, 2005; Sretavan, 2006; Xiao, Roeser, Staub & Baier, 2005). From a “systems” perspective, however, the most impressive advances during the past 25 years have been in our understanding of how the partial decussation is established at the optic chiasm, how retinotopic maps are created in target visual nuclei, and how separate ocular domains are produced within binocular visual structures.
6. How is the partial decussation established at the optic chiasm?
In the mammalian visual pathway, optic axons arising from ganglion cells in the ventro-temporal retina fail to decussate at the optic chiasm, projecting into the ipsilateral optic tract instead, thereby enabling binocular visual processing within each hemisphere without the need for inter-hemispheric connectivity. Fiber tracing studies showed that this uncrossed projection was not segregated from the crossing axons in some species, notably rodents and carnivores, thereby ruling out any simple account in terms of physical guidance (see Reese & Baker, 1992, Jeffery & Erskine, 2005, for reviews; but see also Jeffery, Levitt, & Cooper, 2008). Additionally, in most non-primate mammals, many cells in the temporal retina project contralaterally rather than ipsilaterally, complicating any simple “morphogenetic” account based upon retinotopic fiber order. Birthdating studies showed that the uncrossed component from the temporal retina was generated earlier than was the crossed temporal component (Dräger, 1985; Reese & Colello, 1992), while tract-tracing studies during development confirmed that the uncrossed component reached the brain prior to the crossed component (Baker & Reese, 1993; Godement, Salaun, & Mason, 1990; Sretavan, 1990). Other studies in carnivores confirmed that ganglion cell classes with partial decussation patterns were generated prior to, and gave rise to axons arriving earlier than, those with more complete decussations (Reese & Baker, 1990; Reese, Guillery, & Mallarino, 1992b; Reese, Guillery, Marzi, & Tassinari, 1991; Reese, Thompson, & Peduzzi, 1994; Walsh, Polley, Hickey, & Guillery, 1983). Together, these studies suggested that the factors that guide temporal optic axons to take an uncrossed course at the optic chiasm reflect a time-dependent signaling event in the chiasmatic region (Reese & Baker, 1992). While naturally occurring cell death and inter-ocular interactions have both been shown to influence the formation of the decussation pattern (Guillery, Mason, & Taylor, 1995; Linden & Reese, 2006), the primary mechanism appeared to be one of selective guidance.
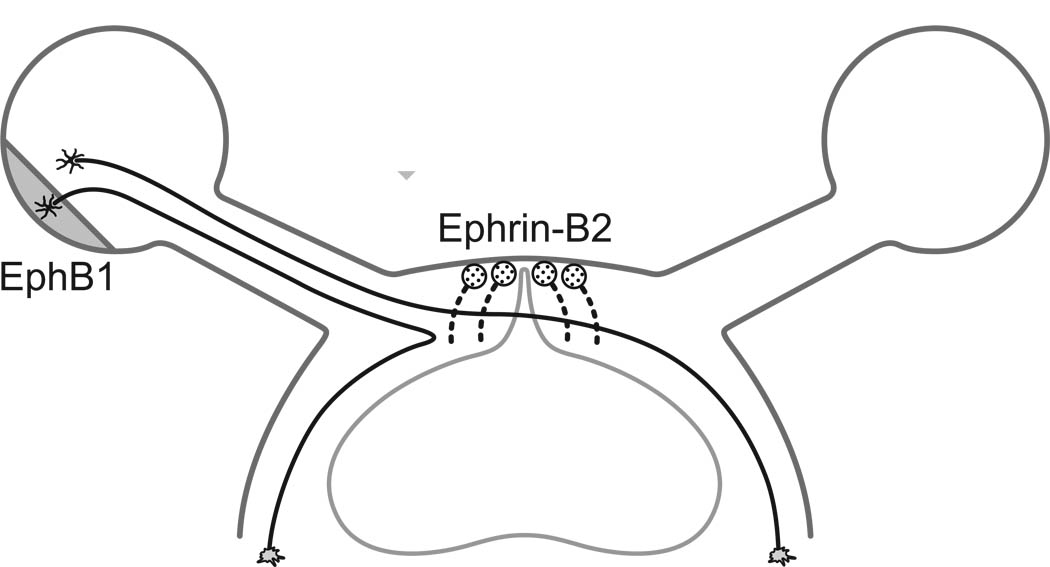
Subsequent explant studies revealed a contact-mediated repulsion of outgrowing processes from ventro-temporal retina by cells of the chiasmatic midline region (Marcus, Blazeski, Godement, & Mason, 1995; Marcus & Mason, 1995; Wang, Dani, Godement, Marcus, & Mason, 1995). The midline cells were determined to be radial glia expressing the Ephrin-B2 ligand, deciphered by the presence of EphB1 receptor on the growth cones of temporal retinal axons, due to the restricted expression of EphB1 within the ventro-temporal retina (figure 5) (Nakagawa Brennan, Johnson, Shewan, Harris & Holt, 2000; Petros, Shrestha, & Mason, 2009; Williams, Mann, Erskine, Sakurai, Wei, Rossi, Gale, Holt, Mason & Henkemeyer, 2003). That expression has been handsomely shown to be controlled by the transcription factor gene Zic2, which, when knocked down leads to a reduced uncrossed projection (Herrera, Brown, Aruga, Rachel, Dolen, Mikoshiba, Brown & Mason, 2003), and when ectopically expressed by nasal retinal cells leads to their upregulation of EphB1 and de novo sensitivity in a repulsion assay (Lee, Petros, & Mason, 2008). Zic2 is itself regulated by Foxd1, as Foxd1-knockout mice lose Zic2 and EphB1 proteins in the ventro-temporal retina, and fail to differentiate an uncrossed retinal projection from this same retinal region Together, these results suggest that a time-dependent modulation of Zic2 expression may be sufficient to reduce EphB-mediated repulsion at the chiasmatic midline at progressively later developmental stages (see also Fabre, Shimogori, & Charron, 2010). Modulating the control of Zic2 expression relative to the neurogenetic period may then account for the major species differences in decussation patterns, including the striking variability in the projection pattern for closely related species (Reese & Baker, 1990; Reese et al., 1991). It remains to be determined, however, whether the mechanisms generating the partial decussation in the mouse visual pathway bear relevance for other mammalian species, where there is a greater tendency for uncrossed axons to remain constrained to the temporal portion of the prechiasmatic nerve, and where early loss of one retina has little consequence upon the trajectory of optic axons from the remaining intact retina (see Jeffery et al., 2008).
Figure 5.
The transcription factor Islet-2 represses Zic2 expression, which in turn regulates EphB1 receptor expression in the ventro-temporal retina. Ganglion cells in this region of the retina are therefore equipped to detect and respond to the presence of Ephrin-B2 on the surface of a select group of radial glia at the midline, deflecting these optic axons ipsilaterally. (Modified from Petros et al., 2009).
These results also suggest that the well-documented abnormal decussation of temporal retinal axons at the optic chiasm in conditions that reduce ocular pigmentation (LaVail, Nixon, & Sidman, 1978; Rachel, Mason, & Beermann, 2002; Sanderson, Guillery, & Shackelford, 1974) may itself be mediated through the modulation of these upstream transcription factors. Explant studies have confirmed that the abnormality in oculo-cutaneous albinism lies within the retina itself, rather than within the chiasm (Marcus, Wang, & Mason, 1996),likely localized to the temporal retina (Rice, Goldowitz, Williams, Hamre, Johnson, Tan, & Reese, 1999), but the mechanism by which the cells of the pigmented epithelium behind the retina might influence these transcription factors regulating EphB receptor expression by retinal ganglion cells is still uncertain (Petros & Mason, 2008). The past 25 years have, however, seen new progress in understanding the signaling events within the cells of the retinal pigment epithelium (RPE). Like mutations in tyrosinase, producing the oculo-cutaneous phenotype and reductions in the size of the uncrossed projection due to ectopic crossing at the chiasmatic midline, so mutations in the Oa1 gene that underlie ocular albinism also have smaller uncrossed projections (Incerti, Cortese, Pizzigoni, Surace, Varani, Coppola, Jeffery, Seeliger, Jaissle, Bennett, Marigo, Schiaffino, Tacchetti & Ballabio, 2000). Oa1 is a novel G-protein coupled receptor situated in the melanosomal membrane within RPE cells, with its N-terminal within the lumen of the melanosome and C-terminal within the cytoplasm (Schiaffino, Baschirotto, Pellegrini, Montalti, Tacchetti, De Luca & Ballabio, 1996). It may therefore serve as a sensor for some intra-melanosomal signal, acting through the G protein Gαi3 (Schiaffino, d'Addio, Alloni, Baschirotto, Valetti, Cortese, Puri, Bassi, Colla, DeLuca, Tacchetti & Ballabio, 1999), since Gαi3 knock-out mice show, like Tyr−/− and Oa1−/− mice, abnormalities in their melanosomes and reductions in the size of their uncrossed pathways (Young, Powelson, Whitney, Raven, Nusinowitz, Jiang, Birnbaumer, Reese & Farber, 2008). These phenotypic similarities suggest a common signaling pathway that is initiated within melanosomes and which is defective in the absence of tyrosinase (Schiaffino & Tacchetti, 2005), perhaps the synthesis of L-dopa itself (Lopez, Decatur, Stamer, Lynch, & McKay, 2008).
7. How is the retinotopic map established within the optic tectum?
How the orderly representation of the retinal surface within central visual structures is established during development had been greatly debated 25 years ago. One camp was preoccupied with evidence supporting a “morphogenetic” account positing that the timing and/or spatial positioning of fibers as they grow along the pathway is critical for the formation of the retinotopic map (Horder & Martin, 1978). According to this view, the retinotopic ordering of optic axons present within the fiber pathway should be sufficient for ensuring their orderly termination within central targets (Bunt & Horder, 1983). Subsequent studies explored in great detail, particularly in mammals, the precision of such ordering, finding that any such order behind the eye appears to be largely a passive consequence of funneling fascicles of axons from the different quadrants of the retinal nerve fiber layer into the optic stalk (FitzGibbon & Reese, 1992, 1996), and, more critically, that such retinotopic fiber order becomes so degraded along the length of the optic nerve as to be nearly absent in the prechiasmatic portion of the pathway (Chan & Guillery, 1993; Horton, Greenwood, & Hubel, 1979; Reese & Baker, 1993; Simon & O'Leary, 1991; Williams, Borodkin, & Rakic, 1991; Williams & Rakic, 1985; but see also Jeffery et al., 2008), independent of the chronotopic reordering of axons that occurs pre-chiasmatically (Reese & Baker, 1992; Reese, 1994). Curiously, the dorso-ventral retinal axis regains some degree of retinotopic fiber order across the width of the optic tract, so that axons enter their target visual nuclei with some degree of pre-sorting in anticipation of the topographic mapping of this retinal axis (Chan & Guillery, 1993; Maggs & Scholes, 1986; Plas, Lopez, & Crair, 2005; Reese & Baker, 1993), but whether this pre-order contributes to map formation has yet to be determined. Axonal timing and pre-formed axonal scaffolds were likewise discounted as critical morphogenetic players (Cornel & Holt, 1992; Holt, 1991). Others had noted that topographic targeting errors were more common during early development, when the population of retinal ganglion cells undergoes naturally occurring cell death, leading to the suggestion that cell death might sculpt the topography from an initially disordered map (O'Leary, Fawcett, & Cowan, 1986). While cell death may act to eliminate some topographic targeting errors, it does not appear to play a major role in establishing the retinotopic map (Linden & Reese, 2006). Rather, targeted elaboration of a terminal field gradually emerges from axonal arbors that are initially diffuse, particularly in mammals (Yates, Roskies, McLaughlin, & O’Leary, 2001).
A second camp was largely made up of researchers favoring some form of chemospecificity underlying this mapping, as originally proposed by Sperry (1963). A major break-through came in 1987, when temporal retinal growth cones were shown to exhibit a clear preference when confronted with the option to elongate in the presence of membrane fragments from rostral versus caudal cells of the chick's optic tectum, using the in vitro “striped carpet assay” (Walter, Henke-Fahle, & Bonhoeffer, 1987b; Walter, Kern-Veits, Huf, Stolze, & Bonhoeffer, 1987a). Temporal axons were shown to avoid growth upon caudal membrane fragments, and the strength of this behavior varied as a function of rostro-caudal position upon the tectum. The membrane-linked molecules, Ephrin-A2 and Ephrin-A5, were subsequently identified to mediate this repulsion and to be expressed in an increasing rostro-caudal gradient, and were detected through a temporal-to-nasal gradient of declining EphA receptor expression upon the retinal ganglion cells (blue to yellow gradients in figure 6; (Cheng, Nakamoto, Bergemann, & Flanagan, 1995; Drescher, Kremoser, Handwerker, Cöschinger, Noda & Bonhoeffer, 1995). Subsequent studies have identified counter-gradients that may explain the preferential growth of nasal axons to the rostral tectum (Rashid, Upton, Blentic, Ciossek, Knöll, Thompson & Drescher, 2005); orthogonal retinal and tectal gradients mediated by Ephrin-B and EphB receptors that participate in the mapping of the dorso-ventral retinal axis across the lateral-to-medial tectal axis (Hindges, McLaughlin, Genoud, Henkemeyer, & O'Leary, 2002); and attractive rather than repulsive interactions mediating the effect of some of those gradients (red to green gradients in figure 6). These have been reviewed extensively recently (Clandinin & Feldheim, 2009; Flanagan, 2007; McLaughlin & O'Leary, 2005; Scicolone, Ortalli, & Carri, 2009); suffice it to say that complementary gradients across both retinal and tectal axes clearly participate in retinotopic map formation by providing retinal growth cones with the means to decipher positional identity upon the tectal surface. While some studies indicate a role for axonal interactions in the mapping mediated by Eph receptors (Brown, Yates, Burrola, Ortuño, Vaidya, Jessell, Pfaff, O'Leary & Lemke, 2000), others have suggested that neighboring ganglion cell axons are not critical for this targeting to the appropriate location upon the tectum (Gosse, Nevin, & Baier, 2008).
Figure 6.
Orthogonal density gradients of EphA3 and EphB2/EphB3 receptors upon the population of retinal ganglion cells enable their axonal growth cones to identify corresponding locations within the optic tectum by virtue of complementary Ephrin-A2/A5 and Ephrin-B1 gradients, mediating the establishment of the retinotopic map.
This is not to say that neighboring optic axons don't participate in the refinement of retinotopic precision as these axons establish their synaptic contacts with tectal cells. A role for correlated visual activity in the refinement of retinotopic mapping in the optic tectum was well-known 25 years ago (see Cline, 1991; Constantine-Paton, Cline, & Debski, 1990, for reviews). Perhaps one of the most significant collections of findings since then have been the demonstrations that, prior to photoreceptor maturation, inner retinal neurons are spontaneously active (Galli & Maffei, 1988); this activity is correlated between neighboring cells (Maffei & Galli-Resta, 1990); this correlated spontaneous activity travels across the retina as “waves” that emerge, traverse and then dissipate, creating a refractory period within the activated region in which subsequent wave-like activity is prohibited (Meister, Wong, Baylor, & Shatz, 1991; Wong, Chernjavsky, Smith, & Shatz, 1995; Wong, Meister, & Shatz, 1993); and that acetylcholine released from starburst amacrine cells plays a critical role in the lateral transmission of this wave-like activity (Feller, Wellis, Stellwagen, Werblin, & Shatz, 1996), as does glutamate released from bipolar cells (Blankenship et al., 2009; Wong, Myhr, Miller, & Wong, 2000) and gap junctional connectivity (Hansen, Torborg, Elstrott, & Feller, 2005; Syed, Lee, Zheng, & Zhou, 2004; Torborg, Hansen, & Feller, 2005), each operating during a distinct temporal window prior to the onset of photo-transduction in the outer nuclear layer and each operating with distinct kinetics (see Blankenship & Feller, 2010; Huberman, Feller, & Chapman, 2008, for reviews). The presence of such spontaneous patterned activity should ensure a correlation between the axonal arbors of neighboring retinal ganglion cells that may then participate in the refinement of their connectivity within target visual structures (figure 7).
Figure 7.
Spontaneous (pre-visual) waves of activity spread across the surface of the developing retina (top), affecting each retina independently. The top five panels show the active regions (shaded) at two-second intervals from left to right. As a consequence, the bursts of action potentials by retinal ganglion cells will be more highly correlated between those axon terminals arising from adjacent locations on the retinal surface (a and b), ensuring a stabilization of their nascent synaptic contacts with target neurons, refining the precision of the retinotopic map (bottom). Much as more distant locations on the retina will have non-synchronous spontaneous activity with these two cells (c), so locations on the opposite retina should generate asynchronous discharges (d) relative to these cells, leading to the loss of binocular innervation upon single cells in the developing lateral geniculate nucleus. (Top modified from Stafford, Sher, Litke, & Feldheim, 2009).
That such spontaneous activity plays a role in retinotopic map refinement has been confirmed using mice in which nicotinic acetylcholine receptor function has been compromised by knocking out the β2-subunit: early studies reported that β2 knockout mice lack retinal waves entirely (Muir-Robinson, Hwang, & Feller, 2002; McLaughlin, Torborg, Feller, & O’Leary, 2003), but more recent studies have demonstrated that such mice show abnormally large and fast retinal waves (Sun, Warland, Ballesteros, van der List, Chalupa, 2008; Stafford, Sher, Litke, & Feldheim, 2009). Nevertheless, these mice have abnormally diffuse ganglion cell axonal arborizations in both the lateral geniculate nucleus and superior colliculus (Grubb, Rossi, Changeux, & Thompson, 2003; McLaughlin, Torborg, Feller, & O'Leary, 2003). These axons still target to their appropriate vicinity, mediated by the above-described Ephrin-A/EphA receptor signaling: knocking out the latter is known to disrupt retinotopic mapping, if not completely abolishing it (Feldheim et al., 2004), while doing so when also knocking out the β2-subunit of the nicotinic receptor near-completely abolishes retinotopic order (Pfeiffenberger, Yamada, & Feldheim, 2006). Indeed, the spontaneous activity not only refines the precision of these retinofugal maps, but also mediates the alignment of these maps between interconnected visual structures, including the projection from the visual cortex upon the superior colliculus (Triplett, Owens, Yamada, Lemke, Cang, Stryker & Feldheim, 2009).
8. How are the separate ocular domains established in the developing lateral geniculate nucleus?
The two hemi-retinas innervating the lateral geniculate nucleus terminate in discrete and complementary ocular territories. The patterning of these ocular domains is species-specific, commonly forming apposed laminae innervated by the opposite eyes, and in every species examined, arises during early development from an initially intermingled distribution of terminations from the two eyes (Jeffery, 1984; Linden, Guillery, & Cucchiaro, 1981; Marotte, 1990; Rakic, 1976; Shatz, 1983), wherein binocular innervation of single geniculate neurons is transiently present (Campbell & Shatz, 1992; Campbell, So, & Lieberman, 1984; Shatz & Kirkwood, 1984; Jaubert-Miazza, Green, Lo, Bui, Mills, & Guido, 2005). The fact that this segregation coincided with the period of naturally occurring ganglion cell loss suggested a causal relationship between the two events (Jeffery, 1984), but other studies examining the developmental change in the axonal arbors of single retinal ganglion cells revealed this process to be mediated by the selective pruning and elaboration of the terminal arbor (Sretavan & Shatz, 1984). The process was also shown to be dependent upon inter-ocular interactions, for early removal of one retina prevented the terminal field from the opposite eye to coalesce into its normal ocular domain (Jeffery, 1984; Rakic, 1981), and corrupted the normal sculpting of single axonal arbors (Sretavan & Shatz, 1986).
Since then, the process by which these overlapping ocular projections segregate into separate ocular domains has been studied extensively, and debated vigorously. As this segregation occurs prior to the onset of visual activity, much attention has focused upon the possibility that, much as correlated spontaneous activity refines the precision of the retinotopic map in visual structures, so it should serve to ensure that activity patterns will be maximally distinct between axons arising from opposite eyes (figure 7). The first suggestion that activity played a role came from studies showing that pharmacological blockade of geniculate spiking by intracranial TTX injections prevented the normal sculpting of optic axons into their characteristically discrete terminal arbors (Sretavan, Shatz, & Stryker, 1988; but see). Subsequent work confirmed that spontaneous retinal activity was effective at driving geniculate activity (Mooney, Penn, Gallego, & Shatz, 1996), and that relative levels of such spontaneous retinal activity between the two eyes influenced the formation of ocular terminal fields in the lateral geniculate nucleus (Penn, Riquelme, Feller, & Shatz, 1998; Stellwagen & Shatz, 2002). Others then attempted to implicate the wave-like transmission of this spontaneous retinal activity by examining the above-mentioned β2-subunit knock-out mice (Muir-Robinson et al.,, 2002; Rossi et al., 2001), as well as connexin 36 knock-out mice, and in double knock-out mice (Torborg et al., 2005). Key to effective binocular segregation, apparently, is the presence the bursts of action potentials that are correlated between neighboring retinal ganglion cells, provided the frequency of such bursting is not so frequent to yield spurious correlations of activity between retinal ganglion cells at homonymous retinal loci in the two eyes (Butts, Kanold, & Shatz, 2007; Huberman, 2007; Torborg & Feller, 2005).
This summary simplifies the extensive debate recently aired on this topic (Chalupa, 2009; Feller, 2009). Doubtless further details of the critical role played by retinal activity in this process will be defined in the near future. But regardless of retinal waves, other additional factors are believed to play a role in this process, to account for the fact that the contralateral and ipsilateral ocular domains form in characteristic locations, and for the fact that animals with abnormal decussations at the optic chiasm produce ocular innervation of the lateral geniculate nucleus wherein the nasal and temporal retina still segregate to their characteristic portions (Guillery, 1974; Williams, Hogan, & Garraghty, 1994). As indicated above, there is a decreasing gradient of EphA receptor across the temporal to nasal retinal axis; there are as well gradients of Ephrin-A across the lateral geniculate nucleus (Feldheim et al., 1998; Huberman, Murray, Warland, Feldheim, & Chapman, 2005). Knocking out Ephrin-A expression in the lateral geniculate nucleus of the mouse, as well as overexpression of EphA within the retina of the ferret, disrupts the normal process of ocular segregation, consistent with the idea that the differential expression of EphA receptor in the temporal versus nasal retina (due to the normal declining gradient across this retinal axis) contributes to the targeting of these axons to distinct ocular domains (Huberman et al., 2005; Pfeiffenberger, Cutforth, Woods, Yamada, Rentería, Copenhagen, Flanagan & Feldheim, 2005). Alas, the similarities between these studies may be more superficial than at first examination, as the effect in the knock-out mouse yields erroneously positioned uncrossed terminal fields within the monocular segment of the lateral geniculate nucleus (that portion of the nucleus normally receiving only input from the far nasal retina viewing the monocular visual field), while EphA overexpression in the ferret retina yields primarily a failure of the ipsilateral and contralateral terminal fields to fully segregate within the binocular segment of the lateral geniculate nucleus (Huberman et al., 2005; Pfeiffenberger et al., 2005). These discrepancies need to be resolved before we can fully understand the relationship between the formation of ocularly-segregated retinotopic maps that are in binocular registration, and the role of activity in this process (Rebsam, Petros, & Mason, 2009). They are particularly vexing for our comprehension of the mapping of the uncrossed retinotopic projection, for this map’s retinal axis runs counter to that for the crossed projection (i.e. the naso-temporal retinal axis for the crossed projection terminates across the lateral-to-medial axis of the LGN, while that for the uncrossed projection terminates across the medial-to-lateral axis). Should one of the EphA receptor gradients peak on the line of decussation, rather than at the far temporal periphery, and a declining Ephrin-A gradient be distributed across the axis of the lateral geniculate nucleus mapping the representation of the peripheral-to-central visual field, then the mouse may prove to construct its retino-geniculate mapping in a fashion comparable to that recently proposed for the human’s visual pathway (Lambot, Depasse, Noel, & Vanderhaeghen, 2005). Ephrin-A2/A5 double knock-out mice show disorderly uncrossed retino-tectal projections (Haustead, Lukehurst, Clutton, Bartlett, Dunlop, Arrese, Sherrard & Rodger, 2008), but the retino-tectal pathway may not be an appropriate model for understanding retino-geniculate organization, particularly with respect to ocular innervation patterns. For now, an adequate account of why the ipsilateral and contralateral ocular domains form where they characteristically reside still remains to be provided, as does an explanation for the polarity of the uncrossed map and the registration of the two half-retinal maps before the onset of vision.
Conclusions
The articles in that 25th Anniversary issue of Vision Research (like others in the present 50th Anniversary issue), described the rich theoretical issues in contemporary vision research then being addressed along with their biological underpinnings. A number of those issues had been the focus for study well before the founding of the journal, and, for a few of the authors writing at the time of the 25th Anniversary, seemed hardly closer to resolution. The subject matter described in the present chapter, in comparison, has a conspicuously different flavor to it, divorced entirely from visual function and relevant to understanding the development of the brain in general, being largely atheoretical in nature and subject to frequent revision in light of new discoveries every few years, focused on the life-history of the various components of the retina and optic pathway, and, where possible, on the genetic, molecular, cellular and activity-dependent mechanisms underlying those developmental changes. These studies inform us hardly at all about visual processing, but they better enable us to conceptualize the principles by which the mature organization comes about, and lead to the design of experiments attempting to validate those accounts, paving the way for further refinements in our understanding. Sperry would likely find the documented breadth of chemo-specific wiring within the nervous system entirely expected, but he would be astonished at the depth of our understanding of the causal chain of events underlying such interactions that produce the architecture and circuitry within the CNS mediating visually guided behavior.
Acknowledgments
The author’s research is supported by the National Institutes of Health (EY-11087 and EY-19968). I thank Andy Huberman, Ross Poché and Rivka Rachel for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
That said, it is interesting to note that roughly one-third of the twelve-hundred fifty citations to Sperry’s 1963 “chemoaffinity” paper (as of 2010) occurred before 1986.
References
- Adler R. A model of retinal cell differentiation in the chick embryo. Prog. Ret. Eye Res. 2000;19:529–557. doi: 10.1016/s1350-9462(00)00008-2. [DOI] [PubMed] [Google Scholar]
- Agathocleous M, Harris WA. Cell determination. In: Sernagor E, Eglen S, Harris B, Wong R, editors. Retinal Development. Cambridge: Cambridge University Press; 2006. pp. 75–98. [Google Scholar]
- Agathocleous M, Harris WA. From progenitors to differentiated cells in the vertebrate retina. Ann. Rev. Cell Develop. Biol. 2009;25:45–69. doi: 10.1146/annurev.cellbio.042308.113259. [DOI] [PubMed] [Google Scholar]
- Agathocleous M, Iordanova I, Willardsen MI, Xue XY, Vetter ML, Harris WA, Moore KB. A directional Wnt/beta-catenin-Sox2-proneural pathway regulates the transition from proliferation to differentiation in the Xenopus retina. Development. 2009;136:3289–3299. doi: 10.1242/dev.040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajioka I, Martins RAP, Bayazitov IT, Donovan S, Johnson DA, Frase S, Cicero SA, Boyd K, Zakharenko SS, Dyer MA. Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell. 2007;131:378–390. doi: 10.1016/j.cell.2007.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi T, Inoue T, Miyoshi G, Bessho Y, Takahashi M, Lee JE, Guillemot F, Kageyama R. Requirement of multiple basic helix-loop-helix genes for retinal neuronal subtype specification. J. Biol. Chem. 2004;279:28492–28498. doi: 10.1074/jbc.M400871200. [DOI] [PubMed] [Google Scholar]
- Atkinson-Leadbeater K, Bertolesi GE, Hehr CL, Webber CA, Cechmanek PB, McFarlane S. Dynamic expression of axon guidance cues required for optic tract development is controlled by fibroblast growth factor signaling. J. Neurosci. 2010;30:685–693. doi: 10.1523/JNEUROSCI.4165-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea TC, Cahill H, Ecker J, Hattar S, Nathans J. Distinct roles of transcription factors brn3a and brn3b in controlling the development, morphology, and function of retinal ganglion cells. Neuron. 2009;61:852–864. doi: 10.1016/j.neuron.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker GE, Reese BE. Chiasmatic course of temporal retinal axons in the developing ferret. J. Comp. Neurol. 1993;330:95–104. doi: 10.1002/cne.903300108. [DOI] [PubMed] [Google Scholar]
- Baye LM, Link BA. Interkinetic nuclear migration and the selection of neurogenic cell divisions during vertebrate retinogenesis. J. Neurosci. 2007;27:10143–10152. doi: 10.1523/JNEUROSCI.2754-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat. Rev. Neurosci. 2010;11:18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Ford KJ, Johnson J, Seal RP, Edwards RH, Copenhagen DR, Feller MB. Synaptic and extrasynaptic factors governing glutamatergic retinal waves. Neuron. 2009;62:230–241. doi: 10.1016/j.neuron.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnarenko SR, Chalupa LM. Stratification of ON and OFF ganglion cell dendrites depends on glutamate-mediated afferent activity in the developing retina. Nature. 1993;36:144–146. doi: 10.1038/364144a0. [DOI] [PubMed] [Google Scholar]
- Bodnarenko SR, Jeyarasasingam G, Chalupa L. Development and regulation of dendritic stratification in retinal ganglion cells by glutamate-mediated afferent activity. J. Neurosci. 1995;15:7037–7045. doi: 10.1523/JNEUROSCI.15-11-07037.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnarenko SR, Yeung G, Thomas L, McCarthy M. The development of retinal ganglion cell dendritic stratification in ferrets. NeuroReport. 1999;10:2955–2959. doi: 10.1097/00001756-199909290-00015. [DOI] [PubMed] [Google Scholar]
- Boije H, Edqvist PH, Hallböök F. Horizontal cell progenitors arrest in G2-phase and undergo terminal mitosis on the vitreal side of the chick retina. Develop. Biol. 2009;330:105–113. doi: 10.1016/j.ydbio.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Bramblett DE, Pennesi ME, Wu SM, Tsai MJ. The transcription factor Bhlhb4 is required for rod bipolar cell maturation. Neuron. 2004;43:779–793. doi: 10.1016/j.neuron.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Brittis P, Canning D, Silver J. Chondroitin sulfate as a regulator of neuronal patterning in the retina. Science. 1992;225:733–736. doi: 10.1126/science.1738848. [DOI] [PubMed] [Google Scholar]
- Brittis PA, Silver J. Multiple factors govern intraretinal axon guidance: A time-lapse study. Mol. Cell. Neurosci. 1995;6:413–432. doi: 10.1006/mcne.1995.1031. [DOI] [PubMed] [Google Scholar]
- Brown A, Yates PA, Burrola P, Ortuño D, Vaidya A, Jessell TM, Pfaff SL, O'Leary DD, Lemke G. Topographic mapping from the retina to the midbrain is controlled by relative but not absolute levels of EphA receptor signaling. Cell. 2000;102:77–88. doi: 10.1016/s0092-8674(00)00012-x. [DOI] [PubMed] [Google Scholar]
- Brown NL, Patel S, Brzezinski J, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development. 2001;128:2497–2508. doi: 10.1242/dev.128.13.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt SM, Horder TJ. Evidence for an orderly arrangement of optic axons within the optic nerves of the major nonmammalian vertebrate classes. J Comp Neurol. 1983;213:94–114. doi: 10.1002/cne.902130109. [DOI] [PubMed] [Google Scholar]
- Burmeister M, Novak J, Liang MY, Basu S, Ploder L, Hawes NL, Vidgen D, Hoover F, Goldman D, Kalnins VI, Roderick TH, Taylor BA, Hankin MH, McInnes RR. The transcription factor Bhlhb4 is required for rod bipolar cell maturation. Nature Genet. 1996;12:376–384. doi: 10.1038/ng0496-376. [DOI] [PubMed] [Google Scholar]
- Butts DA, Kanold PO, Shatz CJ. A burst-based "Hebbian" learning rule at retinogeniculate synapses links retinal waves to activity-dependent refinement. PLoS Biol. 2007;5:e61. doi: 10.1371/journal.pbio.0050061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G, Shatz CJ. Synapses formed by identified retinogeniculate axons during the segregation of eye input. J. Neurosci. 1992;12:1847–1858. doi: 10.1523/JNEUROSCI.12-05-01847.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G, So K-F, Lieberman AR. Normal postnatal development of retinogeniculate axons and terminals and identification of inappropriately-located transient synapses: Electron microscope studies of horseradish peroxidase-labelled retinal axons in the hamster. Neuroscience. 1984;13:743–759. doi: 10.1016/0306-4522(84)90093-9. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. II. Autoradiographic analysis of cell generation using tritiated thymidine. J Comp Neurol. 1979;188:263–272. doi: 10.1002/cne.901880205. [DOI] [PubMed] [Google Scholar]
- Cayouette M, Poggi L, Harris WA. Lineage in the vertebrate retina. TINS. 2006;29:563–570. doi: 10.1016/j.tins.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Chalupa LM. Retinal waves are unlikely to instruct the formation of eye-specific retinogeniculate projections. Neural Develop. 2009;4:25. doi: 10.1186/1749-8104-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SO, Guillery RW. Developmental changes produced in the retinofugal pathways of rats and ferrets by early monocular enucleations: The effects of age and the differences between normal and albino animals. J. Neurosci. 1993;13:5277–5293. doi: 10.1523/JNEUROSCI.13-12-05277.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CW, Chow RL, Lebel M, Sakuma R, Cheung HO, Thanabalasingham V, Zhang X, Bruneau BG, Birch DG, Hui CC, McInnes RR, Cheng SH. The Iroquois homeobox gene, Irx5, is required for retinal cone bipolar cell development. Develop. Biol. 2005;287:48–60. doi: 10.1016/j.ydbio.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Cheng HJ, Nakamoto M, Bergemann AD, Flanagan JG. Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell. 1995;82:371–381. doi: 10.1016/0092-8674(95)90426-3. [DOI] [PubMed] [Google Scholar]
- Cherry TJ, Trimarchi JM, Stadler MB, Cepko CL. Development and diversification of retinal amacrine interneurons at single cell resolution. Proc. Natl. Acad. Sci. 2009;106:9495–9500. doi: 10.1073/pnas.0903264106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RL, Volgyi B, Szilard RK, Ng D, McKerlie C, Bloomfield SA, Birch DG, McInnes RR. Control of late off-center cone bipolar cell differentiation and visual signaling by the homeobox gene Vsx1. Proc. Nat'l. Acad. Sci. 2004;101:1754–1749. doi: 10.1073/pnas.0306520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clandinin TR, Feldheim DA. Making a visual map: mechanisms and molecules. Curr. Opin. Neurobiol. 2009;19:174–180. doi: 10.1016/j.conb.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline HT. Activity-dependent plasticity in the visual systems of frogs and fish. Trends Neurosci. 1991;14:104–111. doi: 10.1016/0166-2236(91)90071-2. [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT, Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Ann. Rev. Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Coombs JL, Van Der List D, Chalupa LM. Morphological properties of mouse retinal ganglion cells during postnatal development. J. Comp. Neurol. 2007;503:803–814. doi: 10.1002/cne.21429. [DOI] [PubMed] [Google Scholar]
- Corbo JC, Cepko CL. A hybrid photoreceptor expressing both rod and cone genes in a mouse model of enhanced S-cone syndrome. PLoS Genet. 2005;1:e11. doi: 10.1371/journal.pgen.0010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornel E, Holt C. Precocious pathfinding: retinal axons can navigate in an axonless brain. Neuron. 1992;9:1001–1011. doi: 10.1016/0896-6273(92)90061-h. [DOI] [PubMed] [Google Scholar]
- Dann JF, Buhl EH, Peichl L. Dendritic maturation in cat retinal ganglion cells: a Lucifer yellow study. Neurosci. Lett. 1987;80:21–26. doi: 10.1016/0304-3940(87)90488-5. [DOI] [PubMed] [Google Scholar]
- Dann JF, Buhl EH, Peichl L. Postnatal dendritic maturation of alpha and beta ganglion cells in cat retina. J. Neurosci. 1988;8:1485–1499. doi: 10.1523/JNEUROSCI.08-05-01485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw NW, Wyatt HJ. Raising rabbits in a moving visual environment: an attempt to modify directional sensitivity in the retina. J. Physiol. 1974;240:309–330. doi: 10.1113/jphysiol.1974.sp010612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–589. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Del Bene F, Wehman AM, Link BA, Baier H. Regulation of neurogenesis by interkinetic nuclear migration through an apical-basal notch gradient. Cell. 2008;134:1055–1065. doi: 10.1016/j.cell.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsky RI, Chang WS, Rapaport DH, Harris WA. Regulation of neuronal diversity in the Xenopus retina by Delta signalling. Nature. 1997;385:67–70. doi: 10.1038/385067a0. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Rapaport DH, Harris WA. Xotch inhibits cell differentiation in the Xenopus retina. Neuron. 1995;14:487–496. doi: 10.1016/0896-6273(95)90305-4. [DOI] [PubMed] [Google Scholar]
- Dräger U. Birth dates of retinal ganglion cells giving rise to the crossed and uncrossed optic projections in the mouse. Proc. R. Soc. Lond. 1985;B 224:57–77. doi: 10.1098/rspb.1985.0021. [DOI] [PubMed] [Google Scholar]
- Drescher U, Kremoser C, Handwerker C, Löschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–270. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- Dunlop SA. Early development of retinal ganglion cell dendrites in the marsupial Setonix brachyurus, Quokka. J. Comp. Neurol. 1990;293:425–447. doi: 10.1002/cne.902930307. [DOI] [PubMed] [Google Scholar]
- Dyer MA, Livesey FJ, Cepko CL, Oliver G. Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat Genet. 2003;34:53–58. doi: 10.1038/ng1144. [DOI] [PubMed] [Google Scholar]
- Dyer MA, Martins R, da Silva Filho M, Muniz JA, Silveira LC, Cepko CL, Finlay BL. Developmental sources of conservation and variation in the evolution of the primate eye. Proc. Nat'l. Acad. Sci. 2009;106:8963–8968. doi: 10.1073/pnas.0901484106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edqvist PH, Hallböök F. Newborn horizontal cells migrate bi-directionally across the neuroepithelium during retinal development. Development. 2004;131:1343–1351. doi: 10.1242/dev.01018. [DOI] [PubMed] [Google Scholar]
- Edqvist PH, Lek M, Boije H, Lindbäck SM, Hallböök F. Axon-bearing and axon-less horizontal cell subtypes are generated consecutively during chick retinal development from progenitors that are sensitive to follistatin. BMC Dev. Biol. 2008;8:46. doi: 10.1186/1471-213X-8-46. doi:10.1186/1471-1213X-1188-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglen SJ. Development of regular cellular spacing in the retina: theoretical models. Math Med Biol. 2006;23:79–99. doi: 10.1093/imammb/dql003. [DOI] [PubMed] [Google Scholar]
- Eglen SJ, Willshaw DJ. Influence of cell fate mechanisms upon retinal mosaic formation: A modelling study. Development. 2002;129:5399–5408. doi: 10.1242/dev.00118. [DOI] [PubMed] [Google Scholar]
- Elshatory Y, Everhart D, Deng M, Xie X, Barlow RB, Gan L. Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. J. Neurosci. 2007;27:12707–12720. doi: 10.1523/JNEUROSCI.3951-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine L, Thompson H. Intraretinal axon guidance. In: Chalupa LM, Williams RW, editors. Eye, Retina, and Visual System of the Mouse. Cambridge: MIT Press; 2008. pp. 379–388. [Google Scholar]
- Eysel UT, Peichl L, Wässle H. Dendritic plasticity in the early postnatal feline retina: Quantitative characteristics and sensitive period. J. Comp. Neurol. 1985;242:134–145. doi: 10.1002/cne.902420109. [DOI] [PubMed] [Google Scholar]
- Fabre PJ, Shimogori T, Charron F. Segregation of ipsilateral retinal ganglion cell axons at the optic chiasm requires the Shh receptor Boc. J. Neurosci. 2010;30:266–275. doi: 10.1523/JNEUROSCI.3778-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajian R, Raven MA, Cusato K, Reese BE. Cellular positioning and dendritic field size of cholinergic amacrine cells are impervious to early ablation of neighboring cells in the mouse retina. Vis. Neurosci. 2004;21:13–22. doi: 10.1017/s0952523804041021. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Perez-Miguelsanz J, Ryder EF, Cepko CL. Clonal analysis in the chicken retina reveals tangential dispersion of clonally related cells. Develop. Biol. 1994;166:666–682. doi: 10.1006/dbio.1994.1346. [DOI] [PubMed] [Google Scholar]
- Feldheim DA, Nakamoto M, Osterfield M, Gale NW, DeChiara TM, Rohatgi R, Yancopoulos GD, Flanagan JG. Loss-of-function analysis of EphA receptors in retinotectal mapping. J. Neurosci. 2004;24:2542–2550. doi: 10.1523/JNEUROSCI.0239-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim DA, Vanderhaeghen P, Hansen MJ, Frisén J, Lu Q, Barbacid M, Flanagan JG. Topographic guidance labels in a sensory projection to the forebrain. Neuron. 1998;21:1303–1313. doi: 10.1016/s0896-6273(00)80650-9. [DOI] [PubMed] [Google Scholar]
- Feller MB. Retinal waves are likely to instruct the formation of eye-specific retinogeniculate projections. Neural Develop. 2009;4:24. doi: 10.1186/1749-8104-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996;272:1182–1187. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- Feng L, Xie X, Joshi PS, Yang Z, Shibasaki K, Chow RL, Gan L. Requirement for Bhlhb5 in the specification of amacrine and cone bipolar subtypes in mouse retina. Development. 2006;133:4815–4825. doi: 10.1242/dev.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGibbon T, Reese BE. Position of growth cones within the retinal nerve fibre layer of fetal ferrets. J. Comp. Neurol. 1992;323:153–166. doi: 10.1002/cne.903230203. [DOI] [PubMed] [Google Scholar]
- FitzGibbon T, Reese BE. Organisation of retinal ganglion cell axons in the optic fibre layer and nerve of fetal ferrets. Vis. Neurosci. 1996;13:847–861. doi: 10.1017/s095252380000910x. [DOI] [PubMed] [Google Scholar]
- Flanagan JG. Neural map specificiation by gradients. Curr. Opin. Neurobiol. 2007;16:59–66. doi: 10.1016/j.conb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Fuerst PG, Bruce F, Tian M, Wei W, Elstrott J, Feller MB, Erskine L, Singer JH, Burgess RW. DSCAM and DSCAML1 function in self-avoidance in multiple cell types in the developing mouse retina. Neuron. 2009;64:484–497. doi: 10.1016/j.neuron.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst PG, Burgess RW. Adhesion molecules in establishing retinal circuitry. Curr. Opin. Neurobiol. 2009;19:389–394. doi: 10.1016/j.conb.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst PG, Koizumi A, Masland RH, Burgess RW. Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature. 2008;451:470–474. doi: 10.1038/nature06514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujitani Y, Fujitani S, Luo H, Qiu F, Burlison J, Long Q, Kawaguchi Y, Edlund H, MacDonald RJ, Furukawa T, Fujikado T, Magnuson MA, Xiang M, Wright CV. Ptf1a determines horizontal and amacrine cell fates during mouse retinal development. Development. 2006;133:4439–4450. doi: 10.1242/dev.02598. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Cepko CL. Crx a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91:531–541. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Mukherjee S, Bao ZZ, Morrow EM, Cepko CL. rax, Hes1, and notch1 promote the formation of Müller glia by postnatal retinal progenitor cells. Neuron. 2000;26:383–394. doi: 10.1016/s0896-6273(00)81171-x. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L, Novelli E. The effects of natural cell loss on the regularity of the retinal cholinergic arrays. J. Neurosci. 2000;20:RC60. doi: 10.1523/JNEUROSCI.20-03-j0005.2000. (61–65) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli L, Maffei L. Spontaneous impulse activity of rat retinal ganglion cells in prenatal life. Science. 1988;242:90–91. doi: 10.1126/science.3175637. [DOI] [PubMed] [Google Scholar]
- Gan L, Wang SW, Huang Z, Klein WH. POU domain factor Brn-3b is essential for retinal ganglion cell differentiation and survival but not for initial cell fate specification. Develop. Biol. 1999;210:469–480. doi: 10.1006/dbio.1999.9280. [DOI] [PubMed] [Google Scholar]
- Geisert EE, Lu L, Freeman-Anderson NE, Templeton JP, Nassr M, Wang X, Gu W, Jiao Y, Williams RW. Gene expression in the mouse eye: an online resource for genetics using 103 trains of mice. Mol. Vis. 2009;15:1730–1763. [PMC free article] [PubMed] [Google Scholar]
- Godement P, Salaun J, Mason CA. Retinal axon pathfinding in the optic chiasma: Divergence of crossed and uncrossed fibers. Neuron. 1990;5:173–186. doi: 10.1016/0896-6273(90)90307-2. [DOI] [PubMed] [Google Scholar]
- Godinho L, Mumm JS, Williams PR, Schroeter EH, Koerber A, Park SW, Leach SD, Wong RO. Targeting of amacrine cell neurites to appropriate synaptic laminae in the developing zebrafish retina. Development. 2005;132:5069–5079. doi: 10.1242/dev.02075. [DOI] [PubMed] [Google Scholar]
- Godinho L, Williams PR, Claassen Y, Provost E, Leach SD, Kamermans M, Wong ROL. Nonapical symmetric divisions underlie horizontal cell layer formation in the developing retina in vivo. Neuron. 2007;56:597–603. doi: 10.1016/j.neuron.2007.09.036. [DOI] [PubMed] [Google Scholar]
- Gosse NJ, Nevin LM, Baier H. Retinotopic order in the absence of axon competition. Nature. 2008;452:892–895. doi: 10.1038/nature06816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Rossi FM, Changeux JP, Thompson ID. Abnormal functional organization in the dorsal lateral geniculate nucleus of mice lacking the beta 2 subunit of the nicotinic acetylcholine receptor. Neuron. 2003;40:1161–1172. doi: 10.1016/s0896-6273(03)00789-x. [DOI] [PubMed] [Google Scholar]
- Guillery RW. Visual pathways in albinos. Sci. Amer. 1974;230:44–54. doi: 10.1038/scientificamerican0574-44. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Mason CA, Taylor JSH. Developmental determinants at the mammalian optic chiasm. J. Neurosci. 1995;15:4727–4737. doi: 10.1523/JNEUROSCI.15-07-04727.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider NB, Naggert JK, Nishina PM. Excess cone cell proliferation due to lack of a functional NR2E3 causes retinal dysplasia and degeneration in rd7/rd7 mice. Hum. Mol. Genet. 2001;10:1619–1626. doi: 10.1093/hmg/10.16.1619. [DOI] [PubMed] [Google Scholar]
- Hansen KA, Torborg CL, Elstrott J, Feller MB. Expression and function of the neuronal gap junction protein connexin 36 in developing mammalian retina. J. Comp. Neurol. 2005;493:309–320. doi: 10.1002/cne.20759. [DOI] [PubMed] [Google Scholar]
- Harman AM, Beazley LD. Patterns of cytogenesis in the developing retina of the wallaby Setonix brachyurus. Anat. Embryol. 1987;177:123–130. doi: 10.1007/BF00572536. [DOI] [PubMed] [Google Scholar]
- Harman AM, Sanderson KJ, Beazley LD. Biphasic retinal neurogenesis in the brush-tailed possum, Trichosurus vulpecula: further evidence for the mechanisms involved in formation of ganglion cell density gradients. J. Comp. Neurol. 1992;325:595–606. doi: 10.1002/cne.903250411. [DOI] [PubMed] [Google Scholar]
- Hattori D, Chen Y, Matthews BJ, Salwinski L, Sabatti C, Grueber WB, Zipursky SL. Robust discrimination between self and non-self neurites requires thousands of Dscam1 isoforms. Nature. 2009;461:644–648. doi: 10.1038/nature08431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haustead DJ, Lukehurst SS, Clutton GT, Bartlett CA, Dunlop SA, Arrese CA, Sherrard RM, Rodger J. Functional topography and integration of the contralateral and ipsilateral retinocollicular projections of ephrin-A−/− mice. J. Neurosci. 2008;28:7376–7386. doi: 10.1523/JNEUROSCI.1135-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera E, Brown L, Aruga J, Rachel RA, Dolen G, Mikoshiba K, Brown S, Mason CA. Zic2 patterns binocular vision by specifying the uncrossed retinal projection. Cell. 2003;114:545–557. doi: 10.1016/s0092-8674(03)00684-6. [DOI] [PubMed] [Google Scholar]
- Herrera E, Marcus R, Li S, Williams SE, Erskine L, Lai E, Mason C. Foxd1 is required for proper formation of the optic chiasm. Development. 2004;131:5727–2739. doi: 10.1242/dev.01431. [DOI] [PubMed] [Google Scholar]
- Hindges R, McLaughlin T, Genoud N, Henkemeyer M, O'Leary DD. EphB forward signaling controls directional branch extension and arborization required for dorsal-ventral retinotopic mapping. Neuron. 2002;35:475–487. doi: 10.1016/s0896-6273(02)00799-7. [DOI] [PubMed] [Google Scholar]
- Hinds JW, Hinds PL. Early ganglion cell differentiation in the mouse retina: An electron microscopic analysis utilizing serial sections. Develop. Biol. 1974;37:381–416. doi: 10.1016/0012-1606(74)90156-0. [DOI] [PubMed] [Google Scholar]
- Hinds JW, Hinds PL. Differentiation of photoreceptors and horizontal cells in the embryonic mouse retina: An electron microscopic, serial section analysis. J. Comp. Neurol. 1979;187:495–512. doi: 10.1002/cne.901870303. [DOI] [PubMed] [Google Scholar]
- Hinds JW, Hinds PL. Development of retinal amacrine cells in the mouse embryo: Evidence for two modes of formation. J. Comp. Neurol. 1983;213:1–23. doi: 10.1002/cne.902130102. [DOI] [PubMed] [Google Scholar]
- Hitchcock PF. Exclusionary dendritic interactions in the retina of the goldfish. Development. 1989;106:589–598. doi: 10.1242/dev.106.3.589. [DOI] [PubMed] [Google Scholar]
- Hojo M, Ohtsuka T, Hashimoto N, Gradwohl G, Guillemot F, Kageyama R. Glial cell fate specification modulated by the bHLH gene Hes5 in mouse retina. Development. 2000;127:2515–2522. doi: 10.1242/dev.127.12.2515. [DOI] [PubMed] [Google Scholar]
- Holt C. Pathfinding and specificity in the early Xenopus retinofugal projection. In: Lam M-K, Shatz CJ, editors. Development of the Visual System. Vol. 3. Cambridge: MIT Press; 1991. pp. 109–122. [Google Scholar]
- Holt CE, Bertsch TW, Ellis HM, Harris WA. Cellular determination in the xenopus retina is independent of lineage and birth date. Neuron. 1988;1:15–26. doi: 10.1016/0896-6273(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Honjo M, Tanihara H, Suzuki S, Tanaka T, Honda Y, Takeichi M. Differential expression of cadherin adhesion receptors in neural retina of the postnatal mouse. Invest. Ophthalmol. Vis. Sci. 2000;41:546–551. [PubMed] [Google Scholar]
- Horder TJ, Martin KAC. Morphogenetics as an alternative to chemospecificity in the formation of nerve connections. In: Curtis ASG, editor. Cell-Cell Recognition, Society for Experimental Biology Symposium. Cambridge: Cambridge University Press; 1978. pp. 275–358. [PubMed] [Google Scholar]
- Horton JC, Greenwood MM, Hubel DH. Nonretinotopic arrangement of fibres in cat optic nerve. Nature. 1979;282:720–722. doi: 10.1038/282720a0. [DOI] [PubMed] [Google Scholar]
- Hsiau TH, Diaconu C, Myers CA, Lee J, Cepko CL, Corbo JC. The cis-regulatory logic of the mammalian photoreceptor transcriptional network. PLoS One. 2007;2:e643. doi: 10.1371/journal.pone.0000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD. Mechanisms of eye-specific visual circuit development. Curr. Opin. Neurobiol. 2007;17:73–80. doi: 10.1016/j.conb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Ann. Rev. Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Murray KD, Warland DK, Feldheim DA, Chapman B. Ephrin-As mediate targeting of eye-specific projections to the lateral geniculate nucleus. Nat. Neurosci. 2005;8:1013–1021. doi: 10.1038/nn1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckfeldt RM, Schubert T, Morgan JL, Godinho L, Di Cristo G, Huang ZJ, Wong ROL. Transient neurites of retinal horizontal cells exhibit columnar tiling via homotypic interactions. Nat. Neurosci. 2009;12:35–43. doi: 10.1038/nn.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufnagela RB, Lea TT, Riesenberga AL, Brown NL. Neurog2 controls the leading edge of neurogenesis in the mammalian retina. Develop. Biol. 2010 doi: 10.1016/j.ydbio.2010.02.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inatani M. Moecular mechanisms of optic axon guidance. Naturwissenschaften. 2005;92:549–561. doi: 10.1007/s00114-005-0042-5. [DOI] [PubMed] [Google Scholar]
- Incerti B, Cortese K, Pizzigoni A, Surace EM, Varani S, Coppola M, Jeffery G, Seeliger M, Jaissle G, Bennett DC, Marigo V, Schiaffino MV, Tacchetti C, Ballabio A. Oa1 knock-out: new insights on the pathogenesis of ocular albinism type 1. Hum. Molec. Gen. 2000;9:2781–2788. doi: 10.1093/hmg/9.19.2781. [DOI] [PubMed] [Google Scholar]
- Jadhav AP, Mason HA, Cepko CL. Notch 1 inhibits photoreceptor production in the developing mammalian retina. Development. 2006;133:913–923. doi: 10.1242/dev.02245. [DOI] [PubMed] [Google Scholar]
- Jadhav AP, Roesch K, Cepko CL. Development and neurogenic potential of Müller glial cells in the vertebrate retina. Prog. Ret. Eye Res. 2009;28:249–262. doi: 10.1016/j.preteyeres.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaubert-Miazza L, Green E, Lo FS, Bui K, Mills J, Guido W. Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis. Neurosci. 2005;22:661–676. doi: 10.1017/S0952523805225154. [DOI] [PubMed] [Google Scholar]
- Jeffery G. Retinal ganglion cell death and terminal field retraction in the developing rodent visual system. Develop. Brain Res. 1984;13:81–97. doi: 10.1016/0165-3806(84)90079-8. [DOI] [PubMed] [Google Scholar]
- Jeffery G, Erskine L. Variations in the architecture and development of the vertebrate optic chiasm. Prog. Ret. Eye Res. 2005;24:721–753. doi: 10.1016/j.preteyeres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Jeffery G, Levitt JB, Cooper HM. Segregated hemispheric pathways through the optic chiasm distinguish primates from rodents. Neuroscience. 2008;157:637–643. doi: 10.1016/j.neuroscience.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Oh EC, Ng L, Srinivas M, Brooks M, Swaroop A, Forrest D. Retinoid-related orphan nuclear receptor RORbeta is an early-acting factor in rod photoreceptor development. Proc. Natl. Acad. Sci. 2009;106:17534–17539. doi: 10.1073/pnas.0902425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PT, Raven MA, Reese BE. Disruption of transient photoreceptor targeting within the inner plexiform layer following early ablation of cholinergic amacrine cells in the ferret. Vis. Neurosci. 2001;18:741–751. doi: 10.1017/s095252380118507x. [DOI] [PubMed] [Google Scholar]
- Johnson PT, Williams RR, Cusato K, Reese BE. Rods and cones project to the inner plexiform layer during development. J. Comp. Neurol. 1999;414:1–12. [PubMed] [Google Scholar]
- Jusuf PR, Harris WA. Ptf1a is expressed transiently in all types of amacrine cells in the embryonic zebrafish retina. Neural Develop. 2009;4:34. doi: 10.1186/1749-8104-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay JN, Finger-Baier KC, Roeser T, Staub W, Baier H. Retinal ganglion cell genesis requires lakritz, a Zebrafish atonal Homolog. Neuron. 2001;30:725–736. doi: 10.1016/s0896-6273(01)00312-9. [DOI] [PubMed] [Google Scholar]
- Keeley PW, Reese BE. Morphology of dopaminergic amacrine cells in the mouse retina: Independence from homotypic interactions. J. Comp. Neurol. 2010a;518:1220–1231. doi: 10.1002/cne.22270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley PW, Reese BE. Role of afferents in the differentiation of bipolar cells in the mouse retina. J. Neurosci. 2010b;30:1677–1685. doi: 10.1523/JNEUROSCI.5153-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley PW, Whitney IE, Raven MA, Reese BE. Dendritic spread and functional coverage of starburst amacrine cells. J. Comp. Neurol. 2007;505:539–546. doi: 10.1002/cne.21518. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner D, Morgan JL, Parker ED, Lewis RM, Wong ROL. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature. 2009;460:1016–1020. doi: 10.1038/nature08236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Matsuda T, Cepko CL. A core paired-type and POU homeodomain-containing transcription factor program drives retinal bipolar cell gene expression. J. Neurosci. 2008b;28:7748–7764. doi: 10.1523/JNEUROSCI.0397-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Ross SE, Trimarchi JM, Aach J, Greenberg ME, Cepko CL. Identification of molecular markers of bipolar cells in the murine retina. J. Comp. Neurol. 2008a;507:1795–1810. doi: 10.1002/cne.21639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Wu HH, Lander AD, Lyons KM, Matzuk MM, Calof AL. GDF11 controls the timing of progenitor cell competence in developing retina. Science. 2005;308:1927–1930. doi: 10.1126/science.1110175. [DOI] [PubMed] [Google Scholar]
- Kirby MA, Chalupa LM. Retinal crowding alters the morphology of alpha ganglion cells. J. Comp. Neurol. 1986;251:532–541. doi: 10.1002/cne.902510408. [DOI] [PubMed] [Google Scholar]
- Lamba D, Nelson B, Karl MI, Reh TA. Specification, histogenesis, and photoreceptor development in the mouse retina. In: Chalupa LM, Williams RW, editors. Eye, Retina, and Visual System of the Mouse. Cambridge: MIT Press; 2008. pp. 299–310. [Google Scholar]
- Lambot MA, Depasse F, Noel JC, Vanderhaeghen P. Mapping labels in the human developing visual system and the evolution of binocular vision. J. Neurosci. 2005;25:7232–7237. doi: 10.1523/JNEUROSCI.0802-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail JH, Nixon RA, Sidman RL. Genetic control of retinal ganglion cell projections. J. Comp. Neurol. 1978;182:399–421. doi: 10.1002/cne.901820304. [DOI] [PubMed] [Google Scholar]
- Lee R, Petros TJ, Mason CA. Zic2 regulates retinal ganglion cell axon avoidance of ephrinB2 through inducing expression of the guidance receptor EphB1. J. Neurosci. 2008;28:5910–5919. doi: 10.1523/JNEUROSCI.0632-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung KM, Taylor JS, Chan SO. Enzymatic removal of chondroitin sulphates abolishes the age-related axon order in the optic tract of mouse embryos. Eur. J. Neurosci. 2003;17:1755–1767. doi: 10.1046/j.1460-9568.2003.02605.x. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Schall JD, Ault SJ. Extrinsic determinants of retinal ganglion cell structure in the cat. J. Neurosci. 1988;8:2028–2038. doi: 10.1523/JNEUROSCI.08-06-02028.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine EM, Fuhrmann S, Reh TA. Soluble factors and the development of rod photoreceptors. Cell. Mol. Life Sci. 2000;57:224–234. doi: 10.1007/PL00000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine EM, Green ES. Cell-intrinsic regulators of proliferation in vertebrate retinal progenitors. Sem. Cell Dev, Biol. 2004;15:63–74. doi: 10.1016/j.semcdb.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Lewis GP, Fisher SK. Retinal plasticity and cellular remodeling in retinal detachment and reattachment. In: Pinaud R, Tremere LA, De Weerd P, editors. Plasticity in the Visual System: From Genes to Circuits. New York: Springer; 2006. pp. 55–78. [Google Scholar]
- Li S, Mo Z, Yang X, Price SM, Shen MM, Xiang M. Foxn4 controls the genesis of amacrine and horizontal cells by retinal progenitors. Neuron. 2004;43:795–807. doi: 10.1016/j.neuron.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Lillien L. Changes in retinal cell fate induced by overexpression of EGF receptor. Nature. 1995;377:158–162. doi: 10.1038/377158a0. [DOI] [PubMed] [Google Scholar]
- Lillien L. Progenitor cells: What do they know and when do they know it? Curr. Biol. 1998;8:R872–R874. doi: 10.1016/s0960-9822(07)00548-9. [DOI] [PubMed] [Google Scholar]
- Lin B, Wang SW, Masland RH. Retinal ganglion cell type, size, and spacing can be specified independent of homotypic dendritic contacts. Neuron. 2004;43:475–485. doi: 10.1016/j.neuron.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Linden DC, Guillery RW, Cucchiaro J. The dorsal lateral geniculate nucleus of the normal ferret and its postnatal development. J. Comp. Neurol. 1981;203:189–211. doi: 10.1002/cne.902030204. [DOI] [PubMed] [Google Scholar]
- Linden R, Perry VH. Ganglion cell death within the developing retina: A regulatory role for retinal dendrites? Neuroscience. 1982;7:2813–2837. doi: 10.1016/0306-4522(82)90104-x. [DOI] [PubMed] [Google Scholar]
- Linden R, Reese BE. Programmed cell death. In: Sernagor E, Eglen S, Harris B, Wong R, editors. Retinal Development. Cambridge: Cambridge University Press; 2006. pp. 208–241. [Google Scholar]
- Ling T-L, Stone J. The development of astrocytes in the cat retina: evidence of migration from the optic nerve. Develop. Brain Res. 1988;44:73–85. doi: 10.1016/0165-3806(88)90119-8. [DOI] [PubMed] [Google Scholar]
- Liu H, Etter P, Hayes S, Jones I, Nelson B, Hartman B, Hartman B, Forrest D, Reh TA. NeuroD1 regulates expression of thyroid hormone receptor 2 and cone opsins in the developing mouse retina. J. Neurosci. 2008;28:749–756. doi: 10.1523/JNEUROSCI.4832-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat. Rev. Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Wong ROL. Cell-type specific dendritic contacts between retinal ganglion cells during development. J. Neurobiol. 2001;48:150–162. [PubMed] [Google Scholar]
- Lopez VM, Decatur CL, Stamer WD, Lynch RM, McKay BS. L-DOPA is an endogenous ligand for OA1. PLoS Biol. 2008;6:e236. doi: 10.1371/journal.pbio.0060236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei L, Galli-Resta L. Correlation in the discharges of neighboring rat retinal ganglion cells during prenatal life. Proc. Natl. Acad. Sci. 1990;87:2861–2864. doi: 10.1073/pnas.87.7.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggs A, Scholes J. Glial domains and nerve fiber patterns in the fish retinotectal pathway. J. Neurosci. 1986;6:424–438. doi: 10.1523/JNEUROSCI.06-02-00424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus RC, Blazeski R, Godement P, Mason CA. Retinal axon divergence in the optic chiasm: Uncrossed axons diverge from crossed axons within a midline glial specialization. J. Neurosci. 1995;15:3716–3729. doi: 10.1523/JNEUROSCI.15-05-03716.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus RC, Mason CA. The first retinal axon growth in the mouse optic chiasm: Axon patterning and the cellular environment. J. Neurosci. 1995;15:6389–6402. doi: 10.1523/JNEUROSCI.15-10-06389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus RC, Wang L-C, Mason CA. Retinal axon divergence in the optic chiasm: Midline cells are unaffected by the albino mutation. Development. 1996;122:859–868. doi: 10.1242/dev.122.3.859. [DOI] [PubMed] [Google Scholar]
- Marotte LR. Development of retinotopy in projections from the eye to the dorsal lateral geniculate nucleus and superior colliculus of the Wallaby (Macropus eugenii) J. Comp. Neurol. 1990;293:524–539. doi: 10.1002/cne.902930403. [DOI] [PubMed] [Google Scholar]
- Martins RA, Pearson RA. Control of cell proliferation by neurotransmitters in the developing vertebrate retina. Brain Res. 2008;1192:37–60. doi: 10.1016/j.brainres.2007.04.076. [DOI] [PubMed] [Google Scholar]
- Maslim J, Webster M, Stone J. Stages in the structural differentiation of retinal ganglion cells. J. Comp. Neurol. 1986;254:382–402. doi: 10.1002/cne.902540310. [DOI] [PubMed] [Google Scholar]
- Mason CA. The development of developmental neuroscience. J. Neurosci. 2009;29:12735–12747. doi: 10.1523/JNEUROSCI.4648-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Cepko CL. Controlled expression of transgenes introduced by in vivo electroporation. Proc. Nat'l. Acad. Sci. 2007;104:1027–1032. doi: 10.1073/pnas.0610155104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe KL, Gunther EC, Reh TA. The development of the pattern of retinal ganglion cells in the chick retina: mechanisms that control differentiation. Development. 1999;126:5713–5724. doi: 10.1242/dev.126.24.5713. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, O'Leary DD. Molecular gradients and development of retinotopic maps. Ann. Rev. Neurosci. 2005;28:327–355. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O'Leary DD. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- McLoon SC, Barnes RB. Early differentiation of retinal ganglion cells: An axonal protein expressed by premigratory and migrating retinal ganglion cells. J. Neurosci. 1989;9:1424–1432. doi: 10.1523/JNEUROSCI.09-04-01424.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, Saunders TL, Sieving PA, Swaroop A. Nrl is required for rod photoreceptor development. Nat. Gen. 2001;29:447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- Mehta V, Sernagor E. Early neural activity and dendritic growth in turtle retinal ganglion cells. Eur. J. Neurosci. 2006;24:773–786. doi: 10.1111/j.1460-9568.2006.04933.x. [DOI] [PubMed] [Google Scholar]
- Meister M, Wong ROL, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Millard SS, Zipursky SL. Dscam-mediated repulsion controls tiling and self-avoidance. Curr. Opin. Neurobiol. 2008;18:84–89. doi: 10.1016/j.conb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Z, Li S, Yang X, Xiang M. Role of the Barhl2 homeobox gene in the specification of glycinergic amacrine cells. Development. 2004;131:1607–1618. doi: 10.1242/dev.01071. [DOI] [PubMed] [Google Scholar]
- Montague PR, Friedlander MJ. Morphogenesis and territorial coverage by isolated mammalian retinal ganglion cells. J. Neurosci. 1991;11:1440–1457. doi: 10.1523/JNEUROSCI.11-05-01440.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R, Penn AA, Gallego R, Shatz CJ. Thalamic relay of spontaneous retinal activity prior to vision. Neuron. 1996;17:863–874. doi: 10.1016/s0896-6273(00)80218-4. [DOI] [PubMed] [Google Scholar]
- Morest DK. The pattern of neurogenesis in the retina of the rat. Z. Anat. Entwickl.-Gesch. 1970;131:45–67. doi: 10.1007/BF00518815. [DOI] [PubMed] [Google Scholar]
- Morgan JL, Dhingra A, Vardi N, Wong RO. Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar cells. Nat. Neurosci. 2006;9:85–92. doi: 10.1038/nn1615. [DOI] [PubMed] [Google Scholar]
- Mu X, Fu X, Sun H, Beremand PD, Thomas TL, Klein WH. A gene network downstream of transcription factor Math5 regulates retinal progenitor cell competence and ganglion cell fate. Develop. Biol. 2005;280:467–481. doi: 10.1016/j.ydbio.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Mu X, Fu X, Sun H, Liang S, Maeda H, Frishman LJ, Klein WH. Ganglion cells are required for normal progenitor- cell proliferation but not cell-fate determination or patterning in the developing mouse retina. Curr. Biol. 2005;15:525–530. doi: 10.1016/j.cub.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Mu X, Klein WH. Gene regulatory networks and retinal ganglion cell development. In: Chlupa LM, Williams RW, editors. Eye, Retina, and Visual System of the Mouse. Cambridge: Massachusetts Institute of Technology; 2008. pp. 321–332. [Google Scholar]
- Muir-Robinson G, Hwang BJ, Feller MB. Retinogeniculate axons undergo eye-specific segregation in the absence of eye-specific layers. J. Neurosci. 2002;22:5259–5264. doi: 10.1523/JNEUROSCI.22-13-05259.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm J, Lohmann C. Dendritic growth. In: Sernagor E, Eglen S, Harris B, Wong R, editors. Retinal Development. Cambridge: Cambridge University Press; 2006. pp. 242–264. [Google Scholar]
- Muto A, Iida A, Satoh S, Watanabe S. The group E Sox genes Sox8 and Sox9 are regulated by Notch signaling and are required for Müller glial cell development in mouse retina. Exp. Eye Res. 2009;89:549–558. doi: 10.1016/j.exer.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Brennan C, Johnson KG, Shewan D, Harris WA, Holt CE. Ephrin-B regulates the Ipsilateral routing of retinal axons at the optic chiasm. Neuron. 2000;25:599–610. doi: 10.1016/s0896-6273(00)81063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BR, Gumuscu B, Hartman BH, Reh TA. Notch activity is downregulated just prior to retinal ganglion cell differentiation. Develop. Neurosci. 2006;28:128–141. doi: 10.1159/000090759. [DOI] [PubMed] [Google Scholar]
- Ng L, Hurley JB, Dierks B, Srinivas M, Saltó C, Vennström B, Reh TA, Forrest D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat. Genet. 2001;27:94–98. doi: 10.1038/83829. [DOI] [PubMed] [Google Scholar]
- Nishida A, Furukawa A, Koike C, Tano Y, Aizawa S, Matsuo I, Furukawa T. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat. Neurosci. 2003;6:1255–1263. doi: 10.1038/nn1155. [DOI] [PubMed] [Google Scholar]
- Norden C, Young S, Link BA, Harris WA. Actomyosin is the main driver of interkinetic nuclear migration in the retina. Cell. 2009;138:1195–1208. doi: 10.1016/j.cell.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary DDM, Fawcett JW, Cowan WM. Topographic targeting errors in the retinocollicular projection and their elimination by selective ganglion cell death. J. Neurosci. 1986;6:3692–3705. doi: 10.1523/JNEUROSCI.06-12-03692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh ECT, Khan N, Novelli E, Khanna H, Strettoi E, Swaroop A. Transformation of cone precursors to functional rod photoreceptors by bZIP transcription factor NRL. Proc. Nat'l. Acad. Sci. 2007;104:1679–1684. doi: 10.1073/pnas.0605934104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma S, Philpott A, Wang K, Holt CE, Harris WA. p27Xic1, a Cdk inhibitor, promotes the determination of glial cells in Xenopus retina. Cell. 1999;99:499–510. doi: 10.1016/s0092-8674(00)81538-x. [DOI] [PubMed] [Google Scholar]
- Ohsawa R, Kageyama R. Regulation of retinal cell fate specification by multiple transcription factors. Brain Res. 2008;1192:90–98. doi: 10.1016/j.brainres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Ott H, Bastmeyer M, Stuermer CA. Neurolin, the goldfish homolog of DM-GRASP, is involved in retinal axon pathfinding to the optic disk. J. Neurosci. 1998;18:3363–3372. doi: 10.1523/JNEUROSCI.18-09-03363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Deng M, Xie X, Gan L. ISL1 and BRN3B co-regulate the differentiation of murine retinal ganglion cells. Development. 2008;135:1981–1990. doi: 10.1242/dev.010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science. 1998;279:2108–2112. doi: 10.1126/science.279.5359.2108. [DOI] [PubMed] [Google Scholar]
- Perry VH, Linden R. Evidence for dendritic competition in the developing retina. Nature. 1982;297:683–685. doi: 10.1038/297683a0. [DOI] [PubMed] [Google Scholar]
- Perry VH, Maffei L. Dendritic competition: competition for what? Develop. Brain Res. 1988;41:195–208. doi: 10.1016/0165-3806(88)90182-4. [DOI] [PubMed] [Google Scholar]
- Petros TJ, Mason CA. Early development of the optic stalk, chiasm, and astrocytes. In: Chalupa LM, Williams RW, editors. Eye, Retina, and Visual System of the Mouse. Cambridge: MIT Press; 2008. pp. 389–400. [Google Scholar]
- Petros TJ, Shrestha BR, Mason C. Specificity and sufficiency of EphB1 in driving the ipsilateral retinal projection. J. Neurosci. 2009;29:3463–3474. doi: 10.1523/JNEUROSCI.5655-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffenberger C, Cutforth T, Woods G, Yamada J, Rentería RC, Copenhagen DR, Flanagan JG, Feldheim DA. Ephrin-As and neural activity are required for eye-specific patterning during retinogeniculate mapping. Nat. Neurosci. 2005;8:1022–1027. doi: 10.1038/nn1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffenberger C, Yamada J, Feldheim DA. Ephrin-As and patterned retinal activity act together in the development of topographic maps in the primary visual system. J. Neurosci. 2006;26:12873–12884. doi: 10.1523/JNEUROSCI.3595-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas DT, Lopez JE, Crair MC. Pretarget sorting of retinocollicular axons in the mouse. J. Comp. Neurol. 2005;491:305–319. doi: 10.1002/cne.20694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plump AS, Erskine L, Sabatier C, Brose K, Epstein CJ, Goodman CS, Mason CA, Tessier-Lavigne M. Slit1 and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron. 2002;33:219–232. doi: 10.1016/s0896-6273(01)00586-4. [DOI] [PubMed] [Google Scholar]
- Poché RA, Furuta Y, Chaboissier MC, Schedl A, Behringer RR. Sox9 is expressed in mouse multipotent retinal progenitor cells and functions in Müller glial cell development. J. Comp. Neurol. 2008;510:237–250. doi: 10.1002/cne.21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poché RA, Kwan KM, Raven MA, Furuta Y, Reese BE, Behringer RR. Lim1 is essential for the correct laminar positioning of retinal horizontal cells. J. Neurosci. 2007;27:14099–14107. doi: 10.1523/JNEUROSCI.4046-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poché RA, Raven MA, Kwan KM, Furuta Y, Behringer RR, Reese BE. Somal positioning and dendritic growth of horizontal cells are regulated by interactions with homotypic neighbors. Europ. J. Neurosci. 2008;27:1607–1614. doi: 10.1111/j.1460-9568.2008.06132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poché RA, Reese BE. Retinal horizontal cells: challenging paradigms of neural development and cancer biology. Development. 2009;136:2141–2151. doi: 10.1242/dev.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi L, Vitorino M, Masai I, Harris WA. Influences on neural lineage and mode of division in the zebrafish retina in vivo. J. Cell Biol. 2005;171:991–999. doi: 10.1083/jcb.200509098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pow DV, Hendrickson AE. Expression of glycine and the glycine transporter Glyt-1 in the developing rat retina. Vis. Neurosci. 2000;17:1–9. doi: 10.1017/s0952523800171019. [DOI] [PubMed] [Google Scholar]
- Prada C, Puelles L, Genis-Gálvez JM, Ramirez G. Two modes of free migration of amacrine cell neuroblasts in the chick retina. Anat. Embryol. 1987;175:281–287. doi: 10.1007/BF00309842. [DOI] [PubMed] [Google Scholar]
- Qiu F, Jiang H, Xiang M. A comprehensive negative regulatory program controlled by Brn3b to ensure ganglion cell specification from multipotential retinal precursors. J. Neurosci. 2008;28:3392–3403. doi: 10.1523/JNEUROSCI.0043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachel RA, Mason CA, Beermann F. Influence of tyrosinase levels on pigment accumulation in the retinal pigment epithelium and on the uncrossed retinal projection. Pigment Cell Res. 2002;15:273–281. doi: 10.1034/j.1600-0749.2002.02019.x. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976;261:467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- Rakic P. Development of visual centers in the primate brain depends on binocular competition before birth. Science. 1981;214:928–931. doi: 10.1126/science.7302569. [DOI] [PubMed] [Google Scholar]
- Ramoa AS, Campbell G, Shatz CJ. Transient morphological features of identified ganglion cells in living fetal and neonatal retina. Science. 1987;237:522–525. doi: 10.1126/science.3603038. [DOI] [PubMed] [Google Scholar]
- Ramoa AS, Campbell G, Shatz CJ. Dendritic growth and remodeling of cat retinal ganglion cells during fetal and postnatal development. J. Neurosci. 1988;8:4239–4261. doi: 10.1523/JNEUROSCI.08-11-04239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport DH. Retinal neurogenesis. In: Sernagor E, Eglen S, Harris B, Wong R, editors. Retinal Development. Cambridge: Cambridge University Press; 2006. pp. 30–58. [Google Scholar]
- Rapaport DH, Fletcher JT, LaVail MM, Rakic P. Genesis of neurons in the retinal ganglion cell layer of the monkey. J. Comp. Neurol. 1992;322:577–588. doi: 10.1002/cne.903220411. [DOI] [PubMed] [Google Scholar]
- Rapaport DW, Wong LL, Wood ED, Yasumura D, LaVail MM. Timing and topography of cell genesis in the rat retina. J. Comp. Neurol. 2004;474:304–324. doi: 10.1002/cne.20134. [DOI] [PubMed] [Google Scholar]
- Rashid T, Upton AL, Blentic A, Ciossek T, Knöll B, Thompson ID, Drescher U. Opposing gradients of ephrin-As and EphA7 in the superior colliculus are essential for topographic mapping in the mammalian visual system. Neuron. 2005;47:57–69. doi: 10.1016/j.neuron.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Raven MA, Eglen SJ, Ohab JJ, Reese BE. Determinants of the exclusion zone in dopaminergic amacrine cell mosaics. J. Comp. Neurol. 2003;461:123–136. doi: 10.1002/cne.10693. [DOI] [PubMed] [Google Scholar]
- Raven MA, Oh ECT, Swaroop A, Reese BE. Afferent control of horizontal cell morphology revealed by genetic re-specification of rods and cones. J. Neurosci. 2007;27:3540–3547. doi: 10.1523/JNEUROSCI.0372-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven MA, Orton NC, Nassar H, Williams GA, Stell WK, Jacobs GH, Bech-Hensen T, Reese BE. Early afferent signaling in the outer plexiform layer regulates development of horizontal cell morphology. J. Comp. Neurol. 2008;506:745–758. doi: 10.1002/cne.21526. [DOI] [PubMed] [Google Scholar]
- Raven MA, Stagg SB, Nassar H, Reese BE. Developmental improvement in the regularity and packing of mouse horizontal cells: Implications for mechanisms underlying mosaic pattern formation. Vis. Neurosci. 2005b;22:569–573. doi: 10.1017/S095252380522504X. [DOI] [PubMed] [Google Scholar]
- Ready DF, Hanson TE, Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Develop. Biol. 1976;53:217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- Rebsam A, Petros TJ, Mason CA. Switching retinogeniculate axon laterality leads to normal targeting but abnormal eye-specific segregation that is activity dependent. J. Neurosci. 2009;29:14855–14863. doi: 10.1523/JNEUROSCI.3462-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese BE. The chronotopic reordering of optic axons. Pers. Develop. Neurobiol. 1994;3:233–242. [PubMed] [Google Scholar]
- Reese BE. Mosaics, tiling and coverage by retinal neurons. In: Masland RH, Albright T, editors. Vision. Ch. 22. Vol. 1. Oxford: Elsevier; 2008a. pp. 439–456. [Google Scholar]
- Reese BE. Mosaic architecture of the mouse retina. In: Chalupa LM, Williams RW, editors. Eye, Retina, and Visual Systems of the Mouse. Ch. 11. Vol. Cambridge: MIT Press; 2008b. pp. 147–155. [Google Scholar]
- Reese BE, Baker GE. The course of fibre diameter classes through the chiasmatic region in the ferret. Eur. J. Neurosci. 1990;2:34–49. doi: 10.1111/j.1460-9568.1990.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Reese BE, Baker GE. Changes in fiber organization within the chiasmatic region in mammals. Vis. Neurosci. 1992;9:527–533. doi: 10.1017/s0952523800001772. [DOI] [PubMed] [Google Scholar]
- Reese BE, Baker GE. The re-establishment of the representation of the dorso-ventral retinal axis in the chiasmatic region of the ferret. Vis. Neurosci. 1993;10:957–968. doi: 10.1017/s0952523800006179. [DOI] [PubMed] [Google Scholar]
- Reese BE, Colello RJ. Neurogenesis in the retinal ganglion cell layer of the rat. Neuroscience. 1992;46:419–429. doi: 10.1016/0306-4522(92)90062-7. [DOI] [PubMed] [Google Scholar]
- Reese BE, Guillery RW, Mallarino C. Time of ganglion cell genesis in relation to the chiasmatic pathway choice of retinofugal axons. J. Comp. Neurol. 1992b;324:336–342. doi: 10.1002/cne.903240304. [DOI] [PubMed] [Google Scholar]
- Reese BE, Guillery RW, Marzi CA, Tassinari G. Position of axons in the cat's optic tract in relation to their retinal origin and chiasmatic pathway. J. Comp. Neurol. 1991;306:539–553. doi: 10.1002/cne.903060402. [DOI] [PubMed] [Google Scholar]
- Reese BE, Harvey AR, Tan S-S. Radial and tangential dispersion patterns in the mouse retina are cell-class specific. Proc. Natl. Acad. Sci. 1995;92:2494–2498. doi: 10.1073/pnas.92.7.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese BE, Necessary BD, Tam PPL, Faulkner-Jones B, Tan S-S. Clonal expansion and cell dispersion in the developing mouse retina. Eur. J. Neurosci. 1999;11:2965–2978. doi: 10.1046/j.1460-9568.1999.00712.x. [DOI] [PubMed] [Google Scholar]
- Reese BE, Raven MA, Giannotti KA, Johnson PT. Development of cholinergic amacrine cell stratification in the ferret retina and the effects of early excitotoxic ablation. Vis. Neurosci. 2001;18:559–570. doi: 10.1017/s0952523801184063. [DOI] [PubMed] [Google Scholar]
- Reese BE, Raven MA, Stagg SB. Afferents and homotypic neighbors regulate horizontal cell morphology, connectivity and retinal coverage. J. Neurosci. 2005;25:2167–2175. doi: 10.1523/JNEUROSCI.4876-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese BE, Raven MA, Whitney IE, Williams RW, Elshatory Y, Gan L. A QTL on chromosome 13 contributes to the two-fold variation in horizontal cell number in the mouse retina. ARVO Abs. 2008;49:S785. [Google Scholar]
- Reese BE, Thompson WF, Peduzzi JD. Birthdates of neurons in the retinal ganglion cell layer of the ferret. J. Comp. Neurol. 1994;341:464–475. doi: 10.1002/cne.903410404. [DOI] [PubMed] [Google Scholar]
- Reh TA. Determination of cell fate during retinal histogenesis: Intrinsic and extrinsic mechanisms. In: Lam DM-K, Shatz CJ, editors. Development of the Visual System. Cambridge: MIT Press; 1991. pp. 79–94. [Google Scholar]
- Reh TA, Tully T. Regulation of tyrosine hydroxylase-containing amacrine cell number in larval frog retina. Develop. Biol. 1986;114:463–469. doi: 10.1016/0012-1606(86)90210-1. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Germer A, Bringmann A, Biedermann B, Pannicke T, Francke M, Kuhrt H, Reichelt W, Mack A. Glio-neuronal interactions in retinal development. In: Chalupa L, Finlay B, editors. Development and Organization of the Retina. New York: Plenum Press; 1998. pp. 121–146. [Google Scholar]
- Reichenbach A, Robinson SR. Phylogenetic constraints on retinal organization and development. Prog. Ret. Eye Res. 1995;15:139–171. [Google Scholar]
- Resta V, Novelli E, Di Virgilio F, Galli-Resta L. Neuronal death induced by endogenous extracellular ATP in retinal cholinergic neuron density control. Development. 2005;132:2873–2882. doi: 10.1242/dev.01855. [DOI] [PubMed] [Google Scholar]
- Rich KA, Zhan Y, Blanks JC. Migration and synaptogenesis of cone photoreceptors in the developing mouse retina. J. Comp. Neurol. 1997;388:47–63. [PubMed] [Google Scholar]
- Rice DS, Goldowitz D, Williams RW, Hamre K, Johnson PT, Tan S-S, Reese BE. Extrinsic modulation of retinal ganglion cell projections: analysis of the albino mutation in pigmentation mosaic mice. Develop. Biol. 1999;216:41–56. doi: 10.1006/dbio.1999.9467. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Srinivas M, Forrest D, Morreale de Escobar G, Reh TA. Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc. Natl. Acad. Sci. 2006;103:6218–6223. doi: 10.1073/pnas.0509981103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompani SB, Cepko CL. Retinal progenitor cells can produce restricted subsets of horizontal cells. Proc. Nat'l. Acad. Sci. 2008;105:192–197. doi: 10.1073/pnas.0709979104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi FM, Pizzorusso T, Porciatti V, Marubio LM, Maffei L, Changeux JP. Requirement of the nicotinic acetylcholine receptor beta 2 subunit for the anatomical and functional development of the visual system. Proc. Nat'l. Acad. Sci. U S A. 2001;98:6453–6458. doi: 10.1073/pnas.101120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson KJ, Guillery RW, Shackelford RM. Congenitally abnormal visual pathways in mink (Mustela vison) with reduced retinal pigment. J. Comp. Neurol. 1974;154:225–248. doi: 10.1002/cne.901540302. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Reh TA, Harris WA. Development of the Nervous System. 2nd edition ed. Burlington: Academic Press; 2006. [Google Scholar]
- Schiaffino MV, Baschirotto C, Pellegrini G, Montalti S, Tacchetti C, De Luca M, Ballabio A. The ocular albinism type 1 gene product is a membrane glycoprotein localized to melanosomes. Proc. Natl. Acad. Sci. U S A. 1996;93:9055–9060. doi: 10.1073/pnas.93.17.9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino MV, d'Addio M, Alloni A, Baschirotto C, Valetti C, Cortese K, Puri C, Bassi MT, Colla C, DeLuca M, Tacchetti C, Ballabio A. Ocular albinism: evidence for a defect in an intracellular signal transduction system. Nature Genet. 1999;23:108–112. doi: 10.1038/12715. [DOI] [PubMed] [Google Scholar]
- Schiaffino MV, Tacchetti C. The ocular albinism type 1 (OA1) protein and the evidence for an intracellular signal transduction system involved in melanosome biogenesis. Pigment Cell Res. 2005;18:227–233. doi: 10.1111/j.1600-0749.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- Schnitzer J, Rusoff AC. Horizontal cells of the mouse retina contain glutamic acid decarboxylase-like immunoreactivity during early developmental stages. J. Neurosci. 1984;4:2948–2955. doi: 10.1523/JNEUROSCI.04-12-02948.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scicolone G, Ortalli AL, Carri NG. Key roles of Ephs and ephrins in retinotectal topographic map formation. Brain Res. Bull. 2009;79:227–247. doi: 10.1016/j.brainresbull.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Shatz CJ. The prenatal development of the cat's retinogeniculate pathway. J. Neurosci. 1983;3:482–499. doi: 10.1523/JNEUROSCI.03-03-00482.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, Kirkwood PA. Prenatal development of functional connections in the cat's retino-geniculate pathway. J. Neurosci. 1984;4:1378–1397. doi: 10.1523/JNEUROSCI.04-05-01378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman RL. Histogenesis of mouse retina studied with thymidine-H3. In: Smelser G, editor. The Structure of the Eye. New York: Academic Press; 1961. pp. 487–505. [Google Scholar]
- Simon DK, O'Leary DDM. Relationship of retinotopic ordering of axons in the optic pathway to the formation of visual maps in central targets. J. Comp. Neurol. 1991;307:393–404. doi: 10.1002/cne.903070305. [DOI] [PubMed] [Google Scholar]
- Singer JB, Hill AE, Burrage LC, OOlszens KR, Song J, Justice M, O'Brien WE, Conti DV, Witte JS, Lander ES, Nadeau JH. Genetic dissection of complex traits with chromosome substitution strains of mice. Science. 2004;304:445–448. doi: 10.1126/science.1093139. [DOI] [PubMed] [Google Scholar]
- Snow RL, Robson JA. Ganglion cell neurogenesis, migration and early differentiation in the chick retina. Neuroscience. 1994;58:399–409. doi: 10.1016/0306-4522(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc. Nat'l. Acad. Sci. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira A, Hudy S, Hannah R. Ectopic photoreceptor cells and cell death in the developing rat retina. Anat. Embryol. 1984;169:293–301. doi: 10.1007/BF00315634. [DOI] [PubMed] [Google Scholar]
- Sretavan DW. Specific routing of retinal ganglion cell axons at the mammalian optic chiasm during embryonic development. J. Neurosci. 1990;10:1995–2007. doi: 10.1523/JNEUROSCI.10-06-01995.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sretavan DW. Optic nerve formation. In: Sernagor E, Eglen S, Harris B, Wong R, editors. Retinal Development. Cambridge: Cambridge University Press; 2006. pp. 150–171. [Google Scholar]
- Sretavan DW, Shatz CJ. Prenatal development of individual retinogeniculate axons during the period of segregation. Nature. 1984;308:845–848. doi: 10.1038/308845a0. [DOI] [PubMed] [Google Scholar]
- Sretavan DW, Shatz CJ. Prenatal development of cat retinogeniculate axon arbors in the absence of binocular interactions. J. Neurosci. 1986;6:990–1003. doi: 10.1523/JNEUROSCI.06-04-00990.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sretavan DW, Shatz CJ, Stryker MP. Modification of retinal ganglion cell axon morphology by prenatal infusion of tetrodotoxin. Nature. 1988;336:468–471. doi: 10.1038/336468a0. [DOI] [PubMed] [Google Scholar]
- Stacy RC, Wong ROL. Developmental relationship between cholinergic amacrine cell processes and ganglion cell dendrites of the mouse retina. J. Comp. Neurol. 2003;456:154–166. doi: 10.1002/cne.10509. [DOI] [PubMed] [Google Scholar]
- Stafford BK, Sher A, Litke AM, Feldheim DA. Spatial-temporal patterns of retinal waves underlying activity-dependent refinement of retinofugal projections. Neuron. 2009;64:200–212. doi: 10.1016/j.neuron.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron. 2002;33:357–367. doi: 10.1016/s0896-6273(02)00577-9. [DOI] [PubMed] [Google Scholar]
- Stone J, Dreher Z. Relationship between astrocytes, ganglion cells and vasculature of retina. J. Comp. Neurol. 1987;255:35–49. doi: 10.1002/cne.902550104. [DOI] [PubMed] [Google Scholar]
- Sugimura K, Shimono K, Uemura T, Mochizuki A. Self-organizing mechanism for development of space-filling neuronal dendrites. PLoS Comput. Biol. 2007;3(e212) doi: 10.1371/journal.pcbi.0030212. doi: 10.1371/journal.pcbi.0030212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Warland DK, Ballesteros JM, van der List D, Chalupa LM. Retinal waves in mice lacking the beta2 subunit of the nicotinic acetylcholine receptor. Proc. Nat’l. Acad. Sci. USA. 2008;105:13638–13643. doi: 10.1073/pnas.0807178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed MM, Lee S, Zheng J, Zhou ZJ. Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina. J Physiol. 2004;560:533–549. doi: 10.1113/jphysiol.2004.066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teller DY, Movshon JA. Visual development. Vision Res. 1986;25:1483–1506. doi: 10.1016/0042-6989(86)90169-0. [DOI] [PubMed] [Google Scholar]
- Tian N. Synaptic activity, visual experience and the maturation of retinal synaptic circuitry. J Physiol. 2008;586:4347–4355. doi: 10.1113/jphysiol.2008.159202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. Visual deprivation alters development of synaptic function in inner retina after eye opening. Neuron. 2001;32:439–449. doi: 10.1016/s0896-6273(01)00470-6. [DOI] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003;39:85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- Tomita K, Moriyoshi K, Nakanishi S, Guillemot F, Kageyama R. Mammalian achaete–scute and atonal homologs regulate neuronal versus glial fate determination in the central nervous system. EMBO J. 2000;19:5460–5472. doi: 10.1093/emboj/19.20.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog. Neurobiol. 2005;76:213–235. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Torborg CL, Hansen KA, Feller MB. High frequency, synchronized bursting drives eye-specific segregation of retinogeniculate projections. Nat. Neurosci. 2005;8:72–78. doi: 10.1038/nn1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi JM, Stadler MB, Cepko CL. Individual retinal progenitor cells display extensive heterogeneity of gene expression. PLoS One. 2008;3:e1588. doi: 10.1371/journal.pone.0001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi JM, Stadler MB, Roska B, Billings N, Sun B, Bartch BC, Cepko CL. Molecular heterogeneity of developing retinal ganglion and amacrine cells revealed through single cell gene expression profiling. J. Comp. Neurol. 2007;502:1047–1065. doi: 10.1002/cne.21368. [DOI] [PubMed] [Google Scholar]
- Triplett JW, Owens MT, Yamada J, Lemke G, Cang J, Stryker MP, Feldheim DA. Retinal input instructs alignment of visual topographic maps. Cell. 2009;139:175–185. doi: 10.1016/j.cell.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Xiong M, Crowley JC, Finlay BL. Factors controlling the dendritic arborization of retinal ganglion cells. Vis. Neurosci. 1996;13:721–733. doi: 10.1017/s0952523800008609. [DOI] [PubMed] [Google Scholar]
- Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Turner DL, Snyder EY, Cepko CL. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron. 1990;4:833–845. doi: 10.1016/0896-6273(90)90136-4. [DOI] [PubMed] [Google Scholar]
- Tyler MJ, Carney LH, Cameron DA. Control of cellular pattern formation in the vertebrate inner retina by homotypic regulation of cell-fate decisions. J. Neurosci. 2005;25:4565–4576. doi: 10.1523/JNEUROSCI.0588-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitorino M, Jusuf PR, Maurus D, Kimura Y, Higashijima S, Harris WA. Vsx2 in the zebrafish retina: restricted lineages through derepression. Neural Dev. 2009;4:14. doi: 10.1186/1749-8104-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waid DK, McLoon SC. Ganglion cells influence the fate of dividing retinal cells in culture. Development. 1998;125:1059–1066. doi: 10.1242/dev.125.6.1059. [DOI] [PubMed] [Google Scholar]
- Walsh C, Polley EH. The topography of ganglion cell production in the cat's retina. J. Neurosci. 1985;5:741–750. doi: 10.1523/JNEUROSCI.05-03-00741.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C, Polley EH, Hickey TL, Guillery RW. Generation of cat retinal ganglion cells in relation to central pathways. Nature. 1983;302:611–614. doi: 10.1038/302611a0. [DOI] [PubMed] [Google Scholar]
- Walter J, Henke-Fahle S, Bonhoeffer F. Avoidance of posterior tectal membranes by temporal retinal axons. Development. 1987b;101:909–913. doi: 10.1242/dev.101.4.909. [DOI] [PubMed] [Google Scholar]
- Walter J, Kern-Veits B, Huf J, Stolze B, Bonhoeffer F. Recognition of position-specific properties of tectal cell membranes by retinal axons in vitro. Development. 1987a;101:685–696. doi: 10.1242/dev.101.4.685. [DOI] [PubMed] [Google Scholar]
- Wang GY, Liets LC, Chalupa LM. Unique functional properties of on and off pathways in the developing mammalian retina. J. Neurosci. 2001;15:4310–4317. doi: 10.1523/JNEUROSCI.21-12-04310.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-C, Dani J, Godement P, Marcus RC, Mason CA. Crossed and uncrossed retinal axons respond differently to cells of the optic chiasm midline in vitro. Neuron. 1995;15:1349–1364. doi: 10.1016/0896-6273(95)90013-6. [DOI] [PubMed] [Google Scholar]
- Wässle H, Peichl L, Boycott BB. Topography of horizontal cells in the retina of the domestic cat. Proc. R. Soc. B (Lond.) 1978;203:269–291. doi: 10.1098/rspb.1978.0105. [DOI] [PubMed] [Google Scholar]
- Wässle H, Puller C, Müller F, Haverkamp S. Cone contacts, mosaics and territories of bipolar cells in the mouse retina. J. Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Riemann HJ. The mosaic of nerve cells in the mammalian retina. Proc. R. Soc. Lond., B. 1978;200:441–461. doi: 10.1098/rspb.1978.0026. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Raff MC. Retinal astrocytes are immigrants from the optic nerve. Nature. 1988;332:834–837. doi: 10.1038/332834a0. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Raff MC. Rod photoreceptor development in vitro: Intrinsic properties of proliferating neuroepithelial cells change as development proceeds in the rat retina. Neuron. 1990;2:461–467. doi: 10.1016/0896-6273(90)90058-n. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Raff MC. Diffusible rod-promoting signals in the developing rat retina. Development. 1992;114:899–906. doi: 10.1242/dev.114.4.899. [DOI] [PubMed] [Google Scholar]
- Wetts R, Fraser SE. Multipotent precursors can give rise to all major cell types of the frog retina. Science. 1988;239:1142–1145. doi: 10.1126/science.2449732. [DOI] [PubMed] [Google Scholar]
- Whitney IE, Raven MA, Ciobanu DC, Williams RW, Reese BE. Multiple genes on chromosome 7 regulate dopaminergic amacrine cell number in the mouse retina. Invest. Ophthalmol. Vis. Sci. 2009;50:1996–2003. doi: 10.1167/iovs.08-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney IE, Raven MA, Keeley PW, Reese BE. Spatial patterning of cholinergic amacrine cells in the mouse retina. J. Comp. Neurol. 2008;508:1–12. doi: 10.1002/cne.21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney IE, Raven MA, Reese BE. Variation in cholinergic amacrine cell number in the INL and GCL of mouse retina. Soc Neurosci. Abs. 2007;68:10. [Google Scholar]
- Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cats lateral geniculate body. J. Neurophysiol. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Willardsen MI, Suli A, Pan Y, Marsh-Armstrong N, Chien CB, El-Hodiri H, Brown NL, Moore KB, Vetter ML. Temporal regulation of Ath5 gene expression during eye development. Develop. Biol. 2009;326:471–481. doi: 10.1016/j.ydbio.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RR, Cusato K, Raven M, Reese BE. Organization of the inner retina following early elimination of the retinal ganglion cell population: Effects on cell numbers and stratification patterns. Vis. Neurosci. 2001;18:233–244. doi: 10.1017/s0952523801182088. [DOI] [PubMed] [Google Scholar]
- Williams RW, Borodkin M, Rakic P. Growth cone distribution patterns in the optic nerve of fetal monkeys: Implications for mechanisms of axon guidance. J. Comp. Neurol. 1991;11:1081–1094. doi: 10.1523/JNEUROSCI.11-04-01081.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RW, Goldowitz D. Lineage versus environment in embryonic retina: A revisionist perspective. TINS. 1992a;15:368–373. doi: 10.1016/0166-2236(92)90181-7. [DOI] [PubMed] [Google Scholar]
- Williams RW, Goldowitz D. Structure of clonal and polyclonal cell arrays in chimeric mouse retina. Proc. Natl. Acad. Sci. USA. 1992b;89:1184–1188. doi: 10.1073/pnas.89.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RW, Gu J, Qi S, Lu L. The genetic structure of recombinant inbred mice: high-resolution consensus maps for complex trait analysis. Gen. Biol. 2001;2 doi: 10.1186/gb-2001-2-11-research0046. research0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RW, Hogan D, Garraghty PE. Target recognition and visual maps in the thalamus of achiasmatic dogs. Nature. 1994;367:637–639. doi: 10.1038/367637a0. [DOI] [PubMed] [Google Scholar]
- Williams RW, Rakic P. Dispersion of growing axons within the optic nerve of the embryonic monkey. Proc. Natl. Acad. Sci. 1985;82:3906–3910. doi: 10.1073/pnas.82.11.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RW, Strom RC, Goldowitz D. Natural variation in neuron number in mice is linked to a major quantitative trait locus on Chr 11. J. Neurosci. 1998;18:138–146. doi: 10.1523/JNEUROSCI.18-01-00138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RW, Strom RC, Rice DS, Goldowitz D. Genetic and environmental control of variation in retinal ganglion cell number in mice. J. Neurosci. 1996;16:7193–7205. doi: 10.1523/JNEUROSCI.16-22-07193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RW, Strom RC, Zhou G, Yan Z. Genetic dissection of retinal development. Sem. Cell Develop. Biol. 1998;9:249–255. doi: 10.1006/scdb.1998.0236. [DOI] [PubMed] [Google Scholar]
- Williams SE, Mann F, Erskine L, Sakurai T, Wei S, Rossi DJ, Gale NW, Holt CE, Mason CA, Henkemeyer M. Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron. 2003;39:919–935. doi: 10.1016/j.neuron.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Wong LL, Rapaport DH. Defining retinal progenitor cell competence in Xenopus laevis by clonal analysis. Development. 2009;136:1707–1715. doi: 10.1242/dev.027607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ROL, Chernjavsky A, Smith SJ, Shatz CJ. Early functional neural networks in the developing retina. Nature. 1995;374:716–718. doi: 10.1038/374716a0. [DOI] [PubMed] [Google Scholar]
- Wong ROL, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat. Rev. Neurosci. 2002;3:803–812. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- Wong ROL, Meister M, Shatz CJ. Transient period of correlated bursting activity during development of the mammalian retina. Neuron. 1993;11:923–938. doi: 10.1016/0896-6273(93)90122-8. [DOI] [PubMed] [Google Scholar]
- Wong WT, Myhr KL, Miller ED, Wong ROL. Developmental changes in the neurotransmitter regulation of correlated spontaneous retinal activity. J. Neurosci. 2000;20:1–10. doi: 10.1523/JNEUROSCI.20-01-00351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WT, Wong RO. Rapid dendritic movements during synapse formation and rearrangement. Curr, Opin, Neurobiol. 2000;10:118–124. doi: 10.1016/s0959-4388(99)00059-8. [DOI] [PubMed] [Google Scholar]
- Wong WT, Wong RO. Changing specificity of neurotransmitter regulation of rapid dendritic re-modeling during synaptogenesis. Nat. Neurosci. 2001;4:351–352. doi: 10.1038/85987. [DOI] [PubMed] [Google Scholar]
- Xiao T, Roeser T, Staub W, Baier H. A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection. Development. 2005;132:2955–2967. doi: 10.1242/dev.01861. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- Yang Z, Ding K, Pan L, Deng M, Gan L. Math5 determines the competence state of retinal ganglion cell progenitors. Develop. Biol. 2003;264:240–254. doi: 10.1016/j.ydbio.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Yaron O, Farhy C, Marquardt T, Applebury M, Ashery-Padan R. Notch1 functions to suppress cone-photoreceptor fate specification in the developing mouse retina. Development. 2006;133:1367–1378. doi: 10.1242/dev.02311. [DOI] [PubMed] [Google Scholar]
- Yates PA, Roskies AL, McLaughlin T, O’Leary DD. Topographic-specific axon branching controlled by ephrin-as is the critical event in retinotectal map development. J. Neurosci. 2001;21:8548–8563. doi: 10.1523/JNEUROSCI.21-21-08548.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Mears AJ, Friedman JS, Carter T, He S, Oh E, Jing Y, Farjo R, Fleury G, Barlow C, Hero AO, Swaroop A. Expression profiling of the developing and mature Nrl−/− mouse retina: identification of retinal disease candidates and transcriptional regulatory targets of Nrl. Hum. Mol. Genet. 2004;13:1487–1503. doi: 10.1093/hmg/ddh160. [DOI] [PubMed] [Google Scholar]
- Young A, Powelson EB, Whitney IE, Raven MA, Nusinowitz S, Jiang M, Birnbaumer L, Reese BE, Farber DB. Involvement of OA1, an intracellular GPCR, and G alpha i3, its binding protein, in melanosomal biogenesis and optic pathway formation. Invest. Ophthalmol. Vis. Sci. 2008;49:3245–3252. doi: 10.1167/iovs.08-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. Cell proliferation during postnatal development of the retina in the mouse. Develop. Brain Res. 1985;21:229–239. doi: 10.1016/0165-3806(85)90211-1. [DOI] [PubMed] [Google Scholar]
- Zhou ZJ, Lee S. Synaptic physiology of direction selectivity in the retina. J. Physiol. 2008;586:4371–4376. doi: 10.1113/jphysiol.2008.159020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber ME, Harris WA. Formation of the eye field. In: Sernagor E, Eglen S, Harris B, Wong R, editors. Retinal Development. Cambridge: Cambridge University Press; 2006. pp. 8–29. [Google Scholar]