Abstract
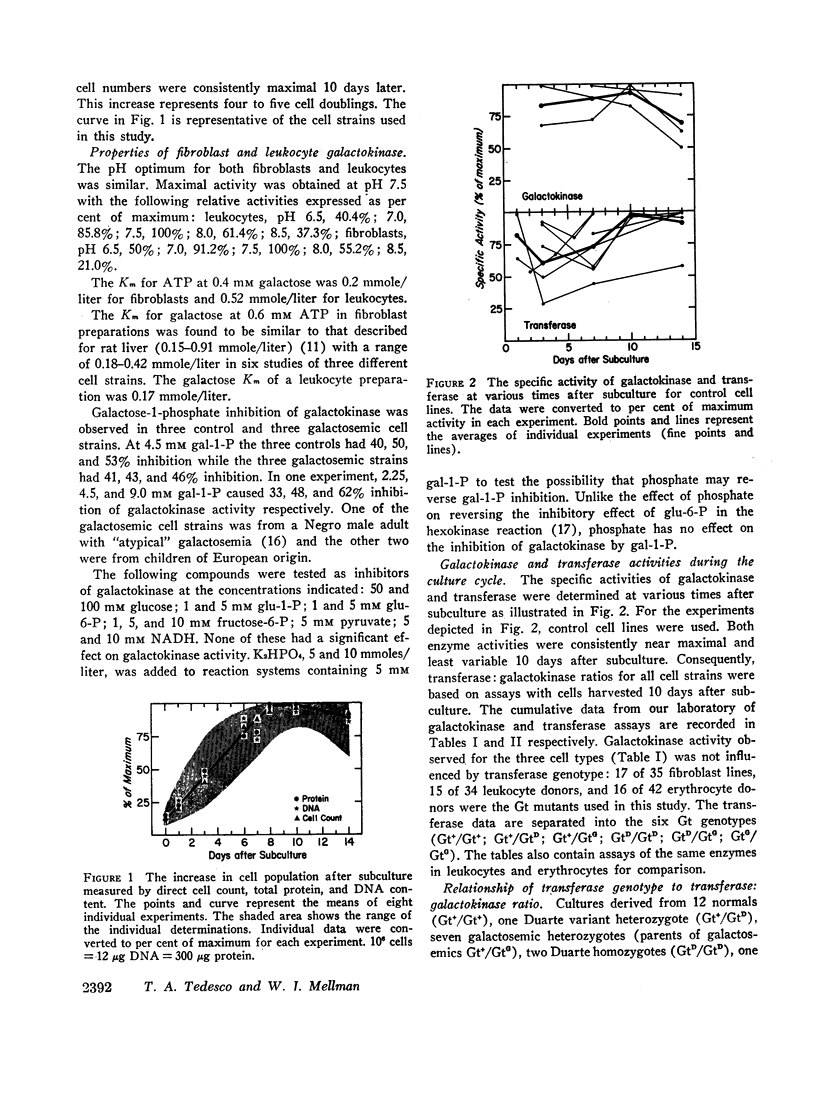
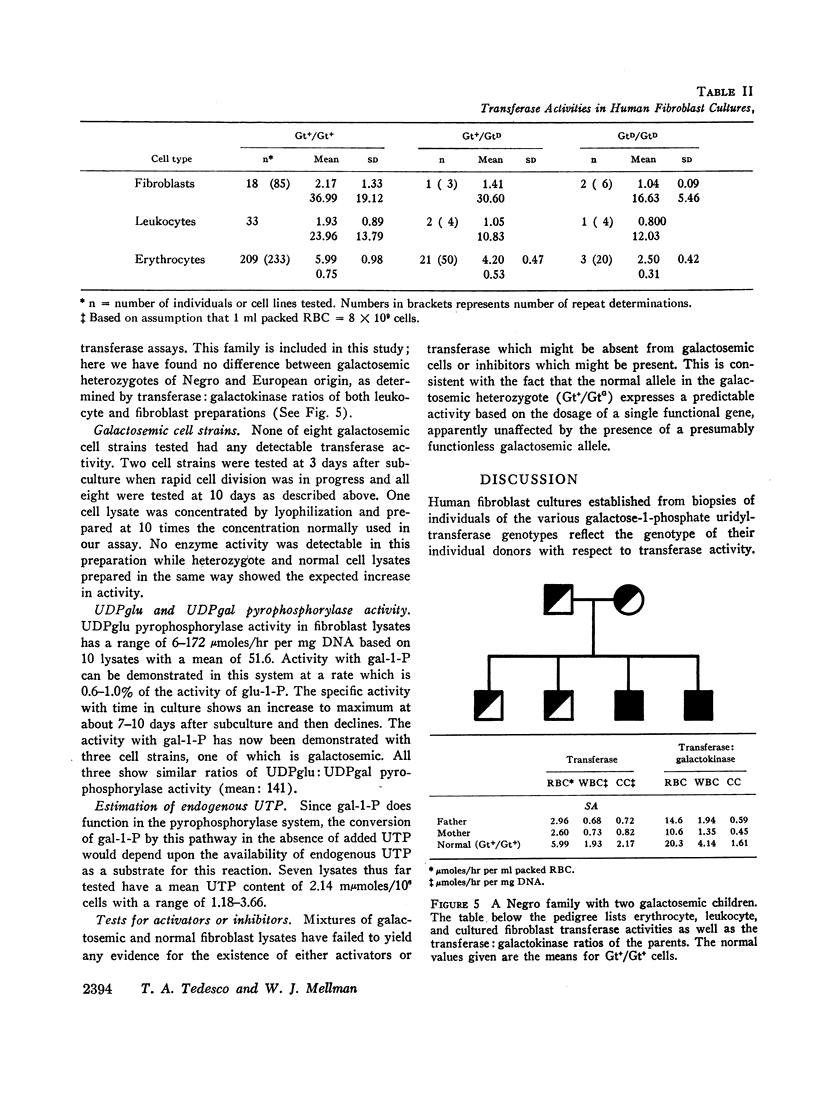
The specific activities of galactokinase and galactose-1-phosphate uridyltransferase were determined in peripheral blood leukocytes directly after separation from whole blood, and in cultured skin fibroblasts at various times during the subculture growth period. Growth curves were obtained for fibroblasts based on three different parameters: direct cell counts, total protein, and total deoxyribonucleic acid (DNA) content. At the time in culture when the specific activity of both enzymes was maximal and least variable, the ratio of transferase to galactokinase correlated well with the transferase genotypes of the original tissue donors. Leukocyte transferase: galactokinase ratios gave a similar distribution pattern.
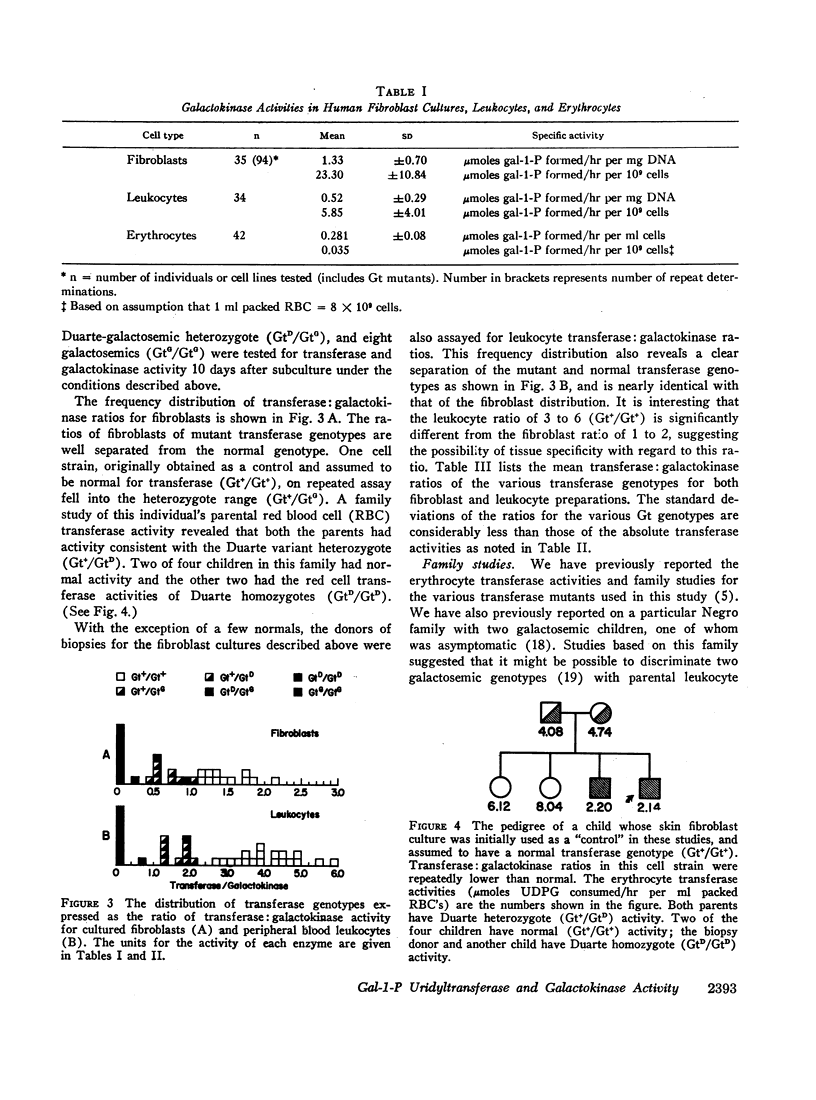
Whereas transferase activity in both fibroblasts and leukocytes was similar, galactokinase was approximately three times as active in fibroblasts as in leukocytes. All fibrobast cell strains tested had similar galactokinase activity regardless of transferase genotype.
The kinetic properties of fibroblast galactokinase were examined. Galactose-1-phosphate inhibits galactokinase activity in both normal and galactosemic cell strains, whereas other glycolytic intermediates have no effect.
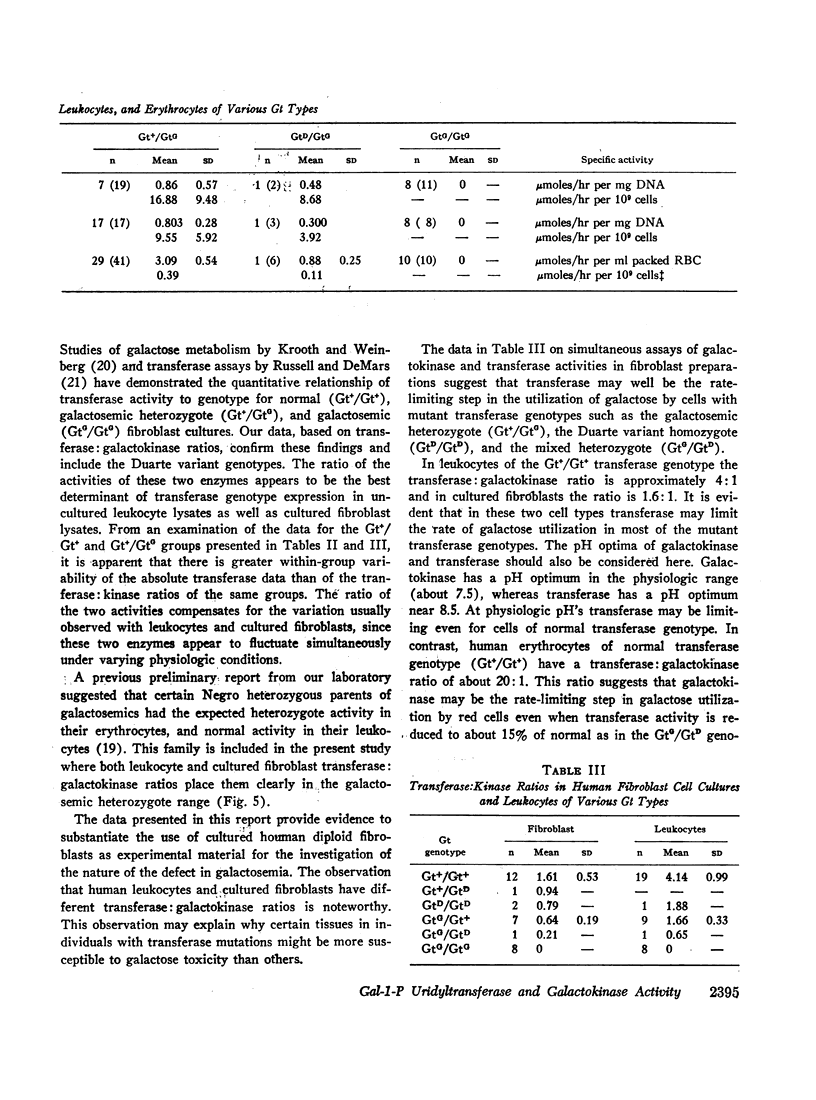
There was no detectable transferase activity in eight galactosemic (GtG/GtG) cell strains when transferase activity was maximal in cell strains of other transferase genotypes. Inhibitors responsible for the absence of transferase activity could not be demonstrated. In addition, transferase activity in galactosemic cell lysates was not observed in cells during logarithmic growth; measurable uridine diphosphate galactose (UDPgal) pyrophosphorylase activity was found in human diploid fibroblast cultures, as well as significant levels of endogenous uridine triphosphate (UTP) in lysates of fibroblast cultures.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham H. D., Howell R. R. Human hepatic uridine diphosphate galactose pyrophosphorylase. Its characterization and activity during development. J Biol Chem. 1969 Feb 25;244(4):545–550. [PubMed] [Google Scholar]
- Beutler E., Baluda M. C., Sturgeon P., Day R. W. The genetics of galactose-1-phosphate uridyl transferase deficiency. J Lab Clin Med. 1966 Oct;68(4):646–658. [PubMed] [Google Scholar]
- CUATRECASAS P., SEGAL S. MAMMALIAN GALACTOKINASE. DEVELOPMENTAL AND ADAPTIVE CHARACTERISTICS IN THE RAT LIVER. J Biol Chem. 1965 Jun;240:2382–2388. [PubMed] [Google Scholar]
- DAVIDSON R. G., BRUSILOW S. W., NITOWSKY H. M. SKIN BIOPSY FOR CELL CULTURE. Nature. 1963 Jul 20;199:296–297. doi: 10.1038/199296b0. [DOI] [PubMed] [Google Scholar]
- Gitzelmann R., Poley J. R., Prader A. Partial galactose-1-phosphate uridyltransferase deficiency due to a variant enzyme. Helv Paediatr Acta. 1967 Jul;22(3):252–257. [PubMed] [Google Scholar]
- ISSELBACHER K. J. A mammalian uridinediphosphate galactose pyrophosphorylase. J Biol Chem. 1958 May;232(1):429–444. [PubMed] [Google Scholar]
- KROOTH R. S., WEINBERG A. N. Studies on cell lines developed from the tissues of patients with galactosemia. J Exp Med. 1961 Jun 1;113:1155–1171. doi: 10.1084/jem.113.6.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mellman W. J., Tedesco T. A. An improved assay of erythrocyte and leukocyte galactose-1-phosphate uridyl transferase: stabilization of the enzyme by a thiol protective reagent. J Lab Clin Med. 1965 Dec;66(6):980–986. [PubMed] [Google Scholar]
- ROSE I. A., O'CONNELL E. L. THE ROLE OF GLUCOSE 6-PHOSPHATE IN THE REGULATION OF GLUCOSE METABOLISM IN HUMAN ERYTHROCYTES. J Biol Chem. 1964 Jan;239:12–17. [PubMed] [Google Scholar]
- Russell J. D., DeMars R. UDP-glucose: alpha-D-galactose-1-phosphate uridylytransferase activity in cultured human fibroblasts. Biochem Genet. 1967 Jun;1(1):11–24. doi: 10.1007/BF00487732. [DOI] [PubMed] [Google Scholar]
- SEGAL S., BLAIR A., ROTH H. THE METABOLISM OF GALACTOSE BY PATIENTS WITH CONGENITAL GALACTOSEMIA. Am J Med. 1965 Jan;38:62–70. doi: 10.1016/0002-9343(65)90160-9. [DOI] [PubMed] [Google Scholar]
- SHERMAN J. R., ADLER J. Galactokinse from Escherichia coli. J Biol Chem. 1963 Mar;238:873–878. [PubMed] [Google Scholar]
- Tedesco T. A., Mellman W. J. Desoxyribonucleic acid assay as a measure of cell number in preparations from monolayer cell cultures and blood leucocytes. Exp Cell Res. 1967 Jan;45(1):230–232. doi: 10.1016/0014-4827(67)90126-7. [DOI] [PubMed] [Google Scholar]



