Abstract
Quantitation of isoprostanes such as 8-iso-PGF2α and 8,12-iso-iPF2α-VI in biological fluids has been proposed as a reliable test of oxidant stress and inflammation in a variety of disorders. This paper presents a liquid chromatography method with tandem mass spectrometry detection for the simultaneous analysis of these two isoprostanes in human CSF and brain tissue samples. An API 5000 triple quadrupole instrument (AB Sciex, Foster City, CA, USA) with an APCI ion source was used in this study. Aliquots of CSF samples (0.25mL) were treated with a methanol:zinc sulfate mixture followed by on-line cleanup on an extraction column (Validated-C18) with 0.1% formic acid. The brain tissue samples were homogenized and lipids were extracted using Folch solution. Solid phase extraction columns (C18) were used for the purification of the brain isoprostane fraction. Chromatographic separation was achieved using an analytical column (Synergi C18 HydroRP) with 0.1% formic acid in water and a mixture of methanol:acetonitrile under isocratic conditions. The mass spectrometer was operated in the MRM scan and negative ion mode. The quadrupoles were set to detect the molecular ions [M-H]− and high mass fragments of isoprostanes: m/z 353→193 amu (8-iso-PGF2α) and m/z 353→115 amu (8,12-iso-iPF2α-VI) and their deuterated internal standards: m/z 357→197 amu (8-iso-PGF2α-d4) and m/z 364 → 115 amu (8,12-iso-iPF2α -VI-d11). The lower limit of quantification was 2.5 pg/mL for 8-iso-PGF2α and 5.0 pg/mL for 8,12-iso-PF2α-VI for the CSF method and 10.0 pg/0.1 g of tissue and 30.0 pg/0.1 g of tissue for 8-iso-PGF2α and 8,12-iso-iPF2α -VI, respectively, for the brain tissue method. No ion suppression or enhancement of the detection of 8-isoPGF2α, 8,12-isoPF2α-VI or both internal standards was found.
Keywords: 8-iso-PGF2α; 8, 12-iso-iPF2α-VI; HPLC-MS/MS; on-line cleanup; CSF; brain tissue samples
1. Introduction
F2-isoprostanes are prostaglandin (PG)-like compounds produced primarily from esterified arachidonic acid, a polyunsaturated fatty acid that is highly oxidizable and abundant in the brain, by non-enzymatic reactions catalyzed by free radicals. F2-isoprostanes are a group of 64 compounds isomeric in structure to F2α prostaglandin. It has been proposed that measurement of F2-isoprostanes is the most reliable approach to assess oxidative stress status in vivo, providing an important tool to explore the role of oxidative stress in the pathogenesis of neurological, cardiovascular, pulmonary, renal and liver diseases [1–4].
F2-isoprostanes have been measured in body fluids such as urine, blood, bile, cerebrospinal fluid (CSF) and lung condensate, as well as in brain and liver tissues [1,5,6]. Currently, gas chromatography (GC)-mass spectrometry [7–15] or liquid chromatography (HPLC)-mass spectrometry [6,16–22] are the most accurate methods for identifying and quantifying isoprostanes in biological fluids. However sample preparation is generally a labor-intensive, time-consuming procedure requiring purification by solid-phase extraction with [12] or without derivatization [7,8,10,11,13,14,16–18,21,23], or liquid-liquid extraction [20] sometimes followed by derivatization [9,19]. For the majority of GC methods SPE is combined with thin layer chromatography (TLC) in order to obtain highly purified samples [8,9,12–15].
In this paper we describe a reliable, accurate and sensitive method together with a very simple and rapid sample preparation procedure for the simultaneous quantification of 8-iso-PGF2α and 8,12-iso-iPF2α -VI in human CSF and brain tissues. We also present here a new procedure for preparing biological fluids free of endogenous isoprostanes for the preparation of standards and quality control samples and a modified method for measurement of matrix effect that is developed specifically for endogenous compound analysis. The potential applicability of this new method for detection of oxidative stress in brain tissue was demonstrated in a pilot study where both isoprostanes were measured in brain tissue samples collected from rats to test the hypothesis that oxidative stress is activated after seizures. The finding that 8-iso-PGF2α concentration in rat hippocampus of animals with induced status epilepticus was significantly higher when compared with control animals [24] supports this hypothesis and provided initial experience in the applicability of this new analysis procedure.
2. Experimental procedures
2.1. Materials
Two isoprostanes 8-iso-PGF2α and 8,12-iso-iPF2α-VI and their internal standards (IS), tetradeuterated 8-iso-PGF2α and 8,12-iso-iPF2α-VI-d11 were obtained from Cayman Chemical Co (Ann Arbor, MI, USA). Acetonitrile, methanol, ethanol, heptane, chloroform and ethyl acetate, all HPLC purity grade, zinc sulfate, sodium chloride, potassium hydroxide and hydrochloric acid, all A.C.S purity grade, were from Fisher Scientific (Pittsburg, PA, USA). Formic acid and 3,5-Di-tert-butyl-4-hydroxytoluene (BHT) were purchased from Sigma –Aldrich (Milwaukee,WI, USA). Solid-phase extraction columns (SPE C18, 200 mg, 4mL) were obtained from Alltech Associates Inc. (Deerfield, Il, USA). All solutions were prepared with type one distilled water (Mar Cor Medical System; Harleysville, PA, USA). Artificial CSF (Na, 150 mM; K, 3.0 mM; Ca, 1.4 mM; Mg, 0.8 mM; P, 1.0 mM; Cl, 155 mM) was purchased from Harvard Apparatus (Holliston, MA, USA). All working solutions of 8-iso-PGF2α and 8,12-iso-iPF2α -VI for standards and quality control (QC) samples preparation, and working solutions of two ISs were prepared by diluting stock solutions of isoprostanes (1mg/mL, each) and ISs (0.1mg/mL, each) with ethanol for CSF analysis and with methanol for brain tissue analysis since methanol was used in the lipid hydrolysis procedure for the latter tissue. The spiking solutions for the calibration standard and quality control samples contained both 8-iso-PGF2α and 8,12-iso-iPF2α -VI. Internal standard spiking solution also contained the two deuterated internal standards in equal concentrations (1ng/mL of ethanol for CSF analysis and 100 ng/mL of methanol for brain tissue analysis).
All calibrators and quality control samples for quantification of isoprostanes in CSF were prepared in artificial CSF, which we validated as a suitable matrix for these samples. The most important limitation for using human CSF as a matrix for standards and QC samples preparation is lack of sufficient volume of this fluid. Additionally, human CSF must be pre-treated to remove endogenous isoprostanes to be used as a biological matrix for standard and QC sample preparation. Thus using artificial CSF for this purpose makes the procedure much simpler and shorter. All calibrators and quality control samples for quantification of isoprostanes in brain tissues were prepared by spiking brain homogenates, previously treated with SPE columns to remove endogenous isoprostanes, with appropriate concentrations of isoprostanes and the respective internal standards.
All CSF samples were obtained by lumbar puncture after informed consent from subjects with a variety of late-life neurodegenerative dementing disorders and cognitively normal elderly undergoing clinical evaluations in the Penn Memory Center. The samples were aliquoted without centrifugation into polypropylene cryovials and stored at −80°C.
Post-mortem frontal lobe brain specimens were obtained from patients who came to autopsy at the Center for Neurodegenerative Disease Research at the University of Pennsylvania following informed consent from the decedent’s family. The samples were stored at −80°C, and only gray matter was used for isoprostane analysis.
2.2. Sample preparation
2.2.1. CSF analysis
CSF sample preparation was limited to a protein precipitation step, achieved by mixing the sample aliquot with a combination of methanol and 0.1 M zinc sulfate (7:3, v/v) followed by a centrifugation step [6]. Six standards that contained both isoprostanes with concentrations ranging from 2.5 to 80 pg/mL (8-iso-PGF2α) and from 5.0 to 250 pg/mL (8,12-iso-iPF2α -VI) together with blank sample were prepared on the day of analysis by spiking artificial CSF (0.25 mL) with an aliquot of standard and internal standard spiking solutions (0.05 mL, each). Quality control sample aliquots (8-iso-PGF2α: 10, 25 and 50 pg/mL; 8,12-iso-iPF2α -VI : 15, 30 and 100 pg/mL) were prepared in bulk by spiking artificial CSF, and then stored in 1.5 mL Eppendorf tubes at −80°C until the day of analysis. On the day of analysis the quality control sample aliquots and patient CSF sample aliquots stored at −80°C, were thawed at +4°C and then 0.25 mL of CSF and QC samples were spiked with 0.05 mL of ethanol and 0.05 mL of internal standards spiking solution. Protein-free supernatant was prepared by adding 0.35 mL of the precipitation mixture to the standards, QC, and subjects’ CSF samples followed by mixing for 10 sec. and then two consecutive centrifugation steps at 9,500 × g (Abbot centrifuge, model 3531) for 15 min. Aliquots of supernatant (370 μL) were injected into the HPLC-MS/MS system.
2.2.2. Brain tissue analysis
Preparation of brain tissue gray matter for analysis of isoprostanes includes three primary steps: lipid extraction, followed by hydrolysis that releases total isoprostanes, and their purification. The extraction and hydrolysis procedure followed the method described by Morrow and Roberts [25]. Briefly, brain tissue samples were homogenized with a blade homogenizer and lipids were extracted using for both procedures Folch solution (chloroform:methanol, 2:1, by volume) containing 0.005% BHT to prevent auto-oxidation. Following evaporation to dryness under nitrogen, lipids were hydrolyzed with KOH (15%) to release F2α-isoprostanes. Solid phase extraction columns (C18) were next used for the purification of the isoprostane fraction which was subsequently analyzed by HPLC with tandem mass spectrometry detection.
In the purification process 2 mL of homogenate was spiked with 0.08 mL of methanol and 0.06 mL of the spiking solution of internal standards and loaded onto pre-conditioned (with 6 mL of methanol, followed by 6 mL of water) C18 solid phase extraction columns. Next the columns were washed with 9 mL of water (pH 3.0) and 9 mL of heptane. Isoprostanes were then eluted with 9 mL of a mixture of ethyl acetate and methanol (1:1, by volume). The eluent from the C18 SPE columns was then evaporated to dryness under a stream of nitrogen at 37°C and the isoprostanes were re-dissolved in 0.5 mL of ethanol and analyzed using the HPLC-tandem mass spectrometry system.
The calibration curve for brain tissue sample analysis included blank sample and five calibrators with the concentration range from 10 to100 pg/0.1g of tissue for 8-iso-PGF2α and from 30 to 200 pg/0.1g of tissue for 8,12-iso-iPF2α -VI. Three QC samples (8-iso-PGF2α: 25, 40 and 80 pg/0.1g of tissue; 8,12-iso-iPF2α : 30, 80 and 160 pg/0.1g of tissue) were included into each run. Brain tissue homogenate free of endogenous isoprostanes, was prepared by running 2 mL of the mixture of homogenate through C18 SPE columns, previously pre-conditioned with methanol and water, 6 mL of each. The homogenate collected from the columns, free of the endogenous isoprostanes which were adsorbed onto the columns, was used as the standard and QC samples’ matrix. For the preparation of standards 2 mL of the matrix was spiked with 0.08 mL of spiking solutions of standards and 0.06 mL of spiking solution of internal standards and loaded on pre-equilibrated SPE columns, in the same way like CSF from patient, for purification process. Quality control samples were prepared in bulk by spiking the matrix, free of endogenous isoprostanes, with QC sample spiking solutions. Then the aliquots of QC samples were stored in 1.5 mL polypropylene Eppendorf tubes at −80°C. On the day of analysis the quality control samples were thawed at +4°C and 2 mL of QC was spiked with 0.08 mL of methanol and 0.06 mL of internal standards spiking solution and loaded onto preconditioned C18 solid phase extraction columns. The next steps of standard and QC sample preparation are the same as for the individual sample preparation.
2.3. HPLC-tandem mass spectrometer and conditions
Analyses of 8-iso-PGF2α and 8,12-iso-iPF2α -VI were carried out on an API 5000™ triple quadrupole mass spectrometer equipped with a QJet™ ion guide and accelerated by a LINAC® collision cell (AB Sciex, Foster City, CA, USA) with an atmospheric pressure chemical ionization probe in a Turbo V™ ion source, interfaced with an Agilent 1100 liquid chromatography system including an autosampler G1313A, isocratic pump G1310A, binary pump G1312A and degasser G1379A. Analyst 1.4.2 software (AB Sciex, Foster City, CA, USA) was used for system control and data processing. The mass spectrometer was operated in the negative ion mode and MRM scan type. The first and third quadrupoles were set to detect the negatively charged molecular ions [M-H]− and a high mass fragment of 8-iso-PGF2α (m/z 353→193 amu) and 8,12-iso-iPF2α -VI (m/z 353→115 amu) and their ISs ( m/z 357→197 amu (8-iso-PGF2α-d4) and m/z 364 → 115 amu (8,12-iso-iPF2α -VI-d11). The fragmentation process was described previously [16,21].
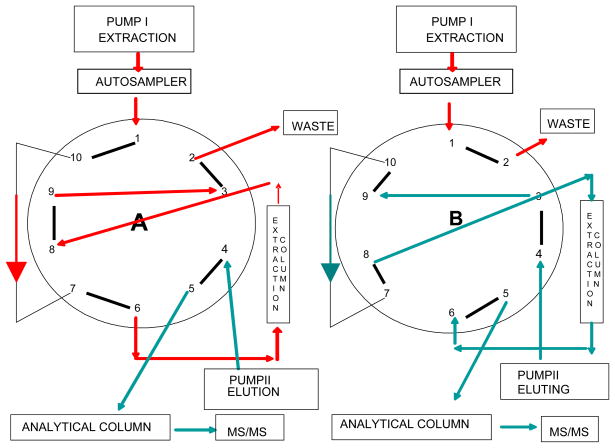
CSF sample cleanup, and the additional sample cleanup for homogenate samples, was achieved on-line with 0.1% formic acid in water (extraction solution) at a flow rate of 1.5 mL/min using an extraction column (Validated-C18, 5μm; Perkin Elmer, MA, USA). After 1.8 min of washing the switching valve was activated and the sample extract was back flushed from the extraction column onto the analytical column (Synergi C18 4μ Hydro-RP 250×3mm, Phenomenex, USA) maintained at 40°C (Fig. 1.). The mobile phase consisted of 0.1% formic acid in water (solvent A) and a mixture of acetonitrile: methanol (20:80, by volume, solvent B), (solvent A: solvent B - 40:60, by volume) adjusted to pH 6.15 with ammonium hydroxide. The flow rate of the mobile phase was 0.6 mL/min and total run time was 17min.
Figure 1.
Switching valve setup for simultaneous analysis of 8-iso-PGF2α and 8,12-iso-iPF2α-VI: A – position for on-line cleanup, B – position for eluting analyte from the extraction column to the analytical one and then to the mass spectrometer.
2.4. Assay validation
Validation of our method followed FDA Guidance for Industry – Bioanalytical Method Validation, May 2001 [26] and was done using standards, QC samples, human CSF and homogenate from postmortem human brain tissues.
2.4.1. LLOQ and linearity
The lower limit of quantification (LLOQ) was defined as the lowest concentration of 8-iso-PGF2α and 8,12-iso-iPF2α -VI that could be measured with a reproducibility of ≤20% and an accuracy of 80–120% [26]. The linearity of the isoprostanes assay was assessed over the concentration range 2.5–300 pg/mL (8-iso-PGF2α) and 5–1000 pg/mL (8,12-iso-iPF2α -VI) for CSF analysis and over the concentration range 10–300 pg/0.1 g of tissue (8-iso-PGF2α) and 30–1000 pg/0.1 g of tissue (8,12-iso-iPF2α -VI) using a linear thru zero non-weighted regression model.
2.4.2. Between- and within-day precision and accuracy
Between-day precision and accuracy was evaluated for all standards and QC samples for CSF and brain tissue analyses. The within-day precision and accuracy was evaluated for three CSF QC samples. All data were collected from a replication experiment in which each sample was analyzed multiple times (10–20) on the same day and in duplicate on different days (5 days). Mean, SD and CV (%) were calculated for the results from the same and different days.
2.4.3. Absolute recovery and matrix effect
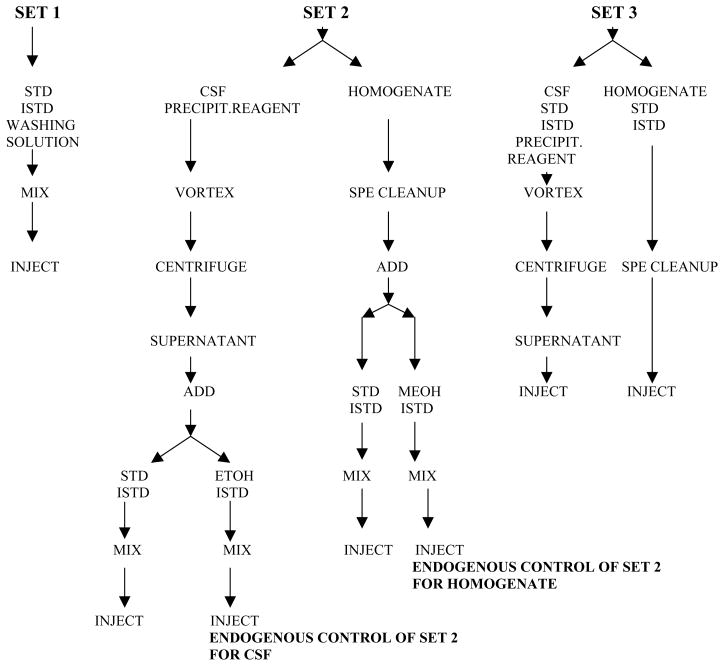
The possibility of a matrix effect (ME) on detection of the two isoprostanes and their internal standards, and absolute recovery (RE) of these four compounds in human CSF and brain tissue homogenate was assessed based on the procedure established by Matuszewski et al.[27] using a mixture of 5 human CSF samples from different patients (to obtain necessary volume) or a mixture of 5 different brain homogenates (to obtain necessary volume) as a biological matrix. The original procedure was modified since we were working with endogenous compounds that are always present in biological material, so in the ME/RE assessment experiment we had to deal with both endogenous and exogenous isoprostanes (see the procedure below and Fig. 2.). For correct calculation of ME on detection of isoprostanes it was necessary to distinguish exogenous from endogenous isoprostanes. Briefly, three sets of three samples obtained by spiking the CSF or homogenate mixture with standard spiking solutions ranged from 5–80 pg/mL (CSF) or 30–70 pg/0.1 g of tissue (homogenate)(8-iso-PGF2α) and from 12.5–250 pg/mL (CSF) or 60–150 pg/0.1 g of tissue (homogenate)(8,12-iso-iPF2α -VI) and the mixture of both internal standards were prepared. Set 1, without biological matrix, contained standards (exogenous isoprostanes) with internal standards in the extraction solution. We used the extraction solution (0.1% formic acid in water) as a solvent for set 1, instead of mobile phase suggested by Matuszewski, since our procedure includes a switching valve technique and 2 dimensional chromatography that incorporates as a first step the binding of the compounds on the extraction column. Thus, addition of mobile phase to set 1 would change this binding. Set 2 contained CSF or homogenate extract spiked with standards and internal standards spiking solutions after protein precipitation (CSF) or clean-up on C18 SPE columns (brain homogenate). This set contained both exo- and endogenous isoprostanes. Additionally, an endogenous control (exogenous blank) sample was included into set 2 since this set was subsequently compared with set 1 that contained only exogenous isoprostanes. This sample contained besides endogenous isoprostanes, and the two internal standards, ethanol/methanol instead of the standards spiking solution. Set 3 contained regular samples i.e. CSF or homogenate spiked with standards and internal standards spiking solutions before protein precipitation (CSF) or cleaning on C18 SPE columns (homogenate). Set 3 contained exo- and endogenous isoprostanes, similar to set 2, permitting direct comparison of set 3 with set 2. This experiment was run on two separate days; all samples were run in triplicate. The peak area for standards and internal standards in these 3 sets was used for the calculations of ME and RE for standards and internal standards according to the following equations:
Figure 2.
Sample preparation for matrix effect and absolute recovery assessment.
for standards:
for internal standards:
where:
set 1 - peak area of standard (exogenous isoprostanes) or internal standard from set 1
-
set 2 - peak area of standard (exo-and endogenous isoprostanes) or internal standard from set 2
endogenous control of set 2 – peak area of endogenous isoprostanes in endogenous control sample from set 2
set 3 - peak area of standard (exo- and endogenous isoprostanes) or internal standard from set 3
To determine the ME for artificial CSF and homogenate extract, without endogenous isoprostanes, on their detection, we used a post column infusion technique [28,29]. An infusion pump was used to deliver a constant flow (10μL/min) of isoprostanes standard contained 50 pg/mL of 8-iso-PGF2α and 8,12-iso-iPF2α -VI each, to HPLC eluent. An artificial CSF sample, or homogenate extract treated to remove endogenous isoprostanes, was injected under the conditions of our method and the response from the infused analyte was recorded.
2.4.4. Analytical recovery of analytes from human CSF and brain tissue homogenate
In addition the analytical recovery of isoprostanes from human CSF and brain tissue homogenates was tested in a spiking experiment. Ten human CSFs and brain tissue homogenate were spiked separately with three different spiking solutions that contained both 8-iso-PGF2α and 8,12-iso-iPF2α -VI (each at concentrations of: 12.5, 25 and 50 pg/mL) for CSF analysis or one spiking solution that contained both 8-iso-PGF2α (40 pg/0.1 g of tissue) and 8,12-iso-iPF2α -VI (80 pg/0.1 g of tissue) for brain tissue analysis. After preparation (protein precipitation for CSF and C18 SPE column cleanup for brain tissue analysis) the samples before and after spiking were run using our method. Analytical recovery of the isoprostanes was expressed as the percentage of the nominal spiked-in concentrations. The final results were calculated as the mean, SD and coefficient of variation.
2.4.5. Carry over
Sample carryover was checked by running blank samples of CSF and brain tissue homogenate after the highest standard and by multiple runs of the lowest standard of CSF (prepared for this experiment both in artificial CSF and CSF pre-treated to remove endogenous isoprostanes)(2.5 pg/mL of 8-iso-PGF2α and 5 pg/mL of 8,12-iso-iPF2α -VI) following the highest one (80 pg/mL of 8-iso-PGF2α and 250 pg/mL of 8,12-iso-iPF2α -VI) to determine if the high concentrations interfere with the subsequent low concentration measurement.
3. Results
3.1. Linearity and LLOQ
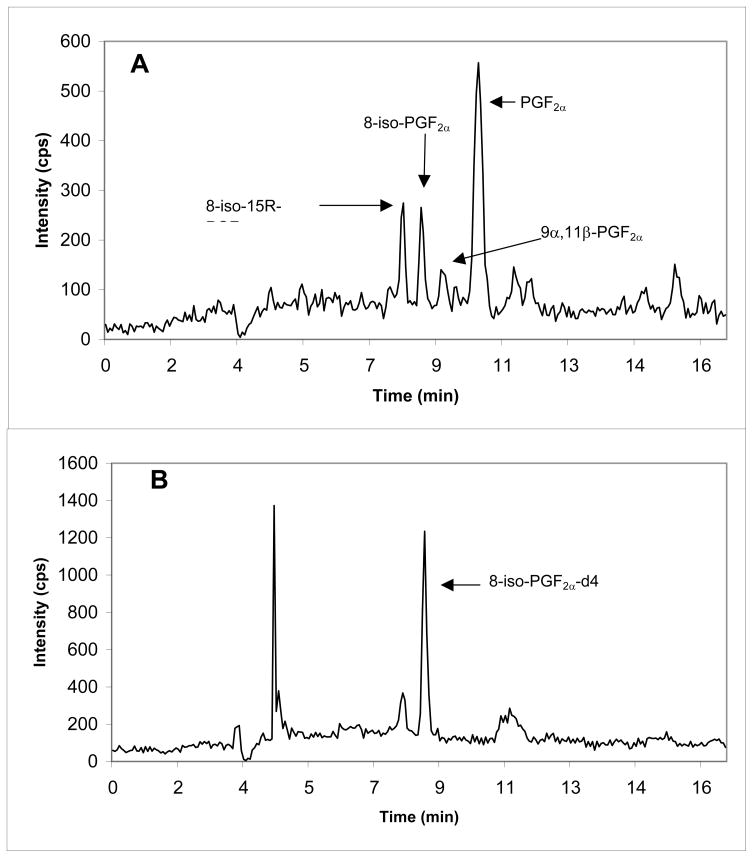
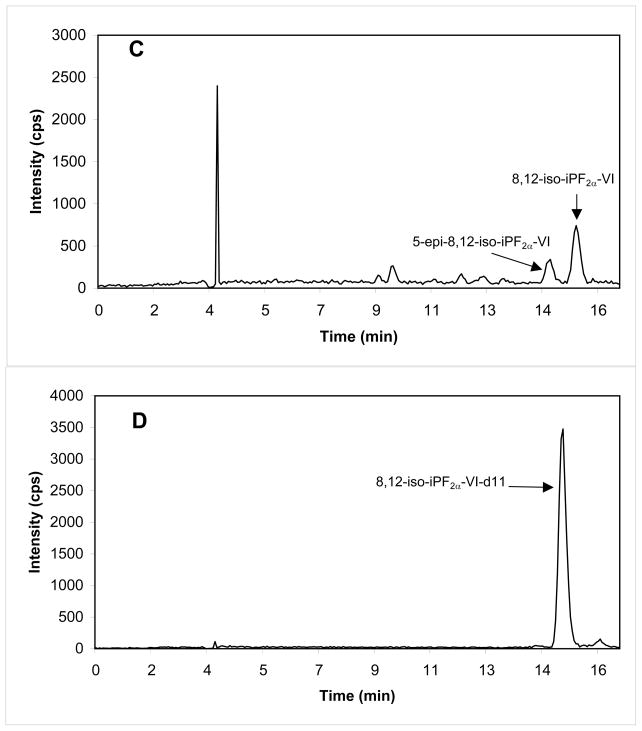
A representative mass chromatogram of 8-iso-PGF2α and 8,12-iso-iPF2α -VI together with their internal standard in a sample of CSF is shown in Fig. 3. The retention times of 8-iso-PGF2α and of 8,12-iso-iPF2α -VI both in CSF and brain tissue sample, were 8.62 ± 0.2 min and 15.56 ± 0.4 min, respectively (n=13 days). The assay was linear for 8-iso-PGF2α over the range 2.5–300 pg/mL (CSF; y = 0.0267(± 0.0034)x, r = 0.9997) or 10–300 pg/0.1 g of tissue (brain tissue; y = 0.0032 (±0.0004)x, r = 0.9989, n = 10), and for 8,12-iso-iPF2α -VI over the range 5–1000 pg/mL (CSF; y = 0.0126 (±0.0008)x, r = 0.9995) or 30–1000 pg/0.1 g of tissue (brain tissue; y = 0.0022(±0.0002)x, r = 0.9985, n = 10). The LLOQ was 2.5 pg/mL and 5.0 pg/mL for 8-iso-PGF2α and 8,12-iso-iPF2α -VI respectively, for the CSF method, and 10.0 pg/0.1 g of tissue and 30.0 pg/0.1 g of tissue for 8-iso-PGF2α and 8,12-iso-iPF2α -VI respectively, for the brain tissue method.
Figure 3.
Mass chromatogram of endogenous 8-iso-PGF2α (A)(5.61 pg/mL) and 8,12-iso-iPF2α-VI (C)(16.0 pg/mL) detected in human CSF. Additional isomers and prostaglandin peak identified using commercial available standards are labeled. Each internal standard (B, D) concentration is 100 pg/mL CSF.
3.2. Between- and within-day precision and accuracy for standards and quality control samples
3.2.1. Standards
Between day precision (CV) for 8-iso-PGF2α standards ranged from 1.8% to 8.5% (CSF) and from 2.4% to 8.9% (brain tissue). For 8,12-iso-iPF2α -VI standards between day precision ranged from 1.1% to 10.6% (CSF) and from 1.5% to 10.2% (brain tissue)(n=5days). Between day accuracy for 8-iso-PGF2α standards ranged from 96.9% to 109.3% (CSF) and from 92.3% to 104.4% (brain tissue) and for 8,12-iso-iPF2α -VI standards from 94.5% to 104.5% (CSF) and 98.0% to 108.3% (brain tissue). See Tab.1 for more details.
Table 1.
Between days (n=5) precision and accuracy for 8-iso-PGF2α and 8,12-iso-iPF2α-VI standards and quality control samples for CSF and brain tissues analysis.
| Concentration | Found amount ± SD (pg/mL) | CV (%) | Accuracy (%) | ||
|---|---|---|---|---|---|
| CSF | Standards | ||||
| 8-iso-PGF 2α (pg/mL) | 2.5 | 2.73 ± 0.2 | 7.42 | 109.3 ± 7.9 | |
| 5.0 | 5.45 ± 0.5 | 8.51 | 109.0 ± 8.9 | ||
| 10.0 | 9.69 ± 0.4 | 3.76 | 96.9 ± 3.6 | ||
| 25.0 | 25.4 ± 0.9 | 3.51 | 101.5 ± 3.5 | ||
| 50.0 | 51.3 ± 2.0 | 3.94 | 102.6 ± 4.2 | ||
| 80.0 | 79.1 ± 1.4 | 1.76 | 99.0 ± 1.8 | ||
| QC Samples | |||||
| 8-iso-PGF 2α (pg/mL) | 10.0 | 10.5 ± 0.8 | 7.78 | 105.1 ± 8.2 | |
| 25.0 | 26.6 ± 1.1 | 4.23 | 106.4 ± 4.4 | ||
| 50.0 | 54.1± 2.3 | 4.25 | 108.1± 4.6 | ||
| Standards | |||||
| 8,12-iso-iPF2α-VI (pg/mL) | 5.0 | 4.97 ± 0.5 | 10.60 | 99.2 ± 10.4 | |
| 12.5 | 13.0 ± 0.6 | 4.36 | 104.5 ± 4.4 | ||
| 25.0 | 24.4 ± 0.8 | 3.23 | 97.6 ± 2.9 | ||
| 50.0 | 47.3 ± 3.9 | 8.34 | 94.5 ± 7.8 | ||
| 125.0 | 120 ± 4.6 | 3.82 | 95.8 ± 3.7 | ||
| 250.0 | 253 ± 2.9 | 1.14 | 101.5 ± 1.3 | ||
| QC Samples | |||||
| 8,12-iso-iPF 2α-VI (pg/mL) | 15.0 | 15.9 ± 1.3 | 8.10 | 105.7 ± 8.4 | |
| 30.0 | 30.4 ± 1.5 | 4.80 | 101.3 ± 4.9 | ||
| 100.0 | 93.8 ± 3.3 | 3.50 | 93.8± 3.3 | ||
| Brain tissue | Standards | ||||
| 8-iso-PGF2α (pg/0.1g of tissue) | 10.0 | 10.4 ± 0.9 | 8.90 | 104.4 ± 9.3 | |
| 30.0 | 29.7 ± 1.0 | 3.60 | 99.1 ± 3.6 | ||
| 50.0 | 50.1 ± 1.4 | 2.89 | 100.3 ± 2.9 | ||
| 70.0 | 64.7 ± 3.8 | 5.80 | 92.3 ± 5.4 | ||
| 100.0 | 101.4 ± 2.5 | 2.44 | 101.4 ± 2.5 | ||
| QC Samples | |||||
| 8-iso-PGF2α (pg/0.1g of tissue) | 25.0 | 26.6 ± 2.2 | 8.20 | 106.5 ± 8.8 | |
| 40.0 | 41.8 ± 1.2 | 2.80 | 104.4 ± 3.0 | ||
| 80.0 | 89.0± 4.5 | 5.10 | 111.3 ± 5.7 | ||
| Standards | |||||
| 8,12-iso-iPF 2α-VI (pg/0.1g of tissue) | 30.0 | 32.5 ± 3.3 | 10.20 | 108.3 ± 11.1 | |
| 60.0 | 61.2 ± 2.6 | 4.24 | 101.9 ± 4.3 | ||
| 100.0 | 98.0 ± 5.8 | 5.95 | 98.0 ± 5.8 | ||
| 150.0 | 151.3 ± 4.9 | 3.33 | 100.7 ± 3.4 | ||
| 200.0 | 199.7 ± 2.3 | 1.53 | 99.7 ± 1.5 | ||
| QC Samples | |||||
| 8,12-iso-iPF 2α-VI (pg/0.1g of tissue) | 30.0 | 31.4 ± 2.8 | 8.90 | 104.6 ± 9.3 | |
| 80.0 | 85.7 ± 9.2 | 9.60 | 107.1 ± 11.5 | ||
| 160.0 | 171.0 ± 17.6 | 10.30 | 106.9 ± 11.0 | ||
3.2.2. Quality control samples
Between day (n=5 days) precision for three CSF QC samples of 8-iso-PGF2α were 7.8%, 4.2% and 4.3%, with accuracy range 105.1%–108.1% and for 8,12-iso-iPF2α -VI were 8.1%, 4.8% and 3.5%, with accuracy range 93.8%–105.7%. Between day (n=5 days) precision for three brain tissue QC samples of 8-iso-PGF2α were 8.2%, 2.8% and 5.1%, with accuracy range 104.4%–111.3% and of 8,12-iso-iPF2α -VI were 8.9%, 9.6% and 10.3%, with accuracy range 104.6%–107.1%. See Tab.1. for more details. Within-day precision (n=10–20) for CSF 8-iso-PGF2α QC samples was lower than 7% with a mean accuracy of 103.6 ± 5.3%, and for 8,12-iso-iPF2α -VI QC samples was lower than 5% with a mean accuracy of 98.3 ± 3.2%.
3.3. Absolute recovery and matrix effect
The mean absolute RE of 8-iso-PGF2α assessed for the concentration range 5–80 pg/mL (CSF) or 30–70 pg/0.1 g of tissue (brain tissue) was 103.2 ± 9.0% (CV =0.1%) or 98.8 ± 13.3% (CV = 6.1%), respectively, and of 8,12-iso-iPF2α -VI for the concentration range 12.5–250 pg/mL (CSF) or 60–150 pg/0.1 g of tissue (brain tissue) was 98.6 ± 6.7 pg/mL (CV=0.1%) or 111.0 ± 11.6% (CV = 10.5%), correspondingly (Tab.2.). Minimal, clinically not significant ME was found for the detection of 8-iso-PGF2α (−6%, CV=0.1% for CSF and −2%, CV=6.6% for brain tissue), and very small ion enhancement for the detection of 8,12-iso-iPF2α -VI in CSF (+1.1%, CV=0.1%). For brain tissue, we found very small, but insignificant ion suppression for detection of 8,12-iso-iPF2α -VI (−2%, CV=7.4%). Data for the RE and ME experiment for both internal standards are summarized in Tab.2. Lack of ME using artificial CSF for detection of 8-iso-PGF2α and 8,12-iso-iPF2α -VI is shown in Fig. 4. There is also no ME for brain tissue homogenate, previously run through the SPE columns, on detection of 8-iso-PGF2α and 8,12-iso-iPF2α -VI (data not shown).
Table 2.
ME (%) and RE (%) (n=18) of 8-iso-PGF2α, 8,12-iso-iPF2α-VI and their internal standards for CSF and brain tissue samples.
| Compound | 8-iso-PGF2α | 8-iso-PGF2α-d4 | 8,12-iso-iPF2α-VI | 8,12-iso-iPF2α-VI-d11 | |
|---|---|---|---|---|---|
| CSF | concentration range (pg/mL) | 5–80 | 100 | 12.5 –250 | 100 |
| ME | 94.0 ± 12.5 | 105.7 ± 5.2 | 101.1 ± 9.7 | 104.3 ± 4.7 | |
| RE | 103.2 ± 9.0 | 101.3 ± 3.7 | 98.6 ± 6.7 | 102.3 ± 5.7 | |
| Brain tissue | concentration range (pg/0.1g of tissue) | 30–70 | 1500 | 60 –150 | 1500 |
| ME | 98.1 ± 6.5 | 96.4 ± 15.6 | 98.1 ± 7.3 | 96.8 ± 4.4 | |
| RE | 98.8 ± 13.0 | 107.9 ± 13.2 | 111.0 ± 11.6 | 110.5 ± 12.7 | |
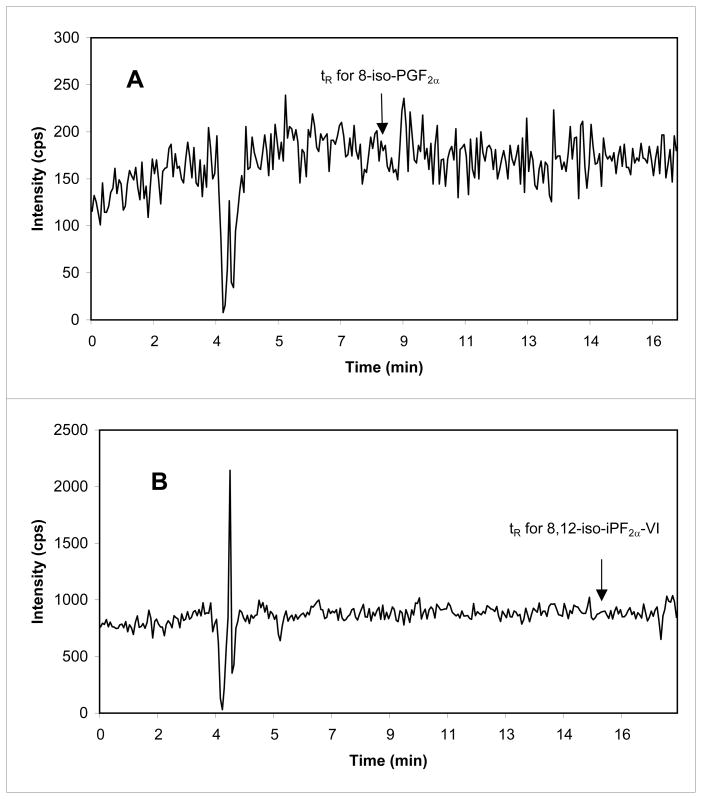
Figure 4.
Lack of ME of artificial CSF on detection of 8-iso-PGF2α (A) and of 8,12-iso-iPF2α –VI (B) by the post-column infusion experiment. The experimental conditions are described in Section 2.4.3. The arrows represent the retention time for 8-iso-PGF2α and 8,12-iso-iPF2α-VI (A and B, respectively).
3.4. Analytical recovery of analytes from human CSF and brain tissue homogenate
Results of the analytical recovery of 8-iso-PGF2α and 8,12-iso-iPF2α -VI after spiking human CSF with three different spiking solutions of the two isoprostanes in each solution are presented in Table 3. Mean analytical recoveries of 8-iso-PGF2α and 8,12-iso-iPF2α -VI from CSF were respectively, from 102 ± 5.3% to 107 ± 6.9% and 95.6 ± 3.1% to 103.1 ± 5.3%. The respective analytical recoveries from brain tissue homogenates were 100.9 ± 12.9% and 103.5 ± 7.2% for the two isomers.
Table 3.
Mean analytical recovery (%) of 8-iso-PGF2α and 8,12-iso-iPF2α-VI used for spiking human CSF and brain tissue homogenate (n=10).
| CSF recovery of (pg/mL) | Brain tissue recovery of (pg/0.1g of tissue) | |||||
|---|---|---|---|---|---|---|
| 8-iso-PGF2α | 12.5 | 25.0 | 50.0 | 40.0 | ||
| Median | 103.5 | 110.1 | 100.8 | 99.0 | ||
| Mean | 102.0 | 107.0 | 102.5 | 100.9 | ||
| SD | 5.3 | 6.9 | 5.8 | 12.9 | ||
| CV (%) | 5.2 | 6.4 | 5.7 | 12.8 | ||
| 8,12-iso- iPF2α-VI | 12.5 | 25.0 | 50.0 | 80.0 | ||
| Median | 96.7 | 103.6 | 95.6 | 105.0 | ||
| Mean | 99.7 | 103.1 | 95.6 | 103.5 | ||
| SD | 6.6 | 5.3 | 3.1 | 7.2 | ||
| CV (%) | 6.6 | 5.1 | 3.3 | 6.9 | ||
3.5. Carry over
No carryover effect was observed. There were no detectable peaks of 8-iso-PGF2α or 8,12-iso-iPF2α -VI in the chromatogram of blank samples of artificial CSF or CSF pre-treated to remove endogenous isoprostanes and brain tissue homogenate (pre-treated to remove endogenous isoprostanes) run immediately after the highest standard. Accuracy of the lowest standard run after the highest one was 98.2 ± 3.9% (CV=4.0%, n=10) for 8-iso-PGF2α and 101.9 ± 2.9% (CV=2.9%, n=10) for 8,12-iso-iPF2α -VI (data for artificial CSF analysis).
4. Discussion
Our goal here was to develop an analytical method that is reliable, accurate and precise with a straightforward simplified sample preparation procedure for quantification of 8-iso-PGF2α and 8,12-iso-iPF2α -VI in CSF and human brain tissue.
Solid phase extraction columns have been used in sample preparation procedures in all published HPLC-MS methods for the analysis of isoprostanes. The first study to use a protein precipitation step followed by on-line cleanup for analysis of isoprostanes is our report of this procedure for analysis of isoprostanes in human urine and plasma [6]. The method for CSF and brain tissue analysis presented in this paper is based on it.
Our method uses an automated column-switching technique together with on-line sample cleanup that reduces manual sample preparation to a simple protein precipitation step, and eliminates the need for complex purification steps. We used 10 port switching valve, instead of simpler six port valve, since this kind of device was available at our laboratory at the time of method development. The connection that we set, one of at least three available for 10 port switching valve, gave satisfactory results based on the data from method validation. For CSF samples, protein precipitation and on-line cleanup were the only steps involved in sample preparation. For brain tissues it was necessary to perform standard procedures for tissue preparation including tissue homogenization with lipid extraction followed by hydrolysis and purification of released isoprostanes on SPE columns. Samples obtained from SPE columns were suitable for injection into the HPLC/MS/MS system without additional on-line cleanup with a column switching technique. However, since we run CSF and brain tissues on the same HPLC-mass spectrometer system to avoid the additional technical work with disconnecting the switching valve and changing the tubing set-up we adjusted the CSF method for analysis of brain tissues samples as well.
The lowest 8,12-iso-iPF2α-VI standard for CSF analysis (the LLOQ) is twice as high as the lowest one for the quantification of 8-iso-PGF2α (5.0 pg/mL vs 2.5 pg/mL) since based on our results the concentration of 8,12-iso-iPF2α-VI in CSF is about 3.1±0.97 (n= 65) times higher compared to the concentration of 8-iso-PGF2α. Also the lowest standards for the quantification of both isoprostanes in the brain tissues assay are higher compared to the assay for CSF, since we found that the concentration of these isoprostanes in brain tissues is 10–12 times higher compared to their concentration in CSF. Based on the recommendations of Shah et al. [30] and the FDA guideline for analytical performance validation [26] the described assay has excellent between- and within-day analytical recovery and precision for all standards and QC samples for both isoprostanes in the two studied biological matrices: human CSF and postmortem human brain tissues. The mean analytical recovery was measured not only for standards and QC samples but also for human CSF and brain tissue homogenates because of the differences in the biological matrices (artificial CSF, used for calibrator and QC samples preparation vs human CSF or brain tissue homogenate “cleaned” of endogenous isoprostanes on SPE columns used for calibrator and QC sample preparation vs brain tissue homogenate containing endogenous isoprostanes). This approach provides assurance that the different matrices used for standard and QC sample preparation do not result in artifactual isoprostane concentrations in human CSF and brain tissue.
The ion suppression/enhancement and absolute recovery were checked for the CSF (artificial and human CSF) and brain tissue matrices. None of these exerted a significant effect on ion detection or recovery. It has already been shown that for small molecules the atmospheric pressure chemical ionization ion source gives less ion suppression when compared to electrospray ionization (ESI)[31–33]. Since we previously reported for the urinary isoprostane analysis method, we observed very high ion suppression using ESI [6], for the current study we selected APCI without taking ESI into consideration. Additionally to further reduce potential inaccuracy caused by changing ionization efficiency from even minimal ion suppression or enhancement from sample to sample we used stable isotope-labeled analytes as internal standards (8-iso-PGF2α–d4 and 8,12-iso-iPF2α-VI-d11).
5. Conclusions
We have described an HPLC –atmospheric pressure chemical ionization- tandem mass spectrometry method with high sensitivity and selectivity for the simultaneous quantification of 8-iso-PGF2α and 8,12-iso-PF2α-VI in human CSF and brain tissue samples. The developed method showed very good performance in terms of accuracy and repeatability. The pretreatment procedure of CSF is extremely simple and did not involve costly, time and labor-consuming sample preparation step. Biological fluids free of endogenous isoprostanes prepared according to developed here procedure are suitable, not expensive matrices for standards and quality control preparation. This methodology meets the criteria of the United States Food and Drug Administration for analytical method validation and qualification. We are utilizing this procedure for the investigation of oxidative stress in neurodegenerative diseases.
Acknowledgments
Supported by (AG024904), from the NIH (National Institute on Aging; National Institute of Biomedical Imaging and Bioengineering), Foundation for the National Institutes of Health, Pfizer, Wyeth Research, Bristol-Myers Squibb, Eli Lilly & Company, GlaxoSmithKline, Merck & Company, AstraZeneca AB, Novartis Pharmaceuticals Corporation, Alzheimer’s Association, Eisai Global Clinical Development, Elan Corporation plc, Forest Laboratories, and Institute for the Study of Aging, U.S. Food and Drug Administration and by AG10124 from the NIH (National Institute on Aging), and SAP4100027296, a health research grant awarded by the Department of Health of the Commonwealth of Pennsylvania from the Tobacco Master Settlement Agreement under Act 2001-77.
Abbreviations
- ME
matrix effect
- RE
recovery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Magdalena Korecka, Email: korecka@mail.med.upenn.edu.
John Q. Trojanowski, Email: trojanow@mail.med.upenn.edu.
References
- 1.Pratico D, Lawson JA, Rokach J, FitzGerald GA. Trends Endocrinol Metab. 2001;12:243. doi: 10.1016/s1043-2760(01)00411-8. [DOI] [PubMed] [Google Scholar]
- 2.Montuschi P, Barnes PJ, Roberts LJ., 2nd Faseb J. 2004;18:1791. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 3.Morrow JD, Roberts LJ. Am J Respir Crit Care Med. 2002;166:S25. doi: 10.1164/rccm.2206011. [DOI] [PubMed] [Google Scholar]
- 4.Roberts LJ, Morrow JD. Free Radic Biol Med. 2000;28:505. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 5.Montine TJ, Montine KS, McMahan W, Markesbery WR, Quinn JF, Morrow JD. Antioxid Redox Signal. 2005;7:269. doi: 10.1089/ars.2005.7.269. [DOI] [PubMed] [Google Scholar]
- 6.Haschke M, Zhang YL, Kahle C, Klawitter J, Korecka M, Shaw LM, Christians U. Clin Chem. 2007;53:489. doi: 10.1373/clinchem.2006.078972. [DOI] [PubMed] [Google Scholar]
- 7.Lawson JA, Li H, Rokach J, Adiyaman M, Hwang SW, Khanapure SP, FitzGerald GA. J Biol Chem. 1998;273:29295. doi: 10.1074/jbc.273.45.29295. [DOI] [PubMed] [Google Scholar]
- 8.Pratico D, Barry OP, Lawson JA, Adiyaman M, Hwang SW, Khanapure SP, Iuliano L, Rokach J, FitzGerald GA. Proc Natl Acad Sci U S A. 1998;95:3449. doi: 10.1073/pnas.95.7.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walter MF, Blumberg JB, Dolnikowski GG, Handelman GJ. Anal Biochem. 2000;280:73. doi: 10.1006/abio.1999.4476. [DOI] [PubMed] [Google Scholar]
- 10.Tsukamoto H, Hishinuma T, Mikkaichi T, Nakamura H, Yamazaki T, Tomioka Y, Mizugaki M. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;774:205. doi: 10.1016/s1570-0232(02)00220-9. [DOI] [PubMed] [Google Scholar]
- 11.Wiswedel I, Hirsch D, Nourooz-Zadeh J, Flechsig A, Luck-Lambrecht A, Augustin W. Free Radic Res. 2002;36:1. doi: 10.1080/10715760210170. [DOI] [PubMed] [Google Scholar]
- 12.Signorini C, Comporti M, Giorgi G. J Mass Spectrom. 2003;38:1067. doi: 10.1002/jms.520. [DOI] [PubMed] [Google Scholar]
- 13.Tsikas D, Schwedhelm E, Suchy MT, Niemann J, Gutzki FM, Erpenbeck VJ, Hohlfeld JM, Surdacki A, Frolich JC. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;794:237. doi: 10.1016/s1570-0232(03)00457-4. [DOI] [PubMed] [Google Scholar]
- 14.Lee CY, Jenner AM, Halliwell B. Biochem Biophys Res Commun. 2004;320:696. doi: 10.1016/j.bbrc.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Morales CR, Terry ES, Zackert WE, Montine TJ, Morrow JD. Clin Chim Acta. 2001;314:93. doi: 10.1016/s0009-8981(01)00637-4. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Lawson JA, Reilly M, Adiyaman M, Hwang SW, Rokach J, FitzGerald GA. Proc Natl Acad Sci U S A. 1999;96:13381. doi: 10.1073/pnas.96.23.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murai Y, Hishinuma T, Suzuki N, Satoh J, Toyota T, Mizugaki M. Prostaglandins Other Lipid Mediat. 2000;62:173. doi: 10.1016/s0090-6980(00)00061-7. [DOI] [PubMed] [Google Scholar]
- 18.Ohashi N, Yoshikawa M. J Chromatogr B Biomed Sci Appl. 2000;746:17. doi: 10.1016/s0378-4347(00)00201-2. [DOI] [PubMed] [Google Scholar]
- 19.Ritov VB, Kelley DE, Kagan VE. Anal Biochem. 2002;311:10. doi: 10.1016/s0003-2697(02)00392-5. [DOI] [PubMed] [Google Scholar]
- 20.Bohnstedt KC, Karlberg B, Wahlund LO, Jonhagen ME, Basun H, Schmidt S. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;796:11. doi: 10.1016/s1570-0232(03)00600-7. [DOI] [PubMed] [Google Scholar]
- 21.Liang Y, Wei P, Duke RW, Reaven PD, Harman SM, Cutler RG, Heward CB. Free Radic Biol Med. 2003;34:409. doi: 10.1016/s0891-5849(02)01018-3. [DOI] [PubMed] [Google Scholar]
- 22.Winnik WM, Kitchin KT. Toxicol Appl Pharmacol. 2008;233:100. doi: 10.1016/j.taap.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Montuschi P, Ragazzoni E, Valente S, Corbo G, Mondino C, Ciappi G, Ciabattoni G. Inflamm Res. 2003;52:502. doi: 10.1007/s00011-003-1212-6. [DOI] [PubMed] [Google Scholar]
- 24.Kelso A, Korecka M, Shaw LM, Cock HR. Epilepsia. 2009;50:329. [Google Scholar]
- 25.Morrow JD, Roberts LJ., 2nd Methods Enzymol. 1999;300:3. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 26.FDA. 2001 http://www.fda.gov/cder/guidance.
- 27.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Anal Chem. 2003;75:3019. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 28.Annesley TM. Clin Chem. 2003;49:1041. doi: 10.1373/49.7.1041. [DOI] [PubMed] [Google Scholar]
- 29.Taylor PJ. Clin Biochem. 2005;38:328. doi: 10.1016/j.clinbiochem.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Shah VP, Midha KK, Findlay JW, Hill HM, Hulse JD, McGilveray IJ, McKay G, Miller KJ, Patnaik RN, Powell ML, Tonelli A, Viswanathan CT, Yacobi A. Pharm Res. 2000;17:1551. doi: 10.1023/a:1007669411738. [DOI] [PubMed] [Google Scholar]
- 31.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Anal Chem. 1998;70:882. doi: 10.1021/ac971078+. [DOI] [PubMed] [Google Scholar]
- 32.King R, Bonfiglio R, Fernandez-Metzler C, Miller-Stein C, Olah T. J Am Soc Mass Spectrom. 2000;11:942. doi: 10.1016/S1044-0305(00)00163-X. [DOI] [PubMed] [Google Scholar]
- 33.Mei H, Hsieh Y, Nardo C, Xu X, Wang S, Ng K, Korfmacher WA. Rapid Commun Mass Spectrom. 2003;17:97. doi: 10.1002/rcm.876. [DOI] [PubMed] [Google Scholar]