Abstract
Filaggrins are an important class of intermediate filament-associated proteins that interact with keratin intermediate filaments of terminally differentiating mammalian epidermis. They show wide species variations and their aberrant expression has been implicated in a number of keratinizing disorders. We have isolated a cDNA clone encoding human filaggrin and used this to demonstrate that the human gene encodes a polyprotein precursor containing numerous tandem filaggrin repeats. This structure is similar to that of mouse; however, the human filaggrin repeat is much longer (972 base pairs; 324 amino acids) and shows little sequence homology to the mouse protein. Also, data presented here reveal that the human filaggrin repeats show considerable sequence variations; such polymorphism is not found in the mouse. Furthermore, chromosomal mapping data revealed that the human gene is located at 1q21, indicating that the polymorphism is confined to a single locus. By peptide mapping, we define a short linker sequence within the human filaggrin repeat that is excised by proteolysis to yield functional molecules. Finally, we show by in situ hybridization that human filaggrin precursor gene expression is tightly regulated at the transcriptional level in terminally differentiating epidermis and that this represents a useful system in which to study intermediate filament-intermediate filament-associated protein interactions as well as disorders of keratinization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baden H. P., Roth S. I., Goldsmith L. A., Baden S. B., Lee L. D. Keratohyalin protein in disorders of keratinization. J Invest Dermatol. 1974 Apr;62(4):411–414. doi: 10.1111/1523-1747.ep12701666. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Gown A. M., Fleckman P., Kimball J. R., Resing K. A. Characterization of two monoclonal antibodies to human epidermal keratohyalin: reactivity with filaggrin and related proteins. J Invest Dermatol. 1987 Mar;88(3):306–313. doi: 10.1111/1523-1747.ep12466185. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Holbrook K. A., Kimball J. R., Hoff M., Sun T. T. Expression of epidermal keratins and filaggrin during human fetal skin development. J Cell Biol. 1985 Oct;101(4):1257–1269. doi: 10.1083/jcb.101.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale B. A., Holbrook K. A., Steinert P. M. Assembly of stratum corneum basic protein and keratin filaments in macrofibrils. Nature. 1978 Dec 14;276(5689):729–731. doi: 10.1038/276729a0. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Resing K. A., Lonsdale-Eccles J. D. Filaggrin: a keratin filament associated protein. Ann N Y Acad Sci. 1985;455:330–342. doi: 10.1111/j.1749-6632.1985.tb50420.x. [DOI] [PubMed] [Google Scholar]
- Fisher C., Haydock P. V., Dale B. A. Localization of profilaggrin mRNA in newborn rat skin by in situ hybridization. J Invest Dermatol. 1987 May;88(5):661–664. doi: 10.1111/1523-1747.ep12470281. [DOI] [PubMed] [Google Scholar]
- Franke W. W. Nuclear lamins and cytoplasmic intermediate filament proteins: a growing multigene family. Cell. 1987 Jan 16;48(1):3–4. doi: 10.1016/0092-8674(87)90345-x. [DOI] [PubMed] [Google Scholar]
- Goldman R., Goldman A., Green K., Jones J., Lieska N., Yang H. Y. Intermediate filaments: possible functions as cytoskeletal connecting links between the nucleus and the cell surface. Ann N Y Acad Sci. 1985;455:1–17. doi: 10.1111/j.1749-6632.1985.tb50400.x. [DOI] [PubMed] [Google Scholar]
- Harding C. R., Scott I. R. Histidine-rich proteins (filaggrins): structural and functional heterogeneity during epidermal differentiation. J Mol Biol. 1983 Nov 5;170(3):651–673. doi: 10.1016/s0022-2836(83)80126-0. [DOI] [PubMed] [Google Scholar]
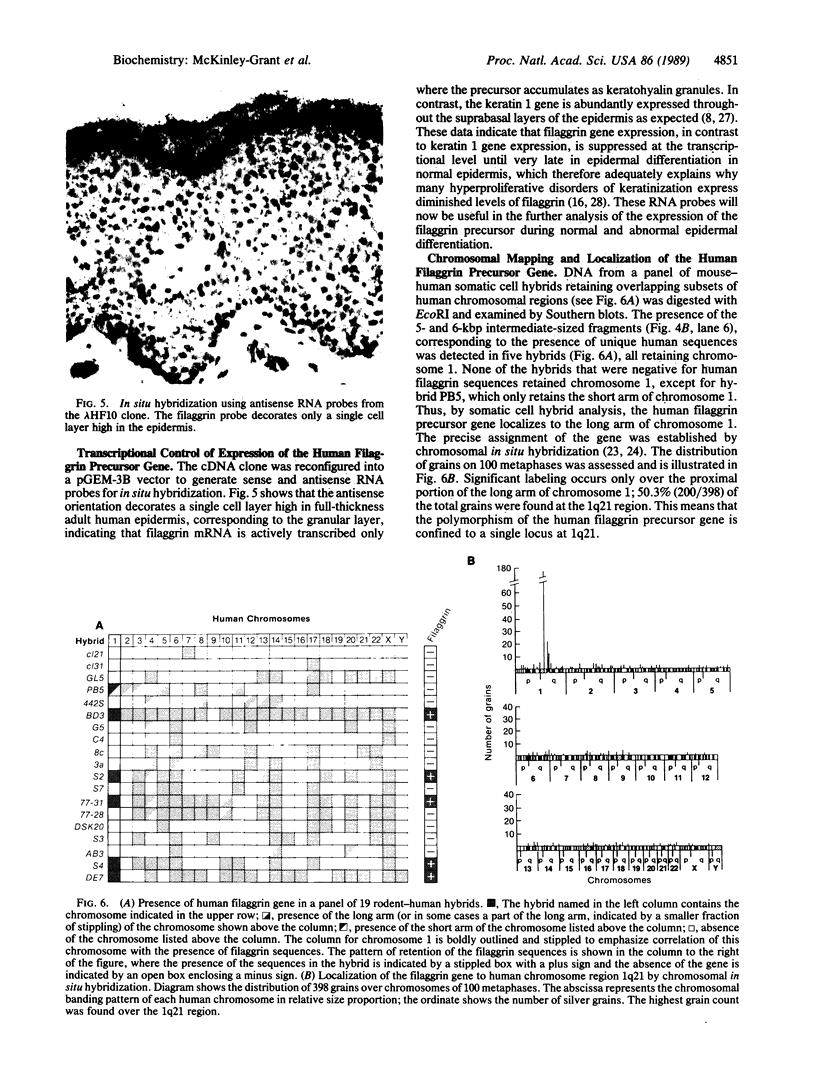
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Haydock P. V., Dale B. A. The repetitive structure of the profilaggrin gene as demonstrated using epidermal profilaggrin cDNA. J Biol Chem. 1986 Sep 25;261(27):12520–12525. [PubMed] [Google Scholar]
- Holbrook K. A., Dale B. A., Brown K. S. Abnormal epidermal keratinization in the repeated epilation mutant mouse. J Cell Biol. 1982 Feb;92(2):387–397. doi: 10.1083/jcb.92.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook K. A., Dale B. A., Sybert V. P., Sagebiel R. W. Epidermolytic hyperkeratosis: ultrastructure and biochemistry of skin and amniotic fluid cells from two affected fetuses and a newborn infant. J Invest Dermatol. 1983 Apr;80(4):222–227. doi: 10.1111/1523-1747.ep12534504. [DOI] [PubMed] [Google Scholar]
- Kubilus J., Scott I., Harding C. R., Yendle J., Kvedar J., Baden H. P. The occurrence of profilaggrin and its processing in cultured keratinocytes. J Invest Dermatol. 1985 Dec;85(6):513–517. doi: 10.1111/1523-1747.ep12277310. [DOI] [PubMed] [Google Scholar]
- Lessin S. R., Huebner K., Isobe M., Croce C. M., Steinert P. M. Chromosomal mapping of human keratin genes: evidence of non-linkage. J Invest Dermatol. 1988 Dec;91(6):572–578. doi: 10.1111/1523-1747.ep12477087. [DOI] [PubMed] [Google Scholar]
- Lynley A. M., Dale B. A. The characterization of human epidermal filaggrin. A histidine-rich, keratin filament-aggregating protein. Biochim Biophys Acta. 1983 Apr 14;744(1):28–35. doi: 10.1016/0167-4838(83)90336-9. [DOI] [PubMed] [Google Scholar]
- Resing K. A., Dale B. A., Walsh K. A. Multiple copies of phosphorylated filaggrin in epidermal profilaggrin demonstrated by analysis of tryptic peptides. Biochemistry. 1985 Jul 16;24(15):4167–4175. doi: 10.1021/bi00336a053. [DOI] [PubMed] [Google Scholar]
- Resing K. A., Walsh K. A., Dale B. A. Identification of two intermediates during processing of profilaggrin to filaggrin in neonatal mouse epidermis. J Cell Biol. 1984 Oct;99(4 Pt 1):1372–1378. doi: 10.1083/jcb.99.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop D. R., Lowy D. R., Tambourin P. E., Strickland J., Harper J. R., Balaschak M., Spangler E. F., Yuspa S. H. An activated Harvey ras oncogene produces benign tumours on mouse epidermal tissue. 1986 Oct 30-Nov 5Nature. 323(6091):822–824. doi: 10.1038/323822a0. [DOI] [PubMed] [Google Scholar]
- Rothnagel J. A., Mehrel T., Idler W. W., Roop D. R., Steinert P. M. The gene for mouse epidermal filaggrin precursor. Its partial characterization, expression, and sequence of a repeating filaggrin unit. J Biol Chem. 1987 Nov 15;262(32):15643–15648. [PubMed] [Google Scholar]
- Steinert P. M., Cantieri J. S., Teller D. C., Lonsdale-Eccles J. D., Dale B. A. Characterization of a class of cationic proteins that specifically interact with intermediate filaments. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4097–4101. doi: 10.1073/pnas.78.7.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Goldman R. D. Intermediate filaments of baby hamster kidney (BHK-21) cells and bovine epidermal keratinocytes have similar ultrastructures and subunit domain structures. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4534–4538. doi: 10.1073/pnas.77.8.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Parry D. A., Idler W. W., Johnson L. D., Steven A. C., Roop D. R. Amino acid sequences of mouse and human epidermal type II keratins of Mr 67,000 provide a systematic basis for the structural and functional diversity of the end domains of keratin intermediate filament subunits. J Biol Chem. 1985 Jun 10;260(11):7142–7149. [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Sybert V. P., Dale B. A., Holbrook K. A. Ichthyosis vulgaris: identification of a defect in synthesis of filaggrin correlated with an absence of keratohyaline granules. J Invest Dermatol. 1985 Mar;84(3):191–194. doi: 10.1111/1523-1747.ep12264813. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]