Abstract
Background
In this study, using a murine model of aortic allotransplantation, the role of blockade of signaling through CD28/B7 and CD40/CD40 ligand co-stimulatory pathways in the evolvement of posttransplant vasculopathy was examined.
Methods
Aortic allografts were transplanted across C57BL/10J (H2b)→C3H (H2k) strain combinations. Transient or more stable blockade of second signaling was achieved by either a single injection or multiple injections of CTLA4-Ig fusion protein (200 μg/dose i.p) and/or anti-CD40 ligand (CD40L) monoclonal antibody (250 μg i.m). At day 30 after transplantation, the grafts were harvested for histopathological and immunohistochemical examination.
Results
Similar to allografts of untreated animals, aortic allografts obtained from recipients treated with either CTLA4-Ig or anti-CD40L monoclonal antibody alone exhibited marked narrowing of the lumen primarily due to concentric intimal thickening caused by proliferation of α-smooth muscle actin-positive cells. Contemporaneous treatment, however, with either a single injection or multiple injections of CTLA4-Ig and anti-CD40L monoclonal antibody resulted in marked diminution of intimal thickening. Interestingly, concurrent prolonged inhibition of CD281B7 and CD401 CD40L pathways resulted in complete abrogation of the development of posttransplant arteriopathy.
Conclusion
These data suggest that a more stable disruption of signaling through costimulatory pathways may be required to obviate the development of posttransplant vasculopathy.
At the cellular interface, the costimulatory events that lead to optimal T-cell activation have been targeted for inhibition of this latter phenomenon (1, 2). In the realm of allotransplantation, the use of CTLA4-Ig fusion protein to block signaling through the CD28/B7 pathway has been shown to enhance allograft survival (3–5) and to prevent or reduce the changes due to chronic rejection (CR*) (3–6).
The recent demonstration of the expression of gp39 (CD40 ligand [CD40L]) on activated T cells and its function as a ligand for CD40 expressed on various antigen-presenting cells has unveiled another dominant costimulatory pathway for T- and B-cell activation (7). The role of signaling through the CD40/CD40L pathway in mediating acute allograft rejection in a fully disparate murine model of heterotopic cardiac transplantation has been documented (8, 9). Furthermore, similar to that of CD28/B7, a transient blockade of CD40/gp39 pathway by the use of monoclonal antibody (mAb) directed against the ligand for CD40 (MR-1) has been shown to enhance allograft survival (8, 9) but with minimal beneficial effect on posttransplant arteriopathy (10). Interestingly, the simultaneous blockage of signaling through the CD28/B7 and CD40/CD40L pathways by the contemporaneous use of CTLA4-Ig and MR-1 resulted not only in prolongation of skin and heart allograft survival but complete abrogation of the changes pathognomonic of CR in a vascularized model of heart allotransplantation (10).
Recognizing the importance of allogeneic immune responses in the etiopathology of CR, we have made an attempt to delineate the role of costimulatory molecules in the evolvement of posttransplant vasculopathy. For this purpose, in-bred 6- to 10-week-old male C57BL/10J (B10; H2b) and C3H (H2k) mice obtained from Jackson Laboratory (Bar Harbor, ME) were maintained in a specific pathogen-free facility with Purina rodent chow and tap water provided ad libitum and used at 10–12 weeks of age. Anti-CD40L (gp39; MR-1), a hamster mAb specific for murine gp39, was purchased from TSD Bioservice (Newark, DE). The human CTLA4-Ig fusion protein, which contains the extracellular domain of human CTLA4 and an immunoglobulin Cγ chain, was generously provided as a gift by Peter Linsley (Bristol-Myers Squibb, Inc., Seattle, WA). Isotype-matched control human IgG (L6) and hamster IgG were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). For detection of α-smooth muscle actin-positive (α-smA+) cells, mouse α-human mAb (IgG2a) was purchased from DAKO Corp. (Carpinteria, CA). Biotinylated horse α-mouse IgG was purchased from Vector Laboratories, Inc. (Burlingame, CA). The ABC immunoperoxidase staining kit (VECTASTIN) was obtained from Vector. The chromogen 3-amino-9-ethylcarbazol was acquired from ScyTek Laboratories, Inc. (Logan, UT).
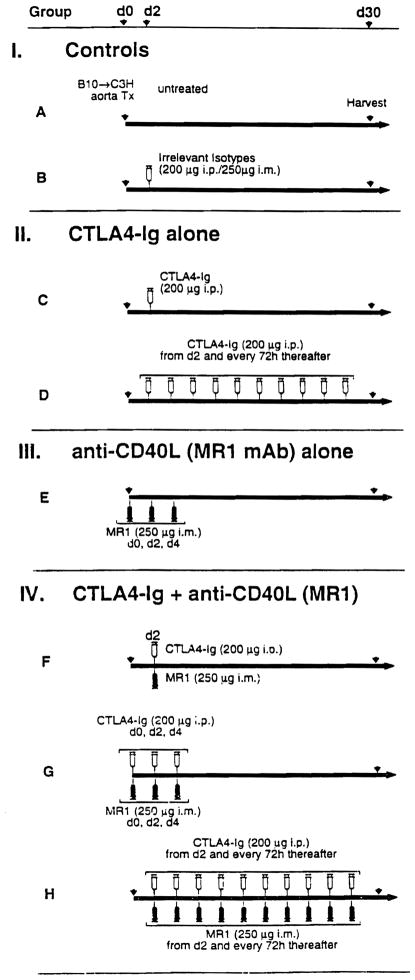
With inhalation anesthesia using methoxyflurane (Metofane; Pitman-Moore, Inc., Mundelein, IL) and with the aid of a dissection microscope, aortic transplantation (AOTx) was performed (11). Briefly, a 6- to 9-mm segment of the descending part of the donor’s thoracic aorta (AO) was harvested and anastomosed (end to side) to the recipient’s abdominal AO. The native abdominal AO was then ligated and severed, thereby converting an end-to-side anastomosis to a quasi-end-to-end anastomosis. The recipients (C3H) of aortic allografts from B10 donors were divided into eight groups (Fig. 1). CTLA4-Ig fusion protein was used at a dose of 200 μg i.p and anti-gp39 (MR-1) at 250 μg i.m. Each group comprised five to seven animals. Group A was untreated. In group B, animals were given a single dose of isotype-matched human IgG (L6, 200 μg i.p) and hamster IgG (250 μg i.m) on day 2 after transplantation. Group C animals were given a single injection of CTLA4-Ig on day 2 after transplantation. In group D, 10 doses of CTLA4-Ig were given starting on day 2 after transplantation and every 72 hr thereafter. In group E, animals were given three doses of anti-gp39 on days 0, 2, and 4 after transplantation. In group F, a single dose of both CTLA4-Ig and MR-1 was given on day 2 after transplantation. In group G, animals were given three doses of both CTLA4-Ig and MR-1 on days 0, 2, and 4 after transplantation. In group H, animals were given 10 doses of both CTLA4-Ig and anti-gp39 mAb starting on day 2 after transplantation and every 72 hr thereafter. AOTx across untreated syngeneic (C3H→C3H) animals served as an additional control. Allografts were harvested on day 30 after transplantation, when distinctive changes of CR are most evident (12).
Figure 1.
Schematic representation of various protocols used to treat B10→C3H aortic allotransplant recipients with CTLA4-Ig fusion protein and/or anti CD40L mAb. Untreated recipients and those treated with the isotype-matched irrelevant mAb served as controls. For details see Materials and Methods.
At day 30 after AOTx, with the animals under anesthesia, the aortic allografts were retrieved, fixed for 48 to 72 hr in 10% buffered formalin, and sectioned (4 μm thick) using a microtome. In addition to hematoxylin and eosin staining (Surgipath, Richmond, IL), Verhoeff-van Gieson (elastic fibers) and Masson’s trichrome (collagen) staining was also performed. Additionally, the presence of α-smA+ cells was determined using a previously described method (12). Briefly, after hydration, endogenous-peroxidase activity was blocked by treating the sections for 45 min with 0.6% solution containing 71% methanol, 24% dH2O, and 5% H2O 2 After two washings, nonspecific binding was blocked by a 20-min incubation in Lipshaw Universal Protein Blocker and then a 45-min incubation with mouse α-human mAb directed against α-smA+ cells; the sections were then washed two times with phosphate-buffered saline. Biotinylated horse α-mouse IgG was used to identify the primary antibody, and after two washings, the sections were incubated for 30 min with avidin-biotin complex (Vector). Coloration was developed using 3-amino-9-ethylcarbazol, and sections were washed twice with phosphate-buffered saline and then counterstained with hematoxylin.
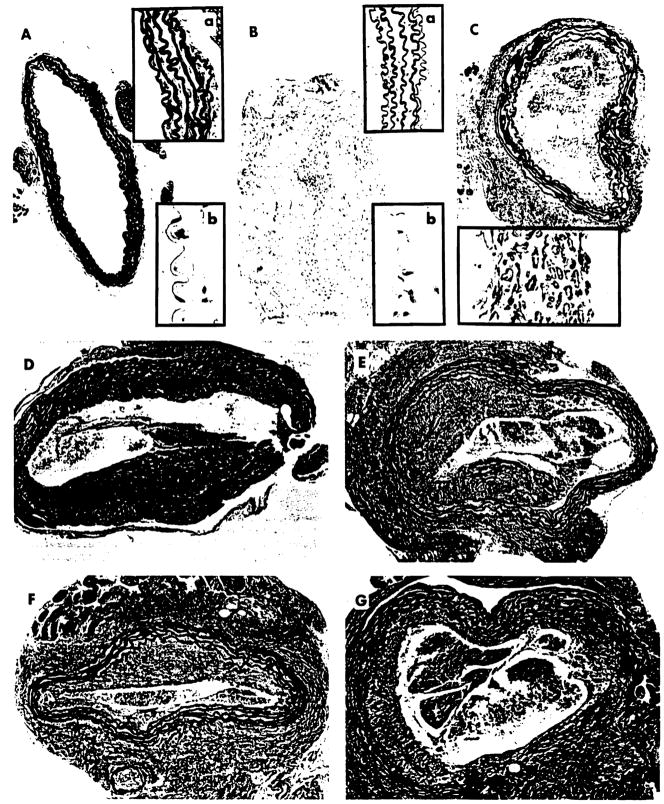
As reported previously (11, 12), aortic allografts obtained at day 30 after transplantation from untreated recipients (group A) exhibited changes characteristic of CR (Fig. 2C). The diffuse intimal thickening, which involved the entire circumference of the vessel, was largely due to proliferation of α-smA+ cells (Fig. 2C, inset). Patchy destruction of the internal elastic membrane was also distinct, as was the intimal deposition of fibrous tissue and collagen (Fig. 2C). In contrast, syngeneic aortic grafts (C3H→C3H) retained normal morphology (Fig. 2B) and were virtually indistinguishable from native AO grafts (Fig. 2A). Furthermore, the histopathological abnormalities observed in irrelevant isotype-matched, mAb-treated C3H recipients of B10 AO (Fig. 1, group B) harvested at day 30 after transplantation (Fig. 2D) were very similar to those of allografts from untreated animals (Fig. 2C).
Figure 2.
Histopathological and immunohistochemical staining of AO obtained at day 30 after transplantation from B10→C3H recipients receiving CTLA4-Ig and/or MR-1. The hematoxylin and eosin-stained section of the native donor (B10) AO exhibited normal morphology (Ab inset); staining for elastic fibers using Verhoeff-van Gieson’s stain suggested that the internal elastic membrane was intact (A and Aa inset). Similar observations have also been made after hematoxylin and eosin staining of transplanted AO obtained from syngeneic (C3H→C3H) animals (B and Bb inset); staining with Verhoeff-van Gieson’s stain showed unimpaired internal elastic membrane (Ba). Intimal thickening with disruption of internal elastic membrane was evident in untreated C3H recipients of B10 AO stained with Verhoeff-van Gieson’s stain (C). The intimal thickening was largely due to proliferation of α-smA+ cells (C, inset). The treatment of aortic allograft; recipients with irrelevant isotype-matched mAb did not mitigate the changes characteristic of CR, as were evident in Verhoeff-van Gieson-stained sections (D). Similarly, Verhoeff-van Gieson-stained sections of the aortas obtained from animals treated with either single (E) or multiple (10) doses (F) of CTLA4-Ig (200 μg/dose i.p.) showed no amelioration of posttransplant arteriopathy. This observation was also made after treatment of recipients with three doses of MR-1, as depicted in Verhoeff-van Gieson-stained sections of aortic allografts (G). Original magnification: ×100. Insets: Aa and Ba, ×400; Ab, Bb, and C, ×1000.
Blockade of signaling through the CD28/B7 pathway by administration of a single dose of CTLA4-Ig given at day 2 after AOTx (Fig. 1, group C) failed to abrogate the development of CR (Fig. 2E). Concentric intimal thickening with disruption of internal elastic limiting membrane was evident in these aortas at day 30 after transplantation. The magnitude of these changes was comparable to changes witnessed in untreated recipients of AOTx (Fig. 2C) and in animals receiving irrelevant mAb (Fig. 2D). Of further importance is the observation that the morphology of the aortic allografts procured from recipients undergoing treatment with 10 consecutive doses of CTLA4-Ig (Fig. 1, group D) was indistinguishable (Fig. 2F) from that of untreated animals (Fig. 2C) and animals treated with isotype-matched irrelevant mAb (Fig. 2D).
Since the transient or more stable blockage of the CD28/B7 pathway did not abrogate the development of CR, we proceeded to ascertain whether interruption of signaling through the CD40/CD40L pathway by the use of α-CD40L mAb (MR-1) alone (Fig. 1, group E) would have any effect on posttransplant vasculopathy. Toward the achievement of this goal, three doses of MR-1 were injected at days 2, 4, and 6 after AOTx. Aortic allografts obtained at day 30 after transplantation revealed the presence of intimal thickening with patchy destruction of internal elastic membrane (Fig. 2G). These changes were comparable to those observed in untreated animals (Fig. 2C) and in animals treated with either irrelevant mAb (Fig. 2D) or with CTLA4-Ig fusion protein (Fig. 2, E and F).
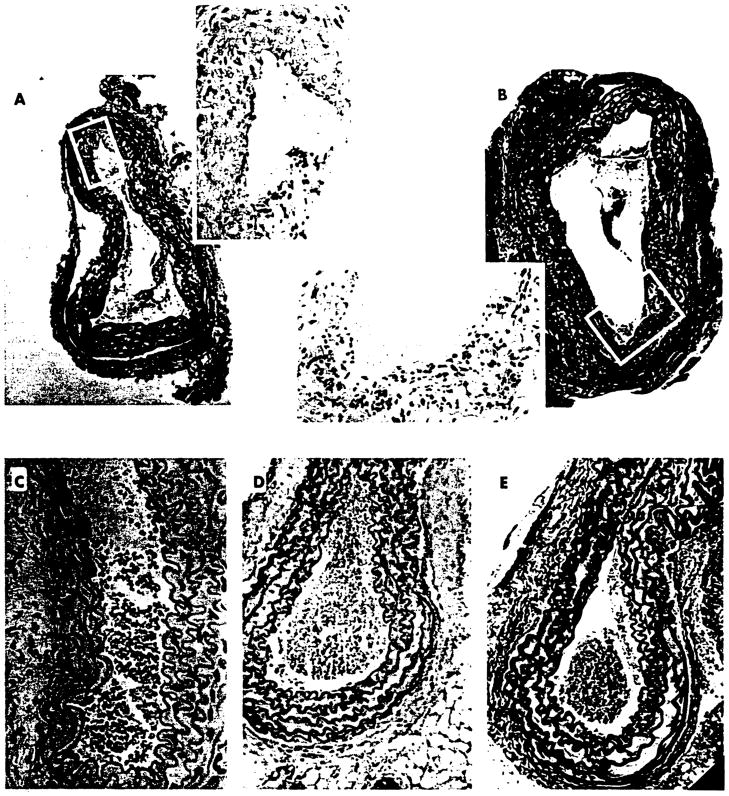
Perioperative concomitant blockade of both the CD28/B7 and the CD40/CD40L pathways by the use of CTLA4-Ig and MR-1, respectively, has previously been shown to prevent the development of CR in a vascularized heart model of allotransplantation in mice (10). To study the corollary of such a treatment on the evolvement of posttransplant arteriopathy in this model, we proceeded to block both costimulatory pathways by treating the aortic allograft recipients with a single dose of CTLA4-Ig and MR-1 (Fig. 1, group F). Unlike the results observed after discrete blockade of the costimulatory pathways, treatment with the latter protocol resulted in a marked decrease in intimal thickening (Fig. 3A, inset); this was in contrast to the diffuse and concentric thickening seen in aortic allografts of untreated animals (Fig. 2C) and in animals treated with irrelevant mAb (Fig. 2D). Despite diminution of intimal thickening, there was still some evidence of residual endothelial damage and minimal (albeit less than that observed in untreated allograft recipients) disruption of the internal elastic limiting membrane (Fig. 3A).
Figure 3.
Microscopic examination of AO obtained at day 30 after transplantation from B10→+C3H recipients in which signaling through both the CD28/B7 and CD40/gp39 pathways was concurrently blocked. Unlike discrete blockage of costimulatory pathways, combined simultaneous treatment with a single dose of both CTLA4-Ig and MR-1 resulted in marked reduction of intimal thickening (A; Verhoeff-van Gieson’s stain), which was patchy and eccentric (A, inset, hematoxylin and eosin). Further amelioration in changes distinctive of CR were evident after three doses of CTLA4-Ig and MR-1 (8; Verhoeff-van Gieson’s stain); nevertheless, minimal endothelial denudation (B, inset; hematoxylin and eosin) was discernible. It is noteworthy that in the majority of the animals, complete abrogation of CR was observed after treatment with 10 doses of CTLA4-Ig and MR-1, as delineated in hematoxylin and eosin (C); Verhoeff-van Gieson (D)- and α-smA+ (E)-stained sections of allogeneic aortas harvested on day 30 after transplantation. Original magnification: A and B, ×100; C, ×400; D and E, ×200; insets, ×400.
In contrast to previous reports (10), treatment with three consecutive doses of CTLA4-Ig and MR-1 (Fig. 1, group G) did not result in complete abrogation of CR (Fig. 3B). However, intimal thickening was markedly reduced with some evidence of endothelial denudation (Fig. 3B, inset). It must be emphasized that these morphological aberrations were less prominent than those observed after a single treatment with CTLA4-Ig and MR-1 (Fig. 3A), which suggests that perhaps a relatively longer blockade of costimulatory pathways may be required to completely abrogate the development of CR.
To test the latter tenet, we proceeded to contemporaneously block signaling through the CD28/B7 and CD40/CD40L pathways by administering 10 consecutive doses ofCTLA4-Ig and anti-gp39 (Fig. 1, group H). Interestingly, the majority of these aortic allografts obtained 30 days after transplantation retained normal morphology (Fig. 3, C, D, and E) indistinguishable from that of native aortas (Fig. 2A). This observation was reminiscent of our previously published data in which prior induction of liver-induced, donor-specific tolerance also abrogated the development of CR (12).
Taken together, these data provide unequivocal evidence for the role for costimulatory molecules in the pathogenesis of posttransplant arteriosclerosis. Furthermore, these data also suggest that mitigation of acute cellular events by transient blockage of signaling through the costimulatory pathway may not provide optimal protection against the development of CR, necessitating a more stable interruption of signaling between antigen-presenting cells and alloreactive T cells. Refinement of this approach for its ultimate clinical application for either prevention or reversal/mitigation of established CR is the objective toward which all future endeavors have converged.
Acknowledgments
The authors acknowledge the assistance of Jo Harnaha and Margie Sigafoos in preparation of the manuscript and Alla Subbotin, M.D., Ph.D. (Department of Pathology, University of Pittsburgh Medical Center), for her expert technical assistance in staining the sections.
Footnotes
Presented at the 16th Annual Meeting of the American Society of Transplant Physicians, May 11–14, 1997, Chicago, IL.
This study was supported by Project Grant DK 29961 from the National Institute of Health, Bethesda, Maryland.
Abbreviations: α-smA+, α-smooth muscle actin-positive cells; AO, aorta; AOTx, aortic transplantation; CD40L, CD40 ligand; CR, chronic rejection; mAb, monoclonal antibody.
References
- 1.Stüber E, Strober W, Neurath W. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996;183:693. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sayegh MH, Akalin E, Hancock WW, et al. CD28–B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J Exp Med. 1995;181:1869. doi: 10.1084/jem.181.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson TC, Alexander DZ, Winn KJ, Linsley PS, Lowry RP, Larsen CP. Transplantation tolerance induced by CTLA4-Ig. Transplantation. 1994;57:1701. [PubMed] [Google Scholar]
- 4.Azuma H, Chandraker A, Nadeau K, et al. Blockade of T cell costimulation prevents development of experimental chronic renal allograft rejection. Proc Natl Acad Sci USA. 1996;93:12439. doi: 10.1073/pnas.93.22.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akalin E, Chandraker A, Russell ME, Turka LA, Hancock WW, Sayegh MH. CD28–B7 cell costimulatory blockade by CTLA4-Ig in the rat renal allograft model. Transplantation. 1996;62:1942. doi: 10.1097/00007890-199612270-00047. [DOI] [PubMed] [Google Scholar]
- 6.Russell ME, Hancock WW, Akalin E, et al. Chronic cardiac rejection in the LEW to F344 rat model. J Clin Invest. 1996;97:833. doi: 10.1172/JCI118483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armitage RJ, Fanslow WC, Strockbine L, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 8.Larsen CP, Alexander DZ, Hollenbaugh D, et al. CD40-gp39 interactions play a critical role during allograft rejection. Transplantation. 1996;61:4. doi: 10.1097/00007890-199601150-00002. [DOI] [PubMed] [Google Scholar]
- 9.Hancock WW, Sayegh MH, Zheng XG, Peach R, Linsley PS, Turka LA. Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc Natl Acad Sci USA. 1996;93:13967. doi: 10.1073/pnas.93.24.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 11.Sun H, Valdivia LA, Subbotin V, et al. An improved surgical technique for the establishment of a murine model of aortic transplantation. Microsurgery. 1996:17. doi: 10.1002/(sici)1098-2752(1998)18:6<368::aid-micr5>3.0.co;2-f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subbotin VM, Sun H, Aitouche A, et al. Abrogation of chronic rejection in a murine model of aortic allotransplantation by prior induction of donor-specific tolerance. Transplantation. 1997;64:1. doi: 10.1097/00007890-199709150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]