Abstract
The medial temporal lobe includes a system of anatomically connected structures that are essential for declarative memory (conscious memory for facts and events). A prominent form of declarative memory is recognition memory (the ability to identify a recently encountered item as familiar). Recognition memory has been frequently assessed in humans and in the experimental animal. This article traces the successful development of an animal model of human medial temporal lobe amnesia, which eventually identified the structures in the medial temporal lobe important for memory. Attention is given to two prominent behavioral paradigms (delayed nonmatching to sample and tests of spontaneous novelty preference).
1. Introduction
In 1899, at a medical meeting in St. Petersburg, Bekhterev (1900) presented the brain of a patient who had exhibited striking memory problems as the most significant clinical symptom. The primary brain pathology was noted to be bilateral softening of the hippocampus and medial temporal cortex. During the following decades a few clinical case studies also suggested a connection between memory impairment and medial temporal lobe damage (Glees & Griffith, 1952; Grünthal, 1947; Hegglin, 1953). Yet a clear connection between memory and medial temporal lobe function would not be achieved until findings were reported for the noted amnesic patient H.M. (Scoville & Milner, 1957).
2. H.M. and the modern era of memory research
The modern era of memory research began with the description of patient H.M. by William Beecher Scoville and Brenda Milner (Scoville & Milner, 1957; Squire, 2009). H.M. had an extensive history of minor and major seizures that were unresponsive to antiepileptic medication. He had minor seizures beginning at 10 years of age, and major seizures began to appear when he was 16. The major seizures occurred without warning as generalized convulsions that involved loss of consciousness followed by prolonged periods of sleep. Despite high doses of medication, the major attacks increased in severity and frequency until eventually he was unable to work or lead a normal life. A decision was then made with the consent of the family to attempt to relieve the seizures through an experimental surgical intervention. On September 1, 1953 at Hartford Hospital in Hartford Connecticut, William Scoville removed H.M.’s medial temporal lobes bilaterally. The surgery involved tissue removal through a supra-orbital trephine with a fine suction tube and attached cautery while the frontal lobe was carefully retracted. The lesion was designed to extend posteriorly for a distance of 8 cm from the tips of the temporal lobes, with the temporal horns constituting the lateral edges of resection. Subsequently, MRI scans showed that the lesion was bilaterally symmetrical and included the medial temporal polar cortex, virtually all of the entorhinal cortex and amygdaloid complex and the anterior half of the intraventricular aspect of the hippocampal formation (i.e., dentate gyrus, hippocampus, and subicular complex). The perirhinal cortex was substantially damaged as well, with some sparing of its ventrocaudal aspect. The rostrocaudal extent of the ablation was approximately 5.4 cm on the left and 5.1 cm on the right (Corkin, Amaral, Gonzalez, Johnson, & Hyman, 1997).
The surgery succeeded in that it reduced the frequency and severity of the seizures. However, H.M. was left with profound amnesia. Although a number of patients had undergone similar removals prior to H.M., those surgeries were performed in an attempt to relieve severe psychosis rather than to relieve seizures. Because the psychosis remained severe in those patients following surgery, the memory problems that must have resulted from the medial temporal lobe resection were not appreciated. For H.M. a devastating memory impairment was readily apparent immediately after surgery (Scoville, 1954).
The subsequent systematic evaluation of H.M. and other patients with similar damage established three fundamental principles. First, memory is a distinct cerebral ability that is separate from other cognitive functions like perception, intelligence, personality and motivation. Second, only long-term memory is disrupted, because information can be maintained and utilized for a short time in immediate memory (and working memory). Third, medial temporal lobe structures are not the ultimate repository of long-term memory because remote memory remains largely intact.
At the time that H.M. was first described, the anatomy of the medial temporal lobe was poorly understood, and it was not known what specific damage (within the large region included in his surgery) was responsible for H.M.’s memory impairment. Accordingly, efforts began to address this question in an animal model of H.M.’s memory impairment.
3. Efforts to develop an animal model of medial temporal lobe amnesia
The findings from H.M. were initially met with some skepticism, especially because early efforts to replicate his memory deficit in animals were unsuccessful. These efforts in fact began almost immediately when Scoville himself came to Montreal and did the same surgery in monkeys that he had done with H.M. (e.g., Correll & Scoville, 1960, 1965). However, these monkeys and others with medial temporal lesions were able to learn tasks that seemed similar to tasks that H.M. could not learn. For example, H.M. was severely impaired on a delayed paired comparison technique consisting of presenting two visual stimuli in succession separated by a short time interval (Milner, 1972). This observation and others suggested that the delay between stimulus presentations was critical and sufficient for observing a memory impairment. However, when monkeys with medial temporal lobe lesions were tested on visual discrimination problems that were designed to approximate the tests given to H.M., the monkeys performed normally (Orbach, Milner, & Rasmussen, 1960). This was true even when long delays were inserted between trials so that the animals could be distracted during the inter-trial interval (Orbach et al., 1960). The difficulty was that it was not yet appreciated that humans and experimental animals can approach ostensibly similar tasks using different strategies. For example, monkeys learn visual discrimination tasks gradually over many trials in a fashion that is now referred to as habit learning. In the monkey, this kind of learning depends on the basal ganglia, not the medial temporal lobe (Mishkin, Malamut, & Bachevalier, 1984; Teng, Stefanacci, Squire, & Zola, 2000).
In the decade that followed H.M.’s initial description, extensive work in the rat and other experimental animals with hippocampal lesions also failed to produce H.M.’s impairment profile. For example, rats with hippocampal lesions, like monkeys, performed normally on simple visual discrimination problems (Kimble, 1963). Lesioned rats were also unimpaired at learning to bar press for food (Clark & Isaacson, 1965; Schmaltz & Isaacson, 1966) and on various forms of active shock avoidance tasks (e.g. Isaacson, Douglas, & Moore, 1961; Kimura, 1958). Yet consistent impairments were observed in passive avoidance tasks (Isaacson & Wickelgren, 1962; Kimble, Kirkby, & Stein, 1966; Snyder & Isaacson, 1965). In addition to the passive avoidance deficits, hippocampal ablations had a profound effect on reversal training, even when the pre-reversal response was readily acquired (Douglas & Pribram, 1966; Kimble & Kimble, 1965; Niki, 1966; Teitelbaum, 1964; Thompson & Langer, 1963; Webster & Voneida, 1964). Further, rats with hippocampal lesions were consistently found to be highly resistant to extinction (Isaacson et al., 1961; Jarrard, Isaacson, & Wickelgren, 1964; Niki, 1962, 1965; Raphelson, Isaacson, & Douglas, 1966).
These patterns of impairment led to the idea that hippocampal lesions impair inhibitory processes. These inhibitory processes could be internal (where a response is actively inhibited) or external (where the processing of irrelevant sensory information is inhibited). Even the hippocampal deficits in maze learning, which were well known by this time, tended to be explained as a failure to inhibit repeated entries into blind alleys (e.g., Kimble, 1963).
It was clear from work during the 1960s that the behavioral impairments observed in rats with hippocampus lesions did not provide an adequate description of memory impairment in humans with hippocampal damage. Accordingly, researchers were less likely to relate their work to human studies of memory-impaired patients and more likely to interpret their findings within the framework of response inhibition as first outlined by Pavlov (1927). In fact, in a review of the literature on the hippocampus and behavior, published a decade after the initial description of H.M., Robert Douglas noted:
“Hippocampal lesions obviously do not impair learning in general, even when the learning involves retention for long periods of time. Thus, the animal and human data would appear to be in contradiction. This contradiction could be ‘resolved’ by postulating that the hippocampus has a different basic function in man and beast. Such a solution to this problem is generally unacceptable to physiological psychologists, however. Another possible resolution of this paradox is that the recent memory loss in man is a secondary effect of a different type of primary disorder. The author has chosen the latter course, and suggests that the recent memory loss in man is a genuine phenomenon, but that it is a byproduct of interference during storage and not due to a lack of ability to store, per se”
(Douglas, 1967, p. 424).
This observation made clear that a large animal literature was substantially out of register with the human work and that experimentalists were beginning to doubt the basic assertion that medial temporal lobe damage produces a primary memory impairment.
4. Multiple memory systems
A major difficultly during the 1960s and 1970s was that it was not appreciated that tasks could be supported by different brain systems. Many of the tasks given to animals with hippocampal lesions were in fact skill-based tasks that amnesic patients would have been able to acquire, or they were tasks that animals could learn as a skill even if humans tended to learn the task by consciously memorizing the material. Establishing an animal model would require developing tasks that assess the type of memory impaired in amnesia.
The kind of memory impaired in amnesia in now typically referred to as declarative memory (Cohen & Squire, 1980). Declarative memory provides conscious access to facts and events and is impaired when structures of the medial temporal lobe are damaged. Nondeclarative memory is an umbrella term that includes various acquired skills and abilities that are not accessible to conscious knowledge but are expressed through performance and depend on different brain systems (Squire, 2004). Nondeclarative memory is independent of the medial temporal lobe.
5. One-trial memory tests and the successful development of an animal model
A key advance in establishing a model of human medial temporal lobe amnesia was the implementation of one-trial memory tests for the monkey that assess what one would now call declarative memory. In 1974, David Gaffan suggested that many tests of memory in animals with hippocampal damage might not be similar to the tests that reveal memory impairment in amnesic patients. Accordingly, if one wants to relate the animal work to work in humans it is not adequate to use any convenient test in which the animal must use memory. Rather one must use “specifically designed animal analogs of those tests that do reveal impairment in human amnesiacs.” (Gaffan, 1974, p. 1101).
One of the tests that Gaffan used was a one-trial test of visual recognition memory, which was refined for use in monkeys by Benjamin Weinstein (Weinstein, 1941) while working in Harry Harlow’s laboratory (earlier animal versions of this method appeared in the Russian literature as early as 1923; cited in Weinstein, 1941). In this test (initially referred to as “matching-from-sample delayed reaction procedure”), monkeys were presented with a single object that they displaced for a food reward (the sample phase). Memory for the sample object was then tested by presenting the sample object together with a new object (choice phase). The monkey was trained to select (i.e., match) the object presented previously during the sample phase. In the Gaffan study (1974), unique objects were used on each trial so that successful judgment of familiarity was sufficient to identify the correct object. This testing protocol involved one-trial learning and in this sense was analogous to the yes/no recognition tests often used in studies of human memory. In addition, increasing the time between the sample and choice phase increased the demand on memory. This task became known as the “delayed matching to sample” task (DMS). Importantly, monkeys with fornix lesions exhibited a pronounced delay-dependent memory impairment. They performed normally at a delay of 10 s and were impaired at the two longer delays of 70 and 130 s (Gaffan, 1974). Gaffan concluded “the present experiments are not primarily concerned with …the question of whether a deficit …is itself a secondary effect of defective inhibitory processes …[the] results justify …only the limited conclusion that fornix transection in monkeys causes a loss of recognition memory closely comparable to that seen in human amnesiacs” (Gaffan, 1974, p. 1109). In this study fornix lesions were studied because they disrupt hippocampal function while preserving extrahippocampal structures. Nonetheless, the lesions differed considerably from the damage sustained by H.M.
The year 1978 marked an important turning point. Mortimer Mishkin trained twelve monkeys on a version of the task described by Gaffan (1974), but in this case the monkeys were trained to select the new object during the choice phase rather the familiar object (Mishkin, 1978). This modification exploited the monkey’s natural tendency to select the novel object, which meant that animals learned this task (delayed nonmatching to sample; DNMS) in about a third of the time needed to learn the matching to sample task (Mishkin & Delacour, 1975; Mishkin, 1978). Following training, the monkeys were given lesions designed to mimic the damage sustained by H.M. (hippocampus plus amygdala, together with the cortex that underlies these two structures) or smaller lesions of the hippocampus (and underlying cortex) or amygdala (and underlying cortex). (Note that the cortical areas damaged by the surgery were not specifically targeted but rather were damaged during the surgical approach to the hippocampus and amygdala). Postoperatively the animals reacquired the nonmatching rule, and then the delay between the sample and choice phase was increased progressively from 10 to 30, 60 and 120 s. In this study, hippocampal or amygdala lesions (and underlying cortical areas) yielded only a mild impairment, but the combined lesion (hippocampus plus amygdala and underlying cortex) produced a marked deficit, especially at the longer delays. This study and subsequent studies, which relied especially on the trial-unique DNMS task (Mishkin, 1982; Squire & Zola-Morgan, 1983; Zola-Morgan, Squire, & Mishkin, 1982), document the successful establishment of an animal model of human medial temporal lobe amnesia in the monkey. At the time, the impairment was interpreted as depending on the combined hippocampus and amygdala damage (Mishkin, 1978). Subsequent work would reveal that the cortical areas damaged during the approach to the hippocampus and amygdala were critically important for memory function and that the amygdala itself was not critical (see below).
The DNMS task with trial-unique stimuli was also explicitly adapted for use with rats so as to closely mimic the key features of the monkey version (Mumby, Pinel, & Wood, 1990). Although earlier work had established the principle that rats could learn a nonmatching rule (e.g. Aggleton, 1985; Rothblat & Hayes, 1987), this new task had the advantage of using trial-unique objects that could be displaced to receive food rewards just as in the monkey task (Mumby et al., 1990). Additionally, the apparatus was designed so as to allow the delay between the sample and choice phase to be as short as 4 s (shorter than the 8–10 s delay used in the monkey). This feature of the task made training the nonmatching rule in the rat more efficient. Finally, the delay interval could be imposed without handling the rat during the delay interval or between trials. This reduced distraction and allowed performance across delays to be comparable to what was achieved in the monkey across similar delays (Mumby et al., 1990). Subsequent work using this task demonstrated that lesions of the hippocampus or the cortical regions near the hippocampus produced a delay-dependent memory impairment similar to what had been observed in the monkey (Mumby & Pinel, 1994), and consistent with the memory impairment seen in H.M. This new task successfully extended the animal model of medial temporal lobe amnesia to the rat. Subsequent work demonstrated that the impairment in animals with hippocampal lesions was unambiguously a delay-dependant memory impairment and not an artifact of the training protocols typically used for monkeys and rats (Clark, West, Zola, & Squire, 2001). Ordinarily, animals receive many more trials at the short delay that is used to train the nonmatching rule than at any other delay. In this study, animals were given even more practice at the longer delay than they had been given at the shortest delay. Nevertheless, despite the extended training, the performance of rats with hippocampal lesions remained impaired at the long delay and remained intact at the short (4-s) delay. This finding ruled out the possibility that good performance was observed at the short delays because extensive training was given at these delays.
The demonstration of delay-dependent impairments in performance has been critical for identifying the impairment as a memory impairment. When a brain lesion spares performance at short delays (when the demand on memory is small) and impairs performance selectively at longer delays (when the demand on memory is larger), this finding rules out a variety of alternative explanations for the impairment (e.g., including the ability to perceptually recognize objects, motivational changes, stress responses, circadian influences, and secondary effects of the lesion including hyper-activity, increased distractibility, motor impairments, and other non-specific effects).
6. The emergence of spontaneous novelty preference tasks (and an easy way to test recognition memory)
Coincidentally, at about the same time that H.M. was first described in 1957, the seeds were being planted for an important new behavioral test for visual recognition memory in the experimental animal. This paradigm would eventually become the most frequently used test of recognition memory in the experimental animal and an important tool for studying MTL amnesia.
In 1956 Robert Frantz described a method to study early visual development in animals (Frantz, 1956). The method had its genesis in his doctoral dissertation work on object preference and pattern vision in newly hatched chicks. Frantz reasoned that, if chicks consistently exhibited a preference for one stimulus over another, it could be inferred that the chick had the visual ability to resolve the differences between the two stimuli. He then went on to show in the infant chimpanzee that these preferences could be measured by observing the differential ocular fixation of the animal (Frantz, 1956). Building on this work, Joseph Fagan reasoned that, while differential fixation to one stimulus over the other is an index of successful perception and discrimination, when differential fixation occurs in a comparison between a novel and a previously exposed target, information about the target must have been successfully acquired and remembered. On the basis of this insight, Fagan developed a paradigm for the human infant whereby two identical stimuli were presented side by side for a period of time (e.g., 1–2 min). Then immediately following presentation, the infant was presented with the recently viewed stimulus and a novel stimulus. In this paradigm, the infants preferentially viewed the novel stimulus, presumably because they remembered the familiar and now less interesting stimulus. Fagan further showed that this preferential viewing of the novel stimulus was present in infants (3–6 months of age) even when a delay of 2 h was interposed before testing of preference (Fagan, 1970). This work established the “visual paired-comparison” task as a reliable test of visual recognition memory. The task takes advantage of an innate or spontaneous preference for novelty (which is preserved across mammalian species), and it has the advantage of not requiring any verbal instruction or rule learning. This feature makes the task an excellent tool for studying preverbal human infants and experimental animals.
The visual paired-comparison test was subsequently adapted for both the monkey (Gunderson & Sackett, 1984) and the rodent (Ennaceur & Delacour, 1988). Note that when used with primates, this task is generally referred to as the visual paired-comparison task (VPC), because it is a test of visual recognition memory. In the rat however, the animal is allowed to physically explore the objects. Visual, olfactory, as well as tactile information is available and could be used to guide performance during the retention test. Accordingly, in the rodent this test is typically referred to with more general descriptors, such as “novel object recognition,” “novel object preference,” “spontaneous object preference”, or “spontaneous object recognition.” Here the term novel object recognition (NOR) is used to describe the rodent version of this test.
Although the VPC task provides a straightforward and simple test of recognition memory, it was not immediately apparent that it would provide a useful tool for studying MTL amnesia in the same way that the DNMS task had been useful. While both the VPC and DNMS tasks could indicate whether an animal retained information about a previously encountered item, it was not initially known whether these two tests depended on the same type of visual memory or whether the two tasks depended on similar neural circuits. For example, in the DNMS task, a nonmatching rule first must be trained, and the animal’s response is purposeful and goal-directed. In the VPC task, by contrast, the response is spontaneous and rather automatic in appearance. Thus, it seemed possible that behavior in the VPC task could be driven by a reflexive or habituation-like memory quite unrelated to the explicit visual recognition that humans exhibit when they encounter familiar objects. Further, in both humans and monkeys, successful performance on the VPC task emerged earlier in development, than did successful performance on the DNMS task (for a more complete discussion of these issues, see Bachevalier, 1990; Diamond, 1990; Fagan, 1990).
Despite these considerations, it was soon demonstrated that the difference between the two tasks with respect to development was due to the greater cognitive demand required by the DNMS task and not to differences in the visual memory requirements of the two tasks (Diamond, 1995). More importantly, it was reported that both amnesic patients and monkeys with damage that included MTL structures exhibited memory impairment on the VPC test (Bachevalier, Brickson, & Hagger, 1993; McKee & Squire, 1993). A similar deficit was also found in the rat (Ennaceur, Neave, & Aggleton, 1996). Later, a mouse version of the NOR task was developed (Tang et al., 1999), and this task was found to be impaired by disruption of the hippocampus (Rampon et al., 2000) and this disruption was delay-dependent (Hammond, Tull, & Stackman, 2004).
The NOR task has now largely supplanted the DNMS test as a test of recognition memory in the experimental animal (Clark & Martin, 2005; Winters, Saksida, & Bussey, 2008). Its widespread use is due to a number of factors. The task exploits the animal’s innate preference for novelty and therefore does not require explicit rule learning. Furthermore, the inherent variability introduced during rule acquisition is avoided. There is also evidence that spontaneous novelty preference tasks are more sensitive to recognition memory impairment than is DNMS (Nemanic, Alvarado, & Bachevalier, 2004; Pascalis, Hunkin, Holdstock, Isaac, & Mayes, 2004). Finally, the NOR task can be administered to humans, monkeys, rats and mice in essentially the same way (humans and monkeys typically view 2-D pictures and rodents are allowed to explore 3-D objects), and the behavioral findings have been remarkably consistent across species (Fig. 3; Clark & Martin, 2005).
Fig. 3.
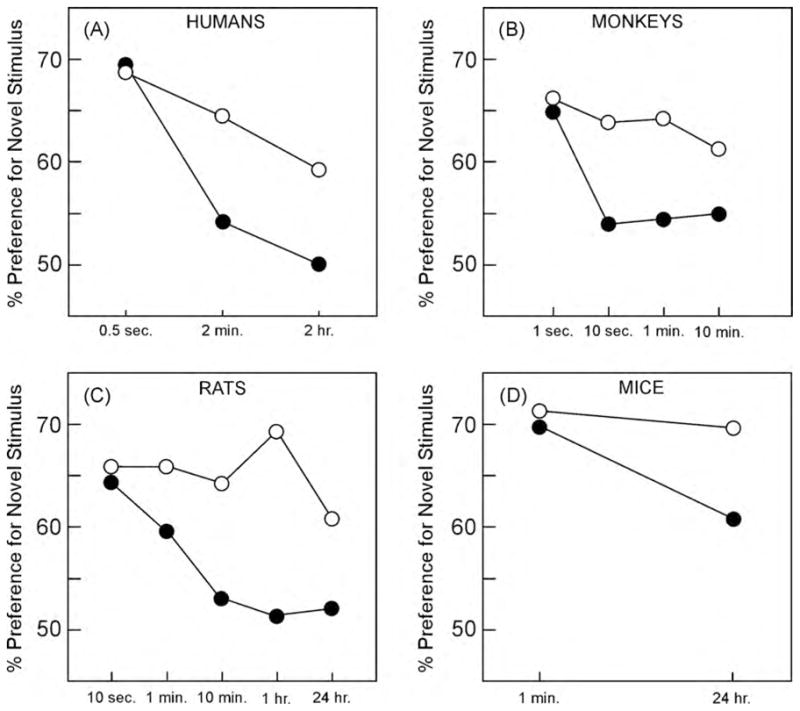
Performance of humans, monkeys, rats, and mice on the VPC/NOR task. In all panels the white circles depict control groups, and the black circles depict groups with hippocampal damage or disruption. (A) Human participants (data from McKee & Squire, 1993). (B) Monkeys (data from Zola et al., 2000). (C) Rats (data from Clark et al., 2000). (D) Mice (data from Hammond et al., 2004). All four studies revealed delay-dependent impairment after hippocampal damage or disruption.
Finally, the NOR test in rodents simple to administer. Rats simply explore objects placed in a small open area. Memory is measured by scoring (usually from a video recording) the amount of time the animal explores the new object compared to the familiar object. This simplicity allows laboratories that are not otherwise equipped to conduct behavioral analysis the opportunity to test recognition memory in their animals.
7. Initial insights from the animal model of human medial temporal lobe amnesia
Early work with the DNMS task revealed severe, delay-dependent memory impairment following large medial temporal lobe lesions that damaged the hippocampus, the amygdala, and the cortex underlying these structures (the H+A+ lesion; Mishkin, 1978). (The “+” denotes that the cortex immediately adjacent to the target structure was damaged.) The H+A+ lesion impaired memory more severely than when damage was restricted to the posterior medial temporal lobe and involved the hippocampus, the posterior entorhinal cortex, and most of the parahippocampal cortex (the H+ lesion; Mishkin, 1978; Zola-Morgan & Squire, 1985, 1986; Zola-Morgan, Squire, & Amaral, 1989a).
8. Amygdala damage is eliminated as a critical structure in amnesia
As noted above, it was initially supposed that the severe memory impairment observed in monkeys on the DNMS task was due to combined damage to the hippocampus and amygdala (Mishkin, 1978). However, subsequent studies revealed that impairment could not be attributed to amygdala damage. Thus, selective damage to the amygdala did not impair performance on the DNMS task and also did not exacerbate the memory impairment associated with the H+ lesion (Zola-Morgan, Squire, & Amaral, 1989b). In contrast, extending the H+ lesion forward to include perirhinal cortex, but not the amygdala, did increase the severity of the memory impairment (the H++ lesion; Zola-Morgan, Squire, Clower, & Rempel, 1993). Moreover, conjoint lesions of the perirhinal and parahippocampal cortices severely impaired memory (Suzuki, Zola-Morgan, Squire, & Amaral, 1993; Zola-Morgan, Squire, Amaral, & Suzuki, 1989). These findings, and others, led to the conclusion that the hippocampal formation (the CA fields of the hippocampus, the dentate gyrus, the subiculum, and the entorhinal cortex) and the adjacent perirhinal and paraphippocampal cortices comprise the major components of the medial temporal lobe memory system (Squire & Zola-Morgan, 1991). Large lesions of this system in the monkey produce a pattern of memory impairment that closely resembles what is observed when similar lesions occur in patients (e.g., patient H.M.; Corkin, 1984; Corkin et al., 1997; Scoville & Milner, 1957; and patient E.P.; Stefanacci, Buffalo, Schmolck, & Squire, 2000).
Subsequent work further characterized the memory impairment that followed damage to MTL structures. The impairment in monkeys is long-lasting (Zola-Morgan & Squire, 1985) and multimodal (Murray & Mishkin, 1984; Suzuki et al., 1993). In addition, monkeys with such lesions exhibit intact skill-based memory and intact habit-like memory (Malamut, Saunders, & Mishkin, 1984; Zola-Morgan & Squire, 1984) as well as intact short-term memory (Overman, Ormsby, & Mishkin, 1990).
9. The anatomy and organization of the medial temporal lobe
The system of structures important for recognition memory includes the hippocampus (dentate gyrus, CA fields and subiculum) and the entorhinal, perirhinal, and parahippocampal cortices (Fig. 1). Note that in the rat, the parahippocampal cortex is referred to as postrhinal cortex.
Fig. 1.
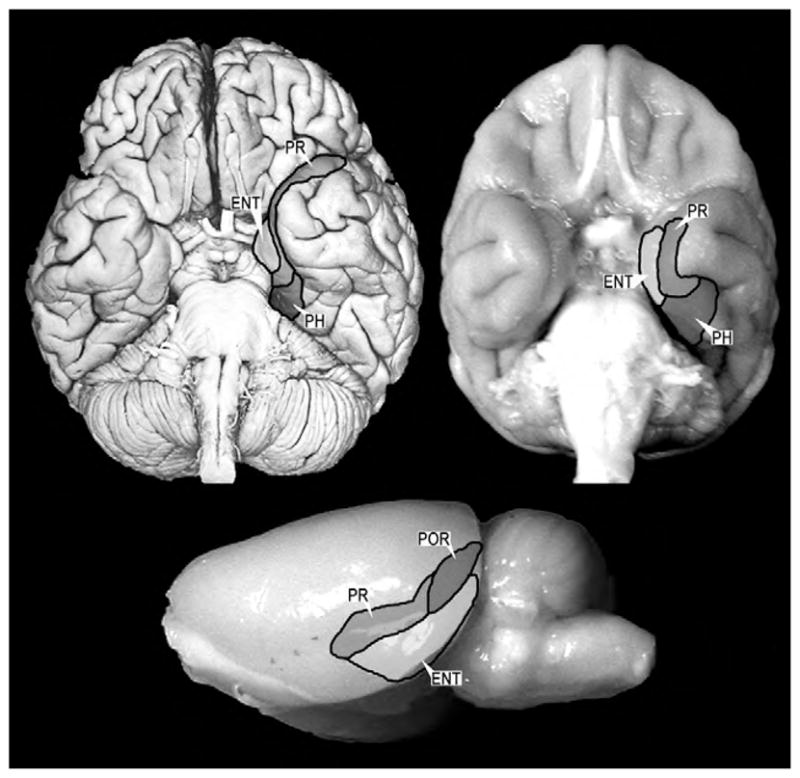
Ventral view of a human brain (upper left), a monkey brain (upper right) and a lateral view of a rat brain (lower center). The major cortical components of the medial temporal lobe are highlighted and outlined. The organization and connections of these structures are highly conserved across these species. Abbreviations: PR: perirhinal cortex, PH: parahippocampal cortex, ENT: entorhinal cortex, POR: postrhinal cortex (referred to as parahippocampal cortex in primates).
The hippocampus lies at the end of the processing hierarchy of the medial temporal lobe, receiving input from both the perirhinal and parahippocampal cortices as well as the entorhinal cortex (Fig. 2). Guided by the anatomy, it seems plausible that the hippocampus extends and combines functions performed by the structures that project to it (Squire, Wixted, & Clark, 2007). Note also that anatomical connections from different regions of neo-cortex enter the medial temporal lobe at different points. Thus, the higher visual areas TE and TEO project preferentially to the perirhinal cortex. Conversely, spatial information that comes to the medial temporal lobe from parietal cortex arrives exclusively at the parahippocampal cortex. Consistent with these anatomical facts, damage to parahippocampal cortex was found to impair spatial memory more did than damage to perirhinal cortex (Malkova & Mishkin, 1997; Parkinson, Murray, & Mishkin, 1988), and damage to perirhinal cortex impaired performance on the visual DNMS task more than did damage to parahippocampal cortex (Ramus, Zola-Morgan, & Squire, 1994).
Fig. 2.
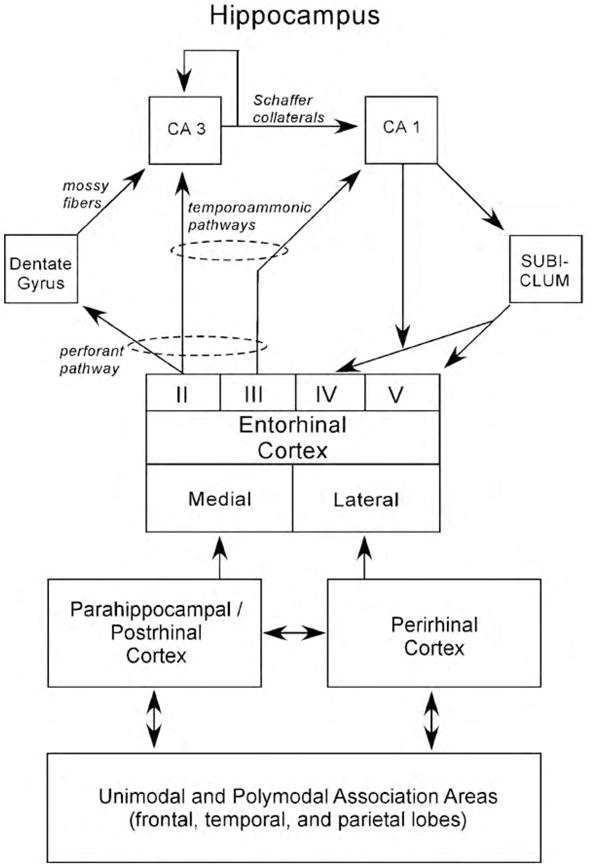
A schematic view of the medial temporal lobe memory system: The hippocampus, defined here as the dentate gyrus (DG), CA3, and CA1, is anatomically situated to receive highly processed information from widespread neocortical regions through three temporal cortical areas: the entorhinal, perirhinal, and parahippocampal cortices (in the rat the postrhinal cortex is used in place of the parahippocampal cortex), and through other direct projections from extra-temporal areas. The main pathway for the transmission of sensory information to the hippocampus is the entorhinal cortex. Layer II of this structure provides the major input to the hippocampus. This unidirectional projection, forming part of the perforant pathway, provides a substantial input to the DG, which, in turn, provides the major input to CA3 via the mossy fiber projection. There is also a smaller unidirectional projection to CA3 from layer II of the entorhinal cortex. CA3 provides the major input to CA1 via the Schaffer collateral/commissural pathway, but there is a substantial recurrent associational projection back to the CA3 field. CA1 also receives a direct temporoammonic projection from layer III of the entorhinal cortex (as does the subiculum, not shown). Both Schaffer collateral and temporoammonic projections to CA1 are unidirectional. CA1 primarily projects to the subiculum, but also sends a projection to entorhinal cortex layer V. The subiculum sends a prominent projection primarily to the entorhinal cortex layers IV and V (see Witter & Amaral, 2004 for review). The figure shows a simplified view of the way in which information enters the hippocampus from the superficial layers of the entorhinal cortex and then flows in a largely unidirectional, feed-forward, clockwise direction to ultimately return predominantly to the deep layers of entorhinal cortex. The perirhinal and parahippocampal/postrhinal cortex account for a substantial portion of the cortical input to the entorhinal cortex. The parahippocampal/postrhinal cortex preferentially projects to medial entorhinal cortex, and the perirhinal cortex preferentially projects to the lateral entorhinal cortex. These structures in turn receive projections from unimodal and polymodal areas in the frontal, temporal, and parietal lobes.
10. The focus moves to individual MTL structures
10.1. The perirhinal cortex
Once a clear understanding that the cortical regions of the MTL were important for memory, it became important to characterize how they contributed. For example, the perirhinal cortex and area TE are immediately adjacent to each other in the temporal lobe and are reciprocally interconnected. These areas are thought to lie at the interface between visual perception and visual memory, but it has been unclear what their separate contributions might be. Studies of monkeys suggest that perirhinal cortex plays an important role in the memory aspect of recognition memory and that area TE is important for visual information processing that is required for successful visual recognition memory (Buffalo et al., 1999; Buffalo, Ramus, Squire, & Zola, 2000). These conclusions are based in part on functional dissociations that have been demonstrated between the effects of damage to the perirhinal cortex and the effects of damage to visual area TE. For example, monkeys with damage limited to the perirhinal cortex exhibited delay-dependent memory impairment on both visual and tactile versions of the DNMS task (normal performance at short delays when the demand on memory is minimal, but impaired performance at longer delays when the demand of memory is greater). In contrast, monkeys with damage limited to area TE were impaired on visual DNMS but not tactile DNMS (Buffalo et al., 1999). That is, the impairment after TE lesions was unimodal, not multimodal.
A second important finding was that monkeys with perirhinal cortical lesions acquired an automated version of visual DNMS as quickly as normal animals when the delay between sample and choice was only 0.5 s (Buffalo et al., 2000). This finding shows that the ability to perceive the stimuli was not affected by perirhinal lesions. By contrast, monkeys with TE lesions were severely impaired at the 0.5 s delay. Thus, monkeys with TE lesions failed even when the memory demands of the task were minimal. The most likely explanation of this result is that the monkeys with TE lesions had difficulty processing the visual stimuli. A similar finding was obtained with the VPC task. Performance after perirhinal lesions was intact when the delay between the familiarization and choice phases was only 1 s but impaired at longer delays. In contrast, monkeys with TE lesions were impaired even at the shortest delay (Buffalo et al., 1999). These findings clearly indicate that perirhinal cortex, like other medial temporal lobe structures, is important for the formation of memory. In contrast, area TE is important for visual perceptual processing.
It is widely agreed that the perirhinal cortex is critically important for recognition memory. Findings in support of this conclusion have been consistently reported for both the DNMS and VPC/NOR task and in both monkeys and rats. For example, monkeys with perirhinal lesions are impaired on the DNMS task (Buffalo et al., 1999, 2000), as are monkeys with perirhinal lesions that include the entorhinal cortex (Eacott, Gaffan, & Murray, 1994; Meunier, Bachevalier, Mishkin, & Murray, 1993; the delayed matching to sample task was used in this study). Furthermore, rats with selective perirhinal lesions (Prusky, Douglas, Nelson, Shabanpoor, & Sutherland, 2004), or perirhinal plus entorhinal lesions (Kornecook, Anzarut, & Pinel, 1999; Mumby & Pinel, 1994), exhibited pronounced delay-dependent impairments on the DNMS or delayed matching to sample task. This same pattern of impairment has also been demonstrated using the VPC task in monkeys (Buffalo et al., 1999; Nemanic et al., 2004) and using the NOR task in rats (Bussey, Duck, Muir, & Aggleton, 2000; Bussey, Muir, & Aggleton, 1999; Ennaceur et al., 1996; Winters & Bussey, 2005).
10.2. The perceptual–mnemonic theory of perirhinal function
Today the dominant view of perirhinal function remains that this cortical structure is critically and primarily involved with memory function. However, during the past several years, a developing literature from work with humans, monkeys, and rats has proposed that the perirhinal cortex is not exclusively involved in memory but may also play a role in particular types of high-level perception (e.g., Bussey & Saksida, 2007; Lee et al., 2005a; Bartko, Winters, Cowell, Saksida, & Bussey, 2007a, 2007b). Specifically, it has been proposed that the perirhinal cortex is involved in visual object perception when there are high amounts of feature ambiguity (Buckley & Gaffan, 2006; Bussey & Saksida, 2005). In other words, the perirhinal cortex is critical in order to resolve differences in stimuli when those stimuli share overlapping (i.e., ambiguous) elements.
Despite the concern that in animal studies testing perceptual ability necessarily embeds the perceptual test within a memory task, several studies reported that monkeys with perirhinal lesions were impaired when discriminations between stimuli with high feature overlap (Buckley & Gaffan, 1998; Buckley, Booth, Rolls, & Gaffan, 2001; Bussey, Saksida, & Murray, 2002). Accordingly, these findings have suggested that the perirhinal cortex is critical for both memory and for the ability to perceptually resolve feature-ambiguous visual stimuli (Bussey, Saksida, & Murray, 2006, 2003). For additional discussion and critical evaluation of this view, drawing on work with humans as well, see Suzuki (2009) and Baxter (2009).
11. Recognition memory and the hippocampus
The title of the classic paper by Scoville and Milner (1957) was “Loss of recent memory after bilateral hippocampal lesions.” The title implied somewhat misleadingly, that the memory loss in patient H.M. was due to the direct damage to the hippocampus. However, the last paragraph of the paper states the point quite correctly: “It is concluded that the anterior hippocampus and hippocampal gyrus, either separately or together, are critically concerned in the retention of current experience.” Despite this indication that the memory impairment should not be attributed to the hippocampus itself, most subsequent work focused on the hippocampus (and amygdala) and ignored the cortical areas of the medial temporal lobe. The work in the rat that began in the early 1960’s readily revealed memory impairments following hippocampal lesions on a variety of behavioral tasks (for review see Douglas, 1967), and these lesions involved a dorsal approach to the hippocampus that spared the cortical areas damaged in H.M. Thus, the hippocampus, and not the MTL cortical areas, became a major focus for memory research.
11.1. Work in the monkey implicates the hippocampus
It was not until the 1990s that investigators began using stereotaxic neurosurgical methods to selectively damage the hippocampus in monkeys and to test for recognition memory impairments. Six studies have assessed the effects of selective hippocampal lesions made in adulthood on recognition memory performance as measured by the DNMS task (Alvarez, Zola-Morgan, & Squire, 1995; Beason-Held, Rosene, Killiany, & Moss, 1999; Murray & Mishkin, 1998; Nemanic et al., 2004; Zola-Morgan, Squire, Rempel, Clower, & Amaral, 1992; Zola et al., 2000). Of these six studies, five found impaired performance following lesions restricted to the hippocampus (all but Murray & Mishkin, 1998). Notably, Zola et al. (2000) brought together data from 18 monkeys with bilateral lesions of the hippocampus made either by an ischemic procedure, by radiofrequency, or by ibotenic acid. Significant recognition memory impairment was observed at all the delays that were tested from 15 s to 40 min. The single study that did not find impaired performance on the DNMS task involved conjoint amygdale–hippocampal lesions (Murray & Mishkin, 1998). This study is a notable exception because the lesioned animals had substantial hippocampal damage (mean of 73%) yet performed normally across all of the DNMS delays (the longest DNMS delay was 2 min) and with reverse-order list lengths that resulted in 40-min delays. It is not clear what factor might account for the good performance in this study. It has been suggested that differences in lesion size could account for the findings (Baxter & Murray, 2001) and that the deficit might be inversely related to lesion size. The monkeys tested by Murray and Mishkin (1998) had relatively large hippocampal lesions. However, when the data from the available studies (Beason-Held et al., 1999; Murray & Mishkin, 1998; Zola et al., 2000) were analyzed together, lesion size was not a significant predictor of performance (Zola & Squire, 2001). In a multiple-regression analysis, most of the variance was explained by differences among the studies, and lesion size itself accounted for only a small amount of the variability within the datasets of the individual experiments. When the effect of hippocampal lesion size on performance was studied directly in large numbers of rats, the impairment was proportionately related to the lesion size. Increasing the size of the lesion increased the behavioral deficit up to a point, beyond which increasing the size of the lesion did not further increase the deficit (Broadbent, Squire, & Clark, 2004; Moser, Moser, & Andersen, 1993). For more detailed discussion of this issue see Baxter and Murray (2001), and Zola and Squire (2001).
Two studies have assessed recognition memory using the VPC task following selective hippocampal lesions in the monkey, and both reported substantial delay-dependent memory impairment (Nemanic et al., 2004; Zola et al., 2000). These data are in register with the two human studies that have tested amnesic patients with the VPC task and found delay-dependent impairment (McKee & Squire, 1993; Pascalis et al., 2004). As noted above, there are some differences in findings between laboratories and these differences might be at least partially related to variation in testing protocols or other unidentified factors. Nonetheless, when one considers the work in primates in its entirety, the majority of the data indicate that selective hippocampal damage impairs recognition memory. Findings in rodents and with the rat in particular, are more mixed.
11.2. Work in rodents and selective hippocampal lesions
A number of studies in the rat have reported that bilateral damage to the hippocampus or the fornix impairs DNMS performance (Clark et al., 2001; Mumby, Pinel, Kornecook, Shen, & Redila, 1995; Mumby, Wood, & Pinel, 1992; Wiig & Bilkey, 1995) or delayed matching-to-sample (Prusky et al., 2004). Other studies of DNMS or similar tasks have failed to find an impairment following bilateral hippocampal or fornix lesions (Aggleton, Hunt, & Rawlins, 1986; Duva et al., 1997; Kesner, Bolland, & Dakis, 1993; Mumby et al., 1996; Rothblat & Kromer, 1991). A consideration of all the studies suggests that impaired performance on the DNMS task typically occurs following hippocampal damage if the delay is sufficiently long and if the hippocampal lesions are sufficiently large—although these factors alone do not reconcile all the available data (see Clark et al., 2001). We also note that the observed impairment is often relatively mild, although nonetheless significant.
Most of the work with selective lesions of the hippocampus has been done with rats and mice using the NOR task. There is a substantial literature reporting that recognition memory impairments following hippocampal damage or disruption in rats and mice (Ainge et al., 2006; Baker & Kim, 2002; Broadbent et al., 2004; Clark, Zola, & Squire, 2000; de Lima, Luft, Roesler, & Schroder, 2006; Gaskin, Tremblay, & Mumby, 2003; Gould et al., 2002; Hammond et al., 2004; Prusky et al., 2004; Rossato et al., 2007; Rampon et al., 2000). These findings all support the notion that the hippocampus is important for familiarity based recognition memory. Yet there is also a literature in the rodents suggesting that the hippocampus is not needed for successful performance on the NOR task, even when large hippocampal lesions are used in conjunction with relatively long retention delays (Forwood, Winters, & Bussey, 2005; Mumby, Tremblay, Lecluse, & Lehmann, 2005; O’Brien, Lehmann, Lecluse, & Mumby, 2006; Winters, Forwood, Cowell, Saksida, & Bussey, 2004). Accordingly, a consensus has not been achieved in the rodent with respect to the role of the hippocampus in recognition memory. It will be important to identify the critical factors that determine when the hippocampus is important for recognition memory and when (or if) normal recognition memory can be accomplished in the absence of the hippocampus (for further discussion of this issue and literature see, Winters et al., 2008).
A possible explanation of the discrepant results is that hippocampal lesions impair recognition memory, but that the impairment is modest and not always detectable with small sample sizes. We recently assessed the anterograde effects of hippocampal lesions on recognition memory using the NOR tasks and a 3-hour delay between the familiarization and test phase (Broadbent, Squire, & Clark, 2010). We used large groups of animals (control animals, n = 47 and animals with hippocampal lesions, n = 44) and multiple behavioral tests (n = 4). Even though a significant deficit did not emerge on every individual test day, a reliable and robust impairment was detected when performance was averaged across the four days of testing (Fig. 4). The finding that individual NOR test sessions did not reliably detect impaired performance in the animals with hippocampal lesions is important because it suggests that a single test of object recognition memory will often be insufficiently sensitive to reveal impaired recognition memory. Given that the recognition memory impairment following hippocampal damage is often only moderately severe, and that the performance measure in this task can range from 0% to 100% even in control animals, reliably detecting impairment may require both multiple test trials and large group sizes. We have previously suggested that lesion size and delay length are critical factors influencing whether impaired object recognition memory is detected after hippocampal damage (Broadbent et al., 2004). Hippocampal lesions are more likely to result in impaired recognition memory when the lesion size is large (>75% of total hippocampal volume; Broadbent et al., 2004) and when the delay length is sufficiently long (>10 min; Clark et al., 2000).
Fig. 4.
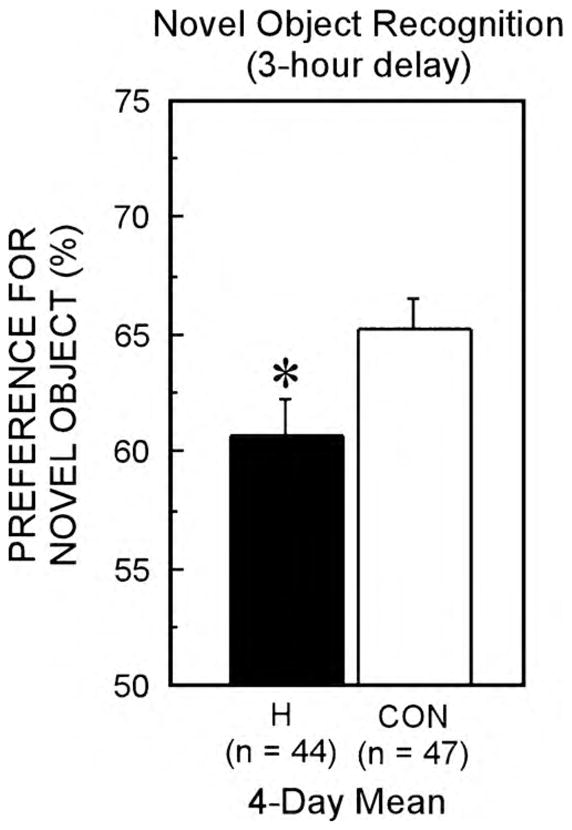
Performance on the NOR task on 4 different days. On each day there was a familiarization and test phase by sham operated animals (CON, n = 47) and animals with hippocampal lesions (H, n = 44). Performance was scored over 30 s of cumulative object exploration. The figure shows the 4-day mean for the hippocampal lesion group (black bar) and the sham group (white bar). Both groups performed above chance (chance = 50%). Group difference is indicated by an asterisk (p < 0.05).
11.3. The hippocampus, perirhinal cortex and the components of recognition memory
Recognition memory is widely viewed as consisting of two components: recollection and familiarity (e.g., Mandler, 1980). Recollection involves remembering specific contextual details about a prior learning episode. This type of memory is sometimes referred to as episodic memory. Familiarity involves simply knowing that an item was previously presented without having available any additional information about the context of the learning episode. Brown and Aggleton (2001) proposed a neuroanatomical basis for these two processes. Their proposal was that recollection depends on the hippocampus, whereas familiarity depends on the adjacent perirhinal cortex. Evidence from neuroimaging, neuropsychological, and neurophysiological studies of humans, and a substantial amount of testing experimental animals with selective damage to the hippocampus or perirhinal cortex have been used to evaluate this proposal. Further, an animal model of episodic memory and amnesia that employs signal detection analyses to characterize recognition memory performance in rats has recently been developed (e.g., Eichenbaum et al., in press, for a comment on this method see Wixted & Squire, 2008). For further discussion of the possible neuroanatomical separation of recollection and familiarity see Aggleton and Brown (2006) and Eichenbaum, Yonelinas, and Ranganath (2007), and for an alternative perspective proposing that recollection and familiarity are typically confounded with memory strength, see Squire et al. (2007).
12. Conclusion
This article provides a brief history of the work that led to the view that the medial temporal lobe is predominately involved in a particular form of memory (declarative memory). The article also outlined how an animal model of amnesia in the monkey and in the rodent has been particularly valuable for evaluating and understanding the anatomy of recognition memory. The perirhinal cortex and hippocampus both appear to contribute in important ways to recognition memory (Squire et al., 2007). But it is also expected that differences in function between these and other structures in the medial temporal lobe will be identified. Clearly, animal models of MTL amnesia will benefit greatly from genetic manipulations now available in the mouse, from the use of viral vectors that can alter cellular function in the experimental animal, and brain imaging methods of immediate early gene activity identification, where regional patterns of activity can be tracked during learning, retention, retrieval and as new memories become old memories.
References
- Aggleton JP. One-trial object recognition by rats. Quarterly Journal of Experimental Psychology. 1985;37B:279–294. [Google Scholar]
- Aggleton JP, Brown MW. Interleaving brain systems for episodic and recognition memory. Trends in Cognitive Science. 2006;10:455–463. doi: 10.1016/j.tics.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Hunt PR, Rawlins JNP. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behavioral Brain Research. 1986;19:133–146. doi: 10.1016/0166-4328(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Ainge JA, Heron-Maxwell C, Theofilas P, Wright P, de Hoz L, Wood ER. The role of the hippocampus in object recognition in rats: examination of the influence of task parameters and lesion size. Behavioral Brain Research. 2006;167:183–195. doi: 10.1016/j.bbr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Alvarez P, Zola-Morgan S, Squire LR. Damage limited to the hippocampal region produces long-lasting memory impairment in monkeys. Journal of Neuroscience. 1995;15:3796–3807. doi: 10.1523/JNEUROSCI.15-05-03796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J. Ontogenetic development of habit and memory formation in primates. Annals of the New York Academy of Sciences. 1990;608:457–474. doi: 10.1111/j.1749-6632.1990.tb48906.x. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Brickson M, Hagger C. Limbic-dependent recognition memory in monkeys develops early in infancy. Learning and Memory. 1993;4:77–80. doi: 10.1097/00001756-199301000-00020. [DOI] [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learning and Memory. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perirhinal cortex resolves feature ambiguity in configural object recognition and perceptual oddity tasks. Learning and Memory. 2007a;14(12):821–832. doi: 10.1101/lm.749207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perceptual functions of perirhinal cortex in rats: zero-delay object recognition and simultaneous oddity discriminations. Journal of Neuroscience. 2007b;27(10):2548–2559. doi: 10.1523/JNEUROSCI.5171-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG. Involvement of medial temporal lobe structures in memory and perception. Neuron. 2009;61(5):667–677. doi: 10.1016/j.neuron.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. Opposite relationship of hippocampal and rhinal cortex damage to delayed nonmatching-to-sample deficits in monkeys. Hippocampus. 2001;11(1):61–71. doi: 10.1002/1098-1063(2001)11:1<61::AID-HIPO1021>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Rosene DL, Killiany RJ, Moss MB. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus. 1999;9:562–574. doi: 10.1002/(SICI)1098-1063(1999)9:5<562::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- von Bekhterev M. Demonstration eines Gehirns mit Zerstö rung der vorderen und inneren Theile der Hirnrinde beider Schlä fenlappen. Neurologisches Zeitblatt. 1900;19:990–991. [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proceedings of National Academy of Science of United States of America. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learning and Memory. 2010;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;1:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Booth MC, Rolls ET, Gaffan D. Selective perceptual impairments after perirhinal cortex ablation. Journal of Neuroscience. 2001;21(24):9824–9836. doi: 10.1523/JNEUROSCI.21-24-09824.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Perirhinal cortex ablation impairs configural learning and paired-associate learning equally. Neuropsychologia. 1998;36(6):535–546. doi: 10.1016/s0028-3932(97)00120-6. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Perirhinal cortical contributions to object perception. Trends in Cognitive Science. 2006;10(3):100–107. doi: 10.1016/j.tics.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Ramus SJ, Clark RE, Teng E, Squire LR, Zola SM. Dissociation between the effects of damage to perirhinal cortex and area TE. Learning and Memory. 1999;6:572–599. doi: 10.1101/lm.6.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo EA, Ramus SJ, Squire LR, Zola SM. Perception and recognition memory in monkeys following lesions of area TE and perirhinal cortex. Learning and Memory. 2000;7:375–382. doi: 10.1101/lm.32100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Aggleton JP. Functionally dissociating aspects of event memory: The effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. Journal of Neuroscience. 1999;19:495–502. doi: 10.1523/JNEUROSCI.19-01-00495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Duck J, Muir JL, Aggleton JP. Distinct patterns of behavioural impairments resulting from fornix transection or neurotoxic lesions of the perirhinal and postrhinal cortices in the rat. Behavioral Brain Research. 2000;111:187–202. doi: 10.1016/s0166-4328(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. Object memory and perception in the medial temporal lobe: An alternative approach. Current Opinion in Neurobiology. 2005;15(6):730–737. doi: 10.1016/j.conb.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. Memory, perception, and the ventral visual-perirhinal-hippocampal stream: Thinking outside of the boxes. Hippocampus. 2007;17(9):898–908. doi: 10.1002/hipo.20320. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Perirhinal cortex resolves feature ambiguity in complex visual discriminations. European Journal of Neuroscience. 2002;15(2):365–374. doi: 10.1046/j.0953-816x.2001.01851.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Impairments in visual discrimination after perirhinal cortex lesions: testing ‘declarative’ vs ‘perceptual-mnemonic’ views of perirhinal cortex function. European Journal of Neuroscience. 2003;17:649–660. doi: 10.1046/j.1460-9568.2003.02475.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Perirhinal cortex and feature-ambiguous discriminations. Learning and Memory. 2006;13(2):103–105. doi: 10.1101/lm.163606. [DOI] [PubMed] [Google Scholar]
- Clark CVH, Isaacson RL. Effect of bilateral hippocampal ablation on DRL performance. Journal of Comparative and Physiological Psychology. 1965;59:137–140. doi: 10.1037/h0021599. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. Journal of Neuroscience. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, West AN, Zola SM, Squire LR. Rats with lesions of the hippocampus are impaired on the delayed nonmatching-to-sample task. Hippocampus. 2001;11:176–186. doi: 10.1002/hipo.1035. [DOI] [PubMed] [Google Scholar]
- Clark RE, Martin SJ. Interrogating rodents regarding their object and spatial memory. Current Opinion in Neurobiology. 2005;15(5):593–598. doi: 10.1016/j.conb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Squire LR. Preserved learning and retention of pattern analyzing skill in amnesia: Dissociation of knowing how and knowing that. Science. 1980;210:207–209. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- Corkin S. Lasting consequences of bilateral medial temporal lobectomy: Clinical course and experimental findings in H.M. Seminars in Neurology. 1984;4:249–259. [Google Scholar]
- Corkin S, Amaral DG, Gonzalez RG, Johnson KA, Hyman BT. H.M.’s medial temporal lobe lesion: Findings from magnetic resonance imaging. Journal of Neuroscience. 1997;17:3964–3980. doi: 10.1523/JNEUROSCI.17-10-03964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll RE, Scoville WB. Effects of medial temporal lesions on visual discrimination performance. Journal of Comparative & Physiological and Psychology. 1965;60:175–181. doi: 10.1037/h0022290. [DOI] [PubMed] [Google Scholar]
- de Lima MN, Luft T, Roesler R, Schroder N. Temporary inactivation reveals an essential role of the dorsal hippocampus in consolidation of object recognition memory. Neuroscience Letters. 2006;405:142–146. doi: 10.1016/j.neulet.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Diamond A. Rate of maturation of the hippocampus and the developmental progression of children’s performance on the delayed non-matching to sample and visual paired comparison tasks. Annals of the New York Academy of Sciences. 1990;608:394–426. doi: 10.1111/j.1749-6632.1990.tb48904.x. [DOI] [PubMed] [Google Scholar]
- Diamond A. Evidence of robust recognition memory early in life even when assessed by reaching behavior. Journal of Experimental Child Psychology. 1995;59(3):419–456. doi: 10.1006/jecp.1995.1020. [DOI] [PubMed] [Google Scholar]
- Douglas RJ. The hippocampus and behavior. Psychology Bulletin. 1967;67(6):416–422. doi: 10.1037/h0024599. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Pribram KH. Learning and limbic lesions. Neuropsychologia. 1966;4:197–220. [Google Scholar]
- Duva CA, Floresco SB, Wunderlich GR, Lao TL, Pinel JPJ, Phillips AG. Disruption of spatial but not object-recognition memory by neurotoxic lesions of the dorsal hippocampus in rats. Behavioral Neuroscience. 1997;111:1184–1196. doi: 10.1037//0735-7044.111.6.1184. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan D, Murray EA. Preserved recognition memory for small sets, and impaired stimulus identification for large sets, following rhinal cortex ablations in monkeys. European Journal of Neuroscience. 1994;6:1466–1478. doi: 10.1111/j.1460-9568.1994.tb01008.x. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Fortin N, Sauvage M, Robitsek RJ, Farovik A. An animal model of amnesia that uses Receiver Operating Characteristics (ROC) analysis to distinguish recollection from familiarity deficits in recognition memory. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2009.09.015. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behavioral Brain Research. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transection in the rat. Behavioral Brain Research. 1996;80:9–25. doi: 10.1016/0166-4328(96)00006-x. [DOI] [PubMed] [Google Scholar]
- Fagan JF. Memory in the infant. Journal of Experimental Child Psychology. 1970;9:217–226. doi: 10.1016/0022-0965(70)90087-1. [DOI] [PubMed] [Google Scholar]
- Fagan JF. The paired-comparison paradigm and infant intelligence. Annals of the New York Academy of Sciences. 1990;608:337–357. doi: 10.1111/j.1749-6632.1990.tb48902.x. [DOI] [PubMed] [Google Scholar]
- Frantz RL. A method for studying early visual development. Perceptual and Mortor Skills. 1956;6:13–15. [Google Scholar]
- Forwood SE, Winters BD, Bussey TJ. Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 h. Hippocampus. 2005;15:347–355. doi: 10.1002/hipo.20059. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Recognition impaired and association intact in the memory of monkeys after transaction of the fornix. Journal of Comparative and Physiological Psychology. 1974;88(6):1100–1109. doi: 10.1037/h0037649. [DOI] [PubMed] [Google Scholar]
- Gaskin S, Tremblay A, Mumby DG. Retrograde and anterograde object recognition in rats with hippocampal lesions. Hippocampus. 2003;13:962–969. doi: 10.1002/hipo.10154. [DOI] [PubMed] [Google Scholar]
- Glees P, Griffith HB. Bilateral destruction of the hippocampus (cornu ammonis) in a case of dementia. Monatsschrift für Psychiatrie und Neurologie. 1952;129:193–204. doi: 10.1159/000140010. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Rowe WB, Heman KL, Mesches MH, Young DA, Rose GM, Bickford PC. Effects of hippocampal lesions on patterned motor learning in the rat. Brain Research Bulletin. 2002;58:581–586. doi: 10.1016/s0361-9230(02)00832-8. [DOI] [PubMed] [Google Scholar]
- Grünthal E. Über das klinische Bild nach umschriebenem beiderseitigem Ausfall der Ammonshornrinde. Monatsschrift für Psychiatrie und Neurologie. 1947;113:1–16. [PubMed] [Google Scholar]
- Gunderson VM, Sackett GP. Development of pattern recognition in infant pigtailed macaques (macaca nemestrina) Developmental Psychology. 1984;20:418–426. [Google Scholar]
- Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiology of Learning and Memory. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hegglin K. Über einen Fall von isolierter linkseitiger Ammonshornerwe-ichung bei präseniler Dementz. Monatsschrift für Psychiatrie und Neurologie. 1953;125:170–186. [PubMed] [Google Scholar]
- Isaacson RL, Douglas RJ, Moore RY. The effect of radical hippocampal ablation on acquisition of avoidance response. Journal of Comparative and Physiological Psychology. 1961;54:62S–628. [Google Scholar]
- Isaacson RL, Wickelgren WO. Hippocampal ablation and passive avoidance. Science. 1962;138:1104–1106. doi: 10.1126/science.138.3545.1104. [DOI] [PubMed] [Google Scholar]
- Jarrard LE, Isaacson RL, Wickelgren WO. Effects of hippocampal ablation and intertribal interval on runway acquisition and extinction. Journal of Comparative and Physiological Psychology. 1964;57:442–444. doi: 10.1037/h0041639. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Bolland BL, Dakis M. Memory for spatial locations, motor responses, and objects: Triple dissociation among the hippocampus, caudate nucleus, and extrastriate visual cortex. Experimental Brain Research. 1993;93:462–470. doi: 10.1007/BF00229361. [DOI] [PubMed] [Google Scholar]
- Kimble DP. The effects of bilateral hippocampal lesions in rats. Journal of Comparative and Physiological Psychology. 1963;56:273–283. doi: 10.1037/h0048903. [DOI] [PubMed] [Google Scholar]
- Kimble DP, Kimble RJ. Hippocampectomy and response perseveration in the rat. Journal of Comparative and Physiological Psychology. 1965;60:474–476. doi: 10.1037/h0022550. [DOI] [PubMed] [Google Scholar]
- Kimble DP, Kirkby RJ, Stein DG. Response perseveration interpretation of passive avoidance deficits in hippocampectomized rats. Journal of Comparative and Physiological Psychology. 1966;61:141–143. doi: 10.1037/h0022858. [DOI] [PubMed] [Google Scholar]
- Kimura D. Effects of selective hippocampal damage on avoidance behavior in the rat. Canadian Journal of Psychology. 1958;12:213–218. doi: 10.1037/h0083740. [DOI] [PubMed] [Google Scholar]
- Kornecook TJ, Anzarut A, Pinel JP. Rhinal cortex, but not medial thalamic, lesions cause retrograde amnesia for objects in rats. Neuroreport. 1999;10:2853–2858. doi: 10.1097/00001756-199909090-00028. [DOI] [PubMed] [Google Scholar]
- Lee AC, Bussey TJ, Murray EA, Saksida LM, Epstein RA, Kapur N, et al. Perceptual deficits in amnesia: challenging the medial temporal lobe “mnemonic” view. Neuropsychologia. 2005;43:1–11. doi: 10.1016/j.neuropsychologia.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Mandler G. Recognizing: the judgment of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- Malamut BL, Saunders RC, Mishkin M. Monkeys with combined amygdalo-hippocampal lesions succeed in object discrimination learning despite 24-hour intertrial intervals. Behavioral Neuroscience. 1984;98:759–769. doi: 10.1037//0735-7044.98.5.759. [DOI] [PubMed] [Google Scholar]
- Malkova L, Mishkin M. Memory for the location of objects after separate lesions of the hippocampus and parahippocampal cortex in Rhesus monkeys. Society for Neursocience Abstracts. 1997;23:14. [Google Scholar]
- McKee RD, Squire LR. On the development of declarative memory. Journal of Experimental Psychology: Learning, Memory and Cognition. 1993;19:397–404. doi: 10.1037//0278-7393.19.2.397. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. Journal of Neuroscience. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clinical Neurosurgery. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature. 1978;273:297–298. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- Mishkin M. A memory system in the monkey. Philosophical Transactions of the Royal Society B: Biological Sciences. 1982;298:83–95. doi: 10.1098/rstb.1982.0074. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Delacour J. An analysis of short-term visual memory in the monkey. Journal of Experimental Psychology: Animal Behavior Processes. 1975;1:326–334. doi: 10.1037//0097-7403.1.4.326. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Malamut B, Bachevalier J. Memories and habits: Two neural systems. In: Lynch G, McGaugh JL, Weinberger NM, editors. Neurobiology of human learning and memory. New York: Guilford; 1984. pp. 65–77. [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. The Journal of Neuroscience. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Pinel JP, Wood ER. Nonrecurring-items delayed nonmatching-to-sample in rats: a new paradigm for testing non-spatial working memory. Psychobiology. 1990;18:321–326. [Google Scholar]
- Mumby DG, Wood ER, Pinel JPJ. Object-recognition memory is only mildly impaired in rats with lesions of the hippocampus and amygdala. Psychobiology. 1992;20:18–27. [Google Scholar]
- Mumby DG, Pinel JP. Rhinal cortex lesions and object recognition in rats. Behavioral Neuroscience. 1994;108:11–18. doi: 10.1037//0735-7044.108.1.11. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Pinel JPJ, Kornecook TJ, Shen MJ, Redila VA. Memory deficits following lesions of hippocampus or amygdala in rat: Assessment by an object-memory test battery. Psychobiology. 1995;23:26–36. [Google Scholar]
- Mumby DG, Wood ER, Duva CA, Kornecook TJ, Pinel JP, Phillips AG. Ischemia-induced object-recognition deficits in rats are attenuated by hippocampal ablation before or soon after ischemia. Behavioral Neuroscience. 1996;110:266–281. doi: 10.1037//0735-7044.110.2.266. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Tremblay A, Lecluse V, Lehmann H. Hippocampal damage and anterograde object-recognition in rats after long retention intervals. Hippocampus. 2005;15:1050–1056. doi: 10.1002/hipo.20122. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Severe tactual as well as visual memory deficits follow combined removal of the amygdala and hippocampus in monkeys. Journal of Neuroscience. 1984;4:2565–2580. doi: 10.1523/JNEUROSCI.04-10-02565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. The Journal of Neuroscience. 1998;18:6568–6582. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: Insights from visual paired comparison versus object-delayed nonmatching in monkeys. Journal of Neuroscience. 2004;24(8):2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki H. The effects of hippocampal ablation on the behavior in the rat. Japanese Psychological Research. 1962;4:139–153. [Google Scholar]
- Niki H. The effects of hippocampal ablation on the inhibitory control of operant behavior in the rat. Japanese Psychological Research. 1965;7:126–137. [Google Scholar]
- Niki H. Response perseveration following the hippocampal ablation in the rat. Japanese Psychological Research. 1966;8:1–9. [Google Scholar]
- Orbach J, Milner B, Rasmussen T. Learning and retention in monkeys after amygdala-hippocampus resection. Archives of Neurology. 1960;3:230–251. doi: 10.1001/archneur.1960.00450030008002. [DOI] [PubMed] [Google Scholar]
- O’Brien N, Lehmann H, Lecluse V, Mumby DG. Enhanced context-dependency of object recognition in rats with hippocampal lesions. Behavioral Brain Research. 2006;170:156–162. doi: 10.1016/j.bbr.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Overman WH, Ormsby G, Mishkin M. Picture recognition vs. picture discrimination learning in monkeys with medial temporal removals. Experimental Brain Research. 1990;79:18–24. doi: 10.1007/BF00228870. [DOI] [PubMed] [Google Scholar]
- Parkinson JK, Murray EA, Mishkin MA. A selective mnemonic role for the hippocampus in monkeys: Memory for the location of objects. Journal of Neuroscience. 1988;8:4159–4167. doi: 10.1523/JNEUROSCI.08-11-04159.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascalis O, Hunkin NM, Holdstock JS, Isaac CL, Mayes AR. Visual paired comparison performance is impaired in a patient with selective hippocampal lesions and relatively intact item recognition. Neuropsychologia. 2004;42(10):1293–1300. doi: 10.1016/j.neuropsychologia.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Pavlov I. In: Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Anrep GV, translator. New York: Oxford University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM, Nelson L, Shabanpoor A, Sutherland RJ. Visual memory task for rats reveals an essential role for hippocampus and perirhinal cortex. Proceedings of National Academy of Science of United States of America. 2004;101:5064–5068. doi: 10.1073/pnas.0308528101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nature Neuroscience. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- Ramus SJ, Zola-Morgan S, Squire LR. Effects of lesions of perirhinal cortex or parahipocampal cortex on memory in monkeys. Society for Neuroscience Abstracts. 1994;20:1074. [Google Scholar]
- Raphelson AC, Isaacson RL, Douglas RJ. The effect of limbic damage on the retention and performance of a runway response. Neuropsychologia. 1966;4:253–264. [Google Scholar]
- Rossato JI, Bevilaqua LR, Myskiw JC, Medina JH, Izquierdo I, Cammarota M. On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learning and Memory. 2007;14:36–46. doi: 10.1101/lm.422607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothblat LA, Hayes LL. Short-term object recognition memory in the rat: Nonmatching with trial-unique junk stimuli. Behavioral Neuroscience. 1987;101:587–590. doi: 10.1037//0735-7044.101.4.587. [DOI] [PubMed] [Google Scholar]
- Rothblat LA, Kromer LR. Object recognition memory in the rat: The role of the hippocampus. Behavioural Brain Research. 1991;42:25–32. doi: 10.1016/s0166-4328(05)80036-1. [DOI] [PubMed] [Google Scholar]
- Schmaltz L, Isaacson RL. The effects of preliminary training conditions under DRL performance in the hippocampectomized rat. Physiology and Behavior. 1966;1:175–182. [Google Scholar]
- Scoville WB. The limbic lobe in man. Journal of Neurosurgery. 1954;11:64–66. doi: 10.3171/jns.1954.11.1.0064. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery and Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder DR, Isaacson RL. The effects of large and small bilateral hippocampal lesions on two types of passive avoidance responses. Psychological Reports. 1965;16:1277–1290. [Google Scholar]
- Squire LR. Memory systems of the brain: A brief history and current perspective. Neurobiology of Learning Memory. 2004;82(2004):171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Squire LR. The legacy of patient H.M. for neuroscience. Neuron. 2009;61(1):6–9. doi: 10.1016/j.neuron.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: A new perspective. Nature Reviews Neuroscience. 2007;8(11):872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The neurology of memory: The case for correspondence between the findings for human and non-human primate. In: Deutsch JA, editor. The Physiological Basis of Memory. 2. New York: Academic Press; 1983. pp. 199–268. [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Buffalo EA, Schmolck H, Squire LR. Profound amnesia after damage to the medial temporal lobe: A neuroanatomical and neuropsychological profile of patient E.P. Journal of Neuroscience. 2000;20:7024–7036. doi: 10.1523/JNEUROSCI.20-18-07024.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA. Perception and the medial temporal lobe: Evaluating the current evidence. Neuron. 2009;61(5):657–666. doi: 10.1016/j.neuron.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Zola-Morgan S, Squire LR, Amaral DG. Lesions of the perirhinal and parahippocampal cortices in the monkey produce long-lasting memory impairment in the visual and tactual modalities. Journal of Neuroscience. 1993;13:2430–2451. doi: 10.1523/JNEUROSCI.13-06-02430.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Teng E, Stefanacci L, Squire LR, Zola SM. Contrasting effects on discrimination learning after hippocampal lesions and conjoint hippocampal-caudate lesions in monkeys. Journal of Neuroscience. 2000;20(10):3853–3863. doi: 10.1523/JNEUROSCI.20-10-03853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum H. A comparison of the effects of orbitofrontal and hippocampal lesions upon discrimination learning and reversal in the cat. Experimental Neurology. 1964;9:452–462. doi: 10.1016/0014-4886(64)90053-6. [DOI] [PubMed] [Google Scholar]
- Thompson R, Langer SK. Deficits in position reversal learning following lesions of the limbic system. Journal of Comparative and Physiological Psychology. 1963;56:987–995. doi: 10.1037/h0045188. [DOI] [PubMed] [Google Scholar]
- Webster DB, Voneida TJ. Learning deficits following hippocampal lesions in split-brain cats. Experimental Neurology. 1964;10:170–182. doi: 10.1016/0014-4886(64)90094-9. [DOI] [PubMed] [Google Scholar]
- Weinstein B. Matching-from-sample by rhesus monkeys and by children. Journal of Comparative Psychology. 1941;31:195–213. [Google Scholar]
- Wiig KA, Bilkey DK. Lesions of rat perirhinal cortex exacerbate the memory deficit observed following damage to the fimbria-fornix. Behavioral Neuroscience. 1995;109:620–630. doi: 10.1037//0735-7044.109.4.620. [DOI] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: Heterogeneity of function within the temporal lobe. Journal of Neuroscience. 2004;24:5901–5908. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. Journal of Neuroscience. 2005;25:52–61. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neuroscience & Biobehavioral Reviews. 2008;32(5):1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. 3. San Diego: Academic Press; 2004. pp. 637–703. [Google Scholar]
- Wixted JT, Squire LR. Constructing receiver operating characteristics (ROCs) with experimental animals: Cautionary notes. Learning and Memory. 2008;15(9):687–690. doi: 10.1101/lm.1077708. [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. Journal of Neuroscience. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola SM, Squire LR. Relationship between magnitude of damage to the hippocampus and impaired recognition memory in monkeys. Hippocampus. 2001;11:92–98. doi: 10.1002/hipo.1027. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Mishkin M. The neuroanatomy of amnesia: Amygdala-hippocampus versus temporal stem. Science. 1982;218:1337–1339. doi: 10.1126/science.6890713. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Preserved learning in monkeys with medial temporal lesions: Sparing of motor and cognitive skills. Journal of Neuroscience. 1984;4:1072–1085. doi: 10.1523/JNEUROSCI.04-04-01072.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Medial temporal lesions in monkeys impair memory on a variety of tasks sensitive to human amnesia. Behavioral Neuroscience. 1985;99:22–34. doi: 10.1037//0735-7044.99.1.22. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Memory impairment in monkeys following lesions limited to the hippocampus. Behavioral Neuroscience. 1986;100:155–160. doi: 10.1037//0735-7044.100.2.155. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG, Suzuki WA. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. Journal of Neuroscience. 1989;9:4355–4370. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Lesions of the hippocampal formation but not lesions of the fornix or the mammillary nuclei produce long-lasting memory impairment in monkeys. Journal of Neuroscience. 1989a;9:898–913. doi: 10.1523/JNEUROSCI.09-03-00898.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Lesions of the amygdala that spare adjacent cortical regions do not impair memory or excerbate the impairment following lesions of the hippocampal formation. Journal of Neuroscience. 1989b;9:1922–1936. doi: 10.1523/JNEUROSCI.09-06-01922.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Rempel NL, Clower RP, Amaral DG. Enduring memory impairment in monkeys after ischemic damage to the hippocampus. Journal of Neuroscience. 1992;12:2582–2596. doi: 10.1523/JNEUROSCI.12-07-02582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Clower RP, Rempel NL. Damage to the perirhinal cortex exacerbates memory impairment following lesions to the hippocampal formation. The Journal of Neuroscience. 1993;13:251–265. doi: 10.1523/JNEUROSCI.13-01-00251.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


