Abstract
From 1976 to 1988, 63 patients received radiation therapy for primary cancers of the extrahepatic biliary system (eight gallbladder and 55 extrahepatic biliary duct). Twelve patients underwent orthotopic liver transplantation. Chemotherapy was administered to 13 patients. Three patients underwent intraluminal brachytherapy alone (range, 28 to 55 Gy). Sixty patients received megavoltage external-beam radiation therapy (range, 5.4 to 61.6 Gy; median, 45 Gy), of whom nine received additional intraluminal brachytherapy (range, 14 to 45 Gy; median, 30 Gy). The median survival of all patients was 7 months. Sixty patients died, all within 39 months of radiation therapy. One patient is alive 11 months after irradiation without surgical resection, and two are alive 50 months after liver transplantation and irradiation. Symptomatic duodenal ulcers developed after radiation therapy in seven patients but were not significantly related to any clinical variable tested. Extrahepatic biliary duct cancers, the absence of metastases, increasing calendar year of treatment, and liver transplantation with postoperative radiation therapy were factors significantly associated with improved survival.
Carcinomas arising in the gallbladder or extrahepatic biliary ducts are uncommon and generally have a poor prognosis; the overall 5-year survival is less than 10%.1–9 Death is usually from locoregional disease rather than distant metastases.10,11 Direct invasion into adjacent structures and metastases to regional lymph nodes, particularly in the porta hepatis are the main routes of spread. The liver is involved in 40% to 50% of case8,10 and retroperitoneal lymph nodes in 20% of patients.8,10,12 Peritoneal spread occurs in 9% of extrahepatic biliary duct cancers and 20% of gallbladder cancers.8,12 Direct extension of disease into the liver or liver metastases occurs commonly, even with small tumors.10,13 Cholangiocarcinomas have a tendency to spread along the biliary tract and are multifocal in as many as 42% of patients.14
Complete surgical resection is often impossible because of tumor spread. Approximately 30% of cases are resectable, but this varies between 5% and 61%, depending on patient selection.1,2,5,9,15–20 The diagnosis of cholangiocarcinoma at the bifurcation of the common duct (Klatskin tumor) is sometimes difficult to make preoperatively; computed tomographic or magnetic resonance scans may not show the tumor, and there are difficulties in establishing a cytologic diagnosis from needle biopsies or brushings. Therefore, some patients undergoing liver transplantation for end-stage liver disease, such as sclerosing cholangitis, are found to have a cholangiocarcinoma that is undetected until liver transplantation is done, and the recipient liver is examined. The initial results reported for liver transplantation of cholangiocarcinoma have been discouraging, primarily because of local tumor recurrence.1,21
Radiation therapy has been used as primary treatment for unresectable cholangiocarcinomas, usually after percutaneous transhepatic biliary drainage has been established for relief of jaundice.4,6,10,11,13,16,22–36 It has also been used as adjuvant therapy after resection. Radiation therapy has been delivered with fractionated external-beam treatment, intracavitary irradiation, or a combination of the two. Intraoperative radiation therapy has also been used with some success.37 We review our recent experience with radiation treatment of these tumors at a major tertiary-care referral center for liver surgery.
Materials and Methods
Between 1976 to 1988, 63 patients were referred to the Joint Radiation Oncology Center at the University of Pittsburgh Medical Center for radiation of primary malignant tumors of the gallbladder or extrahepatic biliary ducts. Twenty-six of these patients were reported earlier.11 A pathologic diagnosis of adenocarcinoma was established before radiation therapy in 54 patients. In the remaining nine patients, the radiographic diagnosis of Klatskin tumor was made by percutaneous transhepatic cholangiogram and/or endoscopic retrograde cholangiopancreatography. Eight patients had unresectable primary or recurrent carcinoma of the gallbladder with involvement of the liver. The other 55 patients had tumors involving the proximal hepatic ducts. Additional clinical characteristics are listed in Table 1.
Table 1.
Clinical Characteristics
| Characteristic | No. | Percent |
|---|---|---|
| Age | ||
| < 60 yr | 32 | 51 |
| ≥ 60 yr | 31 | 49 |
| Sex | ||
| Male | 32 | 51 |
| Female | 31 | 49 |
| Prior liver disease | 14 | 22 |
| No prior liver disease | 49 | 88 |
| Gallbladder primary | 8 | 13 |
| Extrahepatic biliary duct | 55 | 87 |
| Systemic metastases | 10 | 16 |
| No systemic metastases | 53 | 84 |
| Lymphadenopathy | 10 | 16 |
| No lymphadenopathy | 29 | 46 |
| Nodes not assessed | 24 | 38 |
Three patients underwent gross total resection of tumor without liver transplantation. Twelve patients underwent orthotopic liver transplantation. Eight were referred for postoperative radiation therapy, one for preoperative irradiation, and three for radiation therapy after tumor recurrence. To simplify the data analysis, the patient with preoperative radiation therapy was included with the liver transplant plus postoperative radiation therapy group. Twenty-two patients were referred for radiation after partial tumor resection. Twenty-six patients were referred for radiation therapy with no surgical resection attempted. Chemotherapy consisting of 5 fluorouracil (5-FU) or a combination of 5-FU, doxorubicin, and mitomycin C (FAM) was administered to 13 patients.
Total radiation doses administered ranged from 5.40 to 82.37 Gy (median, 47.5 Gy). Three patients received intraluminal brachytherapy alone (28.0, 29.6, and 55.0 Gy at 0.5-cm depth). Sixty patients received megavoltage external-beam irradiation to target absorbed doses of 5.40 to 61.53 Gy (median, 45 Gy). Nine patients received boost intraluminal brachytherapy (range, 14 to 45 Gy to 0.5-cm depth; median, 30 Gy) combined with 26 to 60 Gy (median, 47.6 Gy) of external-beam irradiation. Normalized total doses at 2 Gy per fraction (NTD2) were calculated using linear quadratic factor parameters for small bowel tolerance.38–41 The NTD2 values for external-beam irradiation ranged from 5.83 to 61.56 Gy (median, 45.7 Gy). Two fields were irradiated in ten patients, three in 20 patients, and four in 30 patients. Field sizes ranged from 36 to 702 cm2 (median, 100 cm2). Eight patients were treated with cobalt 60, and 52 patients were irradiated with a 6-MV, 8-MV, or 18-MV photon beam. Fifteen patients were treated with planned split-course irradiation beginning with 25 Gy in ten fractions followed by a 3 to 4-week break.42 Patients with stable medical conditions received another 25 Gy in ten fractions for a total dose of 50 Gy.
Actuarial survival from the start of radiation therapy was calculated using the method of Kaplan and Meier.43 Actuarial ulcer-free survival was calculated in a similar manner with the development of symptomatic ulcers as the end point and patient deaths considered as censored outcomes. The effects of different clinical or treatment parameters on both survival and development of symptomatic ulcers were examined with univariate analysis using the log-rank test.44
Results
Overall survival is illustrated in Figure 1. Sixty patients died 1 to 39 months after starting radiation therapy. Three patients are alive without evidence of disease at 11, 50, and 50 months after therapy (median survival, 7 months). The cause of death was judged to be cancer in all cases except one patient who received preoperative irradiation of 22.5 Gy for a biopsy-proven Klatskin tumor. This patient underwent an orthotopic liver transplant and died within 1 month of surgery from transplant rejection. No evidence of residual cholangiocarcinoma was found in the resected liver. The only two long-term survivors underwent orthotopic liver transplantation and are now disease-free 50 months after postoperative irradiation. Among the nine patients who received postoperative radiation therapy after liver transplantation, the actuarial survival was 22% at both 2 and 4 years.
Fig. 1.
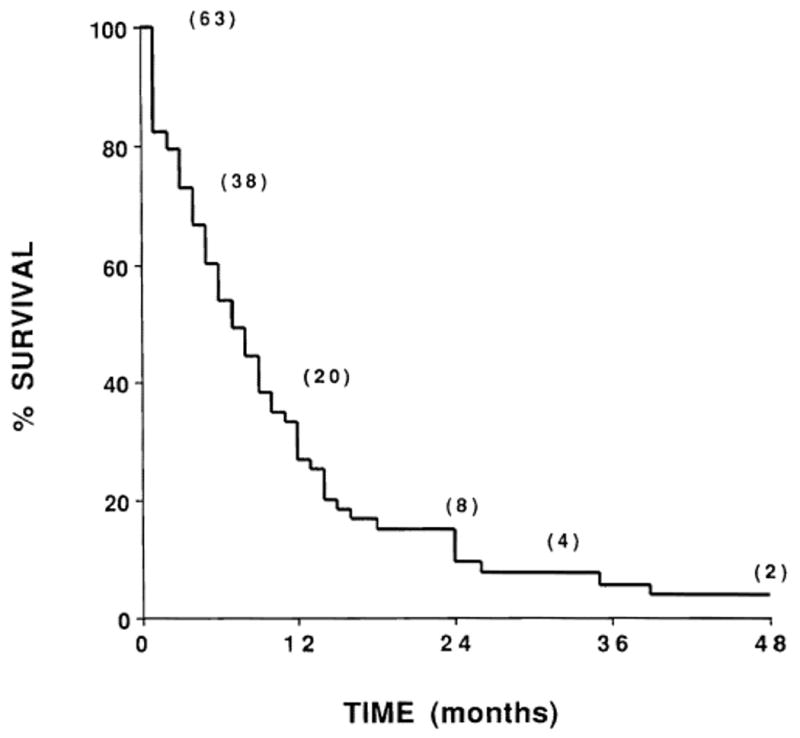
Actuarial survival of all patients receiving radiation therapy for cholangiocarcinoma (n = 63). Numbers in parentheses indicate the number of patients at risk.
Table 2 lists the results of univariate testing of overall survival for 16 clinical parameters using the log-rank test. Neither the total radiation dose nor the external beam NTD2 value administered represented true pretreatment variables because, if a patient's clinical condition deteriorated, his course of radiation was stopped (particularly with the split-course technique), and/or brachytherapy was withheld. To eliminate the bias of this effect on the total radiation dose administered, the analysis was repeated excluding the 13 patients who died within 2 months of starting irradiation. As shown in Table 2, univariate analysis of the entire group of 63 patients demonstrated significantly improved survival with liver transplantation and postoperative radiation therapy (P = 0.031), increasing extent of surgical resection (P = 0.043), increasing total dose (P = 0.039), increasing external beam NTD2 value (P = 0.048), increasing calendar year of radiation treatment (P = 0.022), and extrahepatic duct tumors compared with gallbladder primaries (P = 0.005). When the analysis was repeated excluding those patients living 2 months or less after initiating radiation therapy (to remove dose-selection bias), neither total radiation dose nor external beam NTD2 value were significantly associated with improved survival (P = 0.98 and 0.37, respectively). Extra-hepatic duct primaries (P < 0.0001), increasing calendar year of treatment (P = 0.023), and liver transplantation with postoperative radiation therapy (P = 0.041) were all significantly associated with increased survival. Patients with systemic metastases had a decrease in survival that approached statistical significance (P = 0.052).
Table 2.
Univariate Survival Analyses for All 63 Patients and for 50 Patients Surviving More Than 2 Months
| Factor |
P value (n = 63) |
P value (n = 50) |
|---|---|---|
| Extrahepatic biliary duct | 0.0005* | 0.0001* |
| Year of treatment | 0.0221* | 0.0229* |
| Transplant and postoperative radiotherapy | 0.0313* | 0.0413* |
| Total radiation dose | 0.0394* | 0.9882 |
| Surgery, extent | 0.0430* | 0.0721 |
| NTD2,† external beam | 0.0488* | 0.3659 |
| Sex | 0.0714 | 0.0970 |
| Systemic metastases | 0.1136 | 0.0520 |
| Prior liver disease | 0.1404 | 0.1856 |
| Initial tissue diagnosis | 0.3531 | 0.1611 |
| Age | 0.3937 | 0.8909 |
| Brachytherapy | 0.4340 | 0.8670 |
| Chemotherapy | 0.4454 | 0.1143 |
| Split-course irradiation | 0.5936 | 0.5262 |
| Field size | 0.6502 | 0.6344 |
| Lymphadenopathy | 0.9376 | 0.4889 |
Statistical significance (P < 0.05).
Normalized total dose at 2 Gy per fraction.
The actuarial survival curves for patients with gallbladder primaries (median survival, 3 months) and those with extrahepatic biliary duct primaries (median survival, 9 months) are shown in Figure 2. The actuarial survival curve for patients who underwent liver transplantation and postoperative radiation therapy (n = 9) was compared with the survival curve for the other patients in the series (n = 54) in Figure 3.
Fig. 2.
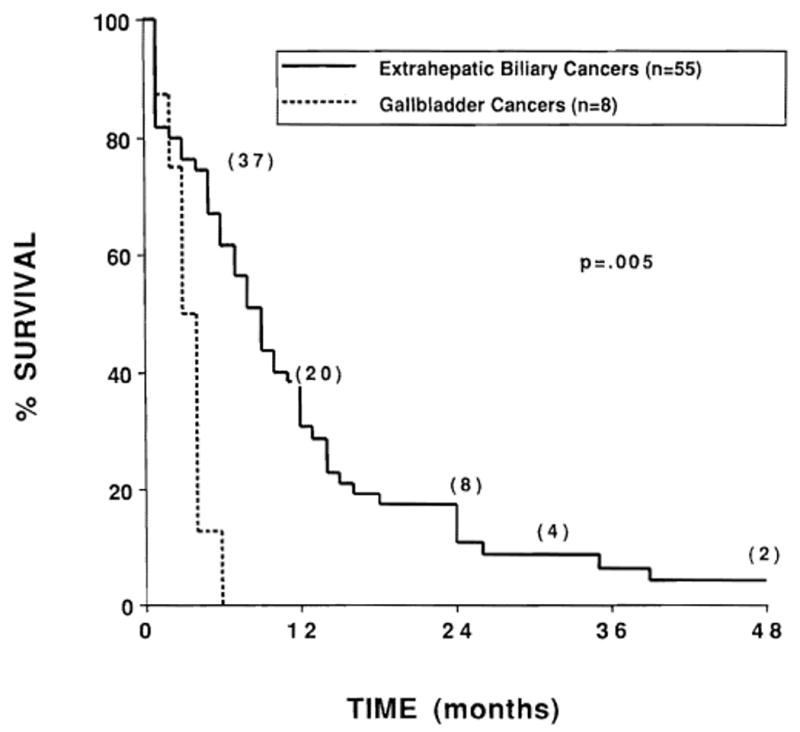
Actuarial survival of patients with gallbladder primaries (n = 8) compared with patients with extrahepatic biliary duct primaries (n = 55). Numbers in parentheses indicate the number of patients at risk.
Fig. 3.
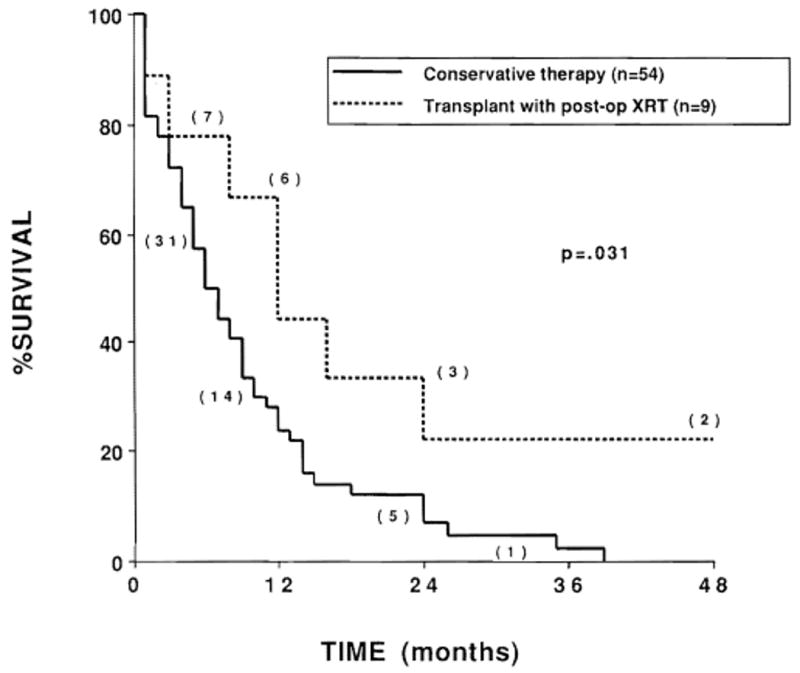
Actuarial survival of patients undergoing liver transplantation with preoperative or postoperative radiation therapy (n = 9) compared with patients receiving more conservative therapy (n = 54). Numbers in parentheses indicate the number of patients at risk.
Seven patients had symptomatic duodenal ulcers 3, 4, 6, 8, 8, 9, and 17 months after radiation. One patient who received split-course radiation therapy to a target absorbed dose of 50 Gy in 20 fractions had a duodenal stricture requiring dilatation. Ulcers in the other six patients were managed successfully with medical therapy. Table 3 shows the results of univariate analysis of ulcer-free survival for 11 different clinical or treatment factors; none was statistically associated with ulcer development. The actuarial risk of developing a symptomatic duodenal ulcer at 2 years was 29% (Fig. 4).
Table 3.
Ulcer-Free Survival Analysis
| Factor | P value (n = 50) |
|---|---|
| Split-course irradiation | 0.1377 |
| Number of fields | 0.2535 |
| Surgery, extent | 0.3334 |
| Total dose | 0.4045 |
| Brachytherapy | 0.4201 |
| NTD2,† external beam | 0.5331 |
| Prior liver disease | 0.6438 |
| Chemotherapy | 0.6264 |
| Liver transplant | 0.8402 |
Normalized total dose at 2 Gy per fraction.
Fig. 4.
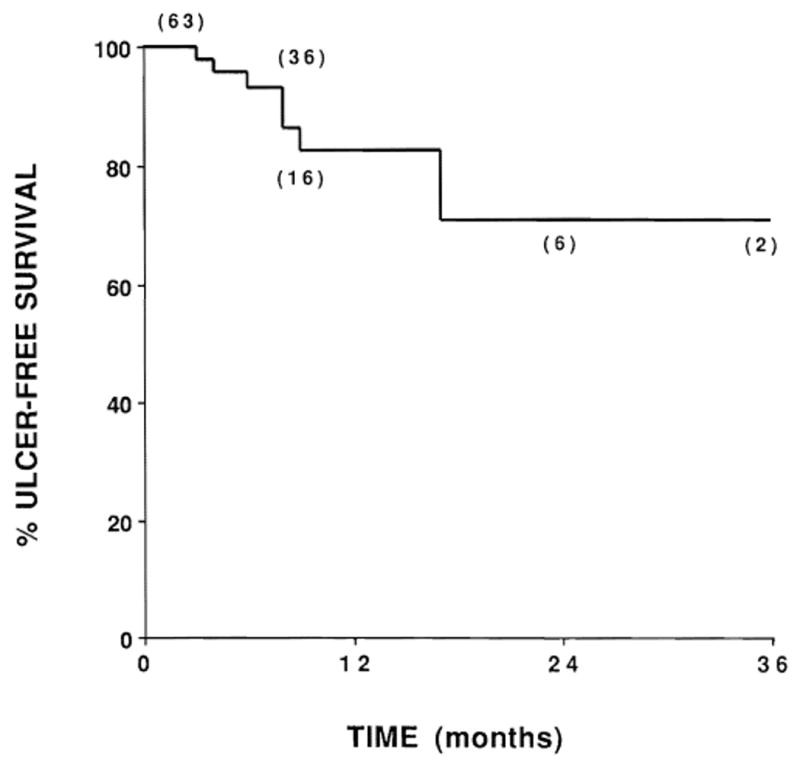
Actuarial survival for freedom from symptomatic duodenal ulcers (n = 63). Numbers in parentheses indicate the number of patients at risk.
Discussion
Primary carcinomas of the extrahepatic biliary passages are aggressive tumors with a poor prognosis. We could not demonstrate significant survival benefits for surgical resection short of liver transplantation, chemotherapy, or high doses of external-beam radiation therapy plus intraluminal brachytherapy. Liver transplantation with postoperative radiation therapy appears to be the only treatment factor studied that improved survival of our cholangiocarcinoma patients. This study did, however, identify two other factors that were of prognostic value. The first factor was primary tumor location. The survival of patients with tumors originating in the gallbladder was inferior to that of patients with tumors originating in the extrahepatic bile ducts. Because the only patients with primary gallbladder tumors referred for radiation therapy had unresectable tumors with liver invasion, our patients with gallbladder tumors were highly selected. This selection factor explains the low proportion of gallbladder tumors (13%) compared with other series (average, 60%).34 The second prognostic factor associated with improved survival was increasing calendar year of radiation treatment. This may be the result of improvements in supportive care for patients treated later.
The optimum treatment approach to cholangiocarcinomas is unclear. Biliary decompression alone appears to improve the mean survival of patients with Klatskin tumors from 3 months with no treatment to about 9 months.15,31,46 In separate reports, others found mean survival rates of 12 to 18 months with fractionated external-beam irradiation.16,18 Using intraluminal brachytherapy alone, Fletcher et al.6,26,31 showed a median survival of 11 months which improved to 17 months in a later update. Several other authors reported on the use of external-beam radiation therapy and brachytherapy for cholangiocarcinoma.24,25,30,34,36 Johnson et al.30 reported median survivals of 9 months after implantation alone and 16 months after external-beam irradiation with and intraluminal brachytherapy (seven patients). Others found a median survival of 7 months in nine patients receiving external-beam radiation therapy alone compared with 15 months in eight patients who received combined brachytherapy and external-beam irradiation25 and average survivals of 3.6 months in five patients receiving intraluminal brachytherapy alone compared with 14 months with external-beam irradiation and intraluminal brachytherapy.34
Selection factors may affect the choice of therapy and lead to survival differences. This appears to be the case in our series. Our initial analysis of the entire group of 63 patients showed that high total radiation dose (which is greatest in the combined brachytherapy and external-beam group) was associated significantly with improved survival. This improvement could not be substantiated, however, when a repeat analysis to eliminate dose-selection bias was limited to those patients surviving more than 2 months after the start of radiation therapy. Thus, the reason higher doses of radiation appeared to improve survival in our original analysis seems to be that patients who would have been treated with high-dose radiation therapy but did not live long enough to complete the treatment were analyzed subsequently in the low-dose group.
The development of symptomatic duodenal ulcers was not statistically significant with either radiation dose or field size, suggesting that other factors such as biliary drainage catheters or surgical biliary diversion contributed to their development. Nevertheless, this lack of association should not be overinterpreted because our analysis was limited by short survival times. Prophylactic treatment to prevent peptic ulcers may be indicated in some patients undergoing radiation therapy for cholangiocarcinoma.
Partial or complete surgical hepatic resection, short of liver transplantation, was not associated with improved survival. Most surgical series report median survival of 20 months with gross total resection compared with 9 months for palliative surgical procedures.2,5,9,12,15–17,20,45,46 The selection of patients with smaller tumors for curative resection may account for the better survival of patients undergoing complete resections. Our series did not contain enough patients who underwent complete resection without liver transplantation to analyze this group separately. Although the extent of surgical resection was prognostically important in the initial analysis (P = 0.043), this did not remain significant in the repeat analysis (P = 0.072) despite the fact that liver transplant patients included most of the patients in the complete resection group. We did find that patients who underwent liver transplantation with postoperative radiation therapy had a modest but significant improvement in survival (P = 0.031). Because of the small number of patients in this study and the large number of clinical variables tested, a valid multivariate analysis could not be done to test for the effects of possible confounding variables (such as primary site or year of treatment) on the better outcome observed with liver transplantation and radiation therapy. That the only two long-term (50-month) survivors in this series underwent liver transplantation with postoperative radiation therapy, however, provides clear encouragement for additional investigation of liver transplantation for cholangiocarcinoma.
Partial response rates of 29% have been reported for systemic chemotherapy with drugs such as 5-FU and mitomycin C as single agents or in combinations, such as FAM.47 Our initial experience with chemotherapy in these tumors was disappointing. On the other hand, Minsky et al.13 reported an encouraging preliminary experience using chemotherapy combined with aggressive radiation therapy. Although they reported five of ten patients alive without disease 16, 17, 17, 48, and 52 months after combined treatment, the two longest survivors had no tissue diagnosis. We found an insignificant trend toward better survival (P = 0.16) in patients without an initial tissue diagnosis, possibly because of smaller tumor size. Nonetheless, tumor progression eventually confirmed the diagnosis of cholangiocarcinoma in all such patients in our series.
In conclusion, we showed a modest but significant improvement in survival using liver transplantation and postoperative radiation therapy but no significant improvement using radiation therapy even with higher doses of radiation, conventional surgical resection, or chemotherapy. Except for patients with small tumors that can be completely resected, the prognosis for patients with cholangiocarcinoma is poor with conventional therapy. More radical treatment strategies, such as upper abdominal exenteration with cluster organ transplantation or liver transplantation with intensive preoperative radiation therapy and/or chemotherapy, may offer a better chance for cure in patients with unresectable cholangiocarcinoma. These strategies are currently being investigated at the University of Pittsburgh Medical Center.
Acknowledgments
The authors thank David Van Thiel, MD, for providing patient follow-up information, William D. Bloomer, MD, and Barry Lembersky, MD, for helpful discussions, and Mrs. Jean Ronick for typing assistance.
References
- 1.Alexander F, Roisi RL, Braasch JW. Biliary carcinoma: A review of 109 cases. Am J Surg. 1984;147:503–508. doi: 10.1016/0002-9610(84)90013-8. [DOI] [PubMed] [Google Scholar]
- 2.Beazley RM, Blumgart LH. Clinicopathological aspects of high bile duct cancer: Experience with resection and bypass surgical treatments. Ann Surg. 1984;199:623–634. doi: 10.1097/00000658-198406000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bismuth H, Malt RA. Carcinoma of the biliary tract. N Engl J Med. 1979;301:704–706. doi: 10.1056/NEJM197909273011307. [DOI] [PubMed] [Google Scholar]
- 4.Buskirk SJ, Gunderson LL, Adson MA, et al. Analysis of failure following curative irradiation of gallbladder and extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1984;10:2013–2023. doi: 10.1016/0360-3016(84)90198-6. [DOI] [PubMed] [Google Scholar]
- 5.Evander A, Fredlund P, Hoevels J, Ihse I, Bengmark SL. Evaluation of aggressive surgery for carcinoma of the extrahepatic bile ducts. Ann Surg. 1980;191:23–29. doi: 10.1097/00000658-198001000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fletcher MS, Dawson JL, Wheeler PG, Brinkley D, Nunnerley H, Williams R. Treatment of high bile duct carcinoma by internal radiotherapy with iridium-192 wire. Lancet. 1981;2:172–174. doi: 10.1016/s0140-6736(81)90357-3. [DOI] [PubMed] [Google Scholar]
- 7.Glenn F, Hays DM. The scope of radical surgery in the treatment of malignant tumors of the extrahepatic biliary tract. Surg Gynecol Obstet. 1959;99:529–541. [PubMed] [Google Scholar]
- 8.Vaittinen E. Carcinoma of the gall bladder: A study of 390 cases diagnosed in Finland 1953–1967. Ann Chir Gynaecol Suppl. 1970;169:1–7. [PubMed] [Google Scholar]
- 9.Vogt PP. Current management of cholangiocarcinoma. Oncology. 1988;2:37–43. [PubMed] [Google Scholar]
- 10.Kopelson G, Harisiadis L, Tretter P. The role of radiation therapy in cancer of the extrahepatic biliary system: An analysis of thirteen patients and a review of the literature of the effectiveness of surgery, chemotherapy, and radiotherapy. Int J Radiat Oncol Biol Phys. 1981;7:413–417. doi: 10.1016/0360-3016(77)90186-9. [DOI] [PubMed] [Google Scholar]
- 11.Mittal B, Deutsch M, Iwatsuki S. Primary cancers of extrahepatic biliary passages. Int J Radiat Oncol Biol Phys. 1985;11:849–854. doi: 10.1016/0360-3016(85)90320-7. [DOI] [PubMed] [Google Scholar]
- 12.Warren KW, Mountain JD, Lloyd-Jones W. Malignant tumors of the bile ducts. Br J Surg. 1972;59:501–505. doi: 10.1002/bjs.1800590702. [DOI] [PubMed] [Google Scholar]
- 13.Minsky BD, Wesson MF, Armstrong JC, et al. Combined modality therapy of extrahepatic biliary system. Int J Radiat Oncol Biol Phys. 1990;(18):1157–1163. doi: 10.1016/0360-3016(90)90453-q. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki M, Takahashi T, Ouchi K, Matsuno S. The development of extension of hepatohilar bile duct carcinoma: A three-dimensional tumor mapping in the intrahepatic biliary tree visualized with the aid of a graphic computer system. Cancer. 1989;64:658–666. doi: 10.1002/1097-0142(19890801)64:3<658::aid-cncr2820640316>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Blumgart LH, Hadgis NS, Benjamin IS, et al. Surgical approaches to cholangiocarcinoma at the confluence of hepatic ducts. Lancet. 1984;1:66–70. doi: 10.1016/s0140-6736(84)90002-3. [DOI] [PubMed] [Google Scholar]
- 16.Cameron JL, Broe P. Proximal bile duct tumors: Surgical management with silastic transhepatic biliary stents. Ann Surg. 1982;196:417–418. doi: 10.1097/00000658-198210000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chitwood WR, Meyers WC, Heaston DK. Diagnosis and treatment of primary extrahepatic bile duct tumors. Am J Surg. 1982;143:99–105. doi: 10.1016/0002-9610(82)90137-4. [DOI] [PubMed] [Google Scholar]
- 18.Lees CD, Hermann RH. Carcinoma of the bile ducts. Surg Gynecol Obstet. 1980;151:193–198. [PubMed] [Google Scholar]
- 19.Tompkins RK, Longmire WP. Prognostic factors in bile duct carcinoma: Analysis of 96 cases. Ann Surg. 1981;194:447–456. doi: 10.1097/00000658-198110000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuzuki T, Ozato Y. Carcinoma of the bifurcation of the hepatic ducts. Arch Surg. 1983;118:1147–1181. doi: 10.1001/archsurg.1983.01390100021006. [DOI] [PubMed] [Google Scholar]
- 21.Iwatsuki S, Gordon RD, Shaw BW, Starzl TE. Role of liver transplantation in cancer therapy. Ann Surg. 1985;202:401. doi: 10.1097/00000658-198510000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pichlmayr R, Ringe B, Lauchart W, Bechstein WO, Gugernatis G, Wagner E. Radical resection and liver grafting as the two main components of surgical strategy in the treatment of proximal bile duct cancer. World J Surg. 1988;12:68. doi: 10.1007/BF01658489. [DOI] [PubMed] [Google Scholar]
- 23.Conroy RM, Shahbazian AA, Edwards KC, et al. A new method for treating carcinomatous biliary obstruction with intracatheter radium. Cancer. 1982;49:1321–1327. doi: 10.1002/1097-0142(19820401)49:7<1321::aid-cncr2820490702>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 24.Dobelbower RR, Merrick HW, Ahuja RK, Skeel RT. 125I Interstitial implant, precision high-dose external beam therapy, and 5-FU for unresectable adenocarcinoma of pancreas and extrahepatic biliary tree. Cancer. 1986;58:2185–2195. doi: 10.1002/1097-0142(19861115)58:10<2185::aid-cncr2820581004>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 25.Fields JN, Emami B. Carcinoma of the extrahepatic biliary system-results of primary and adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 1987;13:331–338. doi: 10.1016/0360-3016(87)90006-x. [DOI] [PubMed] [Google Scholar]
- 26.Fletcher MS, Brinkely D, Dawson JL, Nunnerly H, Williams R. Treatment of hilar carcinoma by bile drainage combined with internal radiotherapy using 192iridium wire. Br J Surg. 1983;70:733–735. doi: 10.1002/bjs.1800701213. [DOI] [PubMed] [Google Scholar]
- 27.Fogel TD, Weissberg JB. The role of radiation therapy in carcinoma of the extrahepatic bile ducts. Int J Radiat Oncol Biol Phys. 1984;10:2251–2258. doi: 10.1016/0360-3016(84)90230-x. [DOI] [PubMed] [Google Scholar]
- 28.Hershkovic AM, Engler MJ, Noell KT. Radical radiotherapy for bile duct carcinoma. Endocur/Hyperth Oncol. 1985;1:119–124. [Google Scholar]
- 29.Hishikawa Y, Shimada T, Miura T, Imojyo Y. Radiation therapy of carcinoma of the extrahepatic bile ducts. Radiology. 1983;146:787–789. doi: 10.1148/radiology.146.3.6402803. [DOI] [PubMed] [Google Scholar]
- 30.Johnson DW, Safai C, Goffinet DR. Malignant obstructive jaundice: Treatment with external beam and intracavitary radiotherapy. Int J Radiat Oncol Biol Phys. 1985;11:411–416. doi: 10.1016/0360-3016(85)90166-x. [DOI] [PubMed] [Google Scholar]
- 31.Karani J, Fletcher M, Brinkley D, Dawson JL, Williams R, Nunnerly H. Internal biliary drainage and local radiotherapy with iridium-192 wire in treatment of hilar cholangiocarcinoma. Clin Radiol. 1985;36:603–606. doi: 10.1016/s0009-9260(85)80242-7. [DOI] [PubMed] [Google Scholar]
- 32.Kopelson G, Gunderson LL. Primary and adjuvant radiation therapy in gallbladder and extrahepatic biliary tract carcinoma. J Clin Gastroenterol. 1983;5:43–50. doi: 10.1097/00004836-198302000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Kopelson G, Harisiadis L, Tretter P, Chang CH. The role of radiation therapy in cancer of the extra-hepatic biliary system. Int J Radiat Oncol Biol Phys. 1977;2:883–894. doi: 10.1016/0360-3016(77)90186-9. [DOI] [PubMed] [Google Scholar]
- 34.Meyers WC, Jones RS. Internal irradiation for bile duct cancer. World J Surg. 1988;12:99–104. doi: 10.1007/BF01658493. [DOI] [PubMed] [Google Scholar]
- 35.Molt P, Hopfan S, Watson RC, Botet JF, Brennan MF. Intraluminal radiation therapy in the management of malignant biliary obstruction. Cancer. 1986;57:536–544. doi: 10.1002/1097-0142(19860201)57:3<536::aid-cncr2820570322>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 36.Prempree T, Cox EF, Sewchand W, Tank CK. Cholangiocarcinoma: A place for brachytherapy. Acta Radiol Oncol. 1983;22:353–359. doi: 10.3109/02841868309134053. [DOI] [PubMed] [Google Scholar]
- 37.Iwasaki Y, Todorok T, Fukao K, Ohara K, Okamura T, Nishimura A. The role of intraoperative radiation therapy in the treatment of bile duct cancer. World J Surg. 1988;12:91–98. doi: 10.1007/BF01658492. [DOI] [PubMed] [Google Scholar]
- 38.Flickinger JC, Kalend A. Use of normalized total dose to represent the biological effect of fractionated radiotherapy. Radiother Oncol. 1990;17:339–347. doi: 10.1016/0167-8140(90)90007-j. [DOI] [PubMed] [Google Scholar]
- 39.Maciejewski B, Taylor JM, Withers HR. Alpha/beta value and the importance of size of dose per fraction for later complications in the supraglottic larynx. Radiother Oncol. 1986;7:332–326. doi: 10.1016/s0167-8140(86)80061-5. [DOI] [PubMed] [Google Scholar]
- 40.Orton CG, Cohen L. An unified approach to dose-effect relationships in radiotherapy: I. Modified TDF and linear quadratic equations. Int J Radiat Oncol Biol Phys. 1988;14:549–556. doi: 10.1016/0360-3016(88)90273-8. [DOI] [PubMed] [Google Scholar]
- 41.Withers HR, Thames HD, Peters LJ. A new isoeffect curve for change in dose per fraction. Radiother Oncol. 1983;1:187–191. doi: 10.1016/s0167-8140(83)80021-8. [DOI] [PubMed] [Google Scholar]
- 42.Flickinger JC, Jawalekar J, Deutsch M, Webster J. Split course radiation therapy for adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 1988;15:359–364. doi: 10.1016/s0360-3016(98)90016-5. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan ES, Meier P. Non-parametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–480. [Google Scholar]
- 44.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient: II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Launois B, Champion JP, Brissot P, Gosselin M. Carcinoma of the hepatic hilus: Surgical management of the case for resection. Ann Surg. 1979;190:151–157. doi: 10.1097/00000658-197908000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mizumoto R, Kawarada Y, Suzuki H. Surgical treatment of hilar carcinoma of the bile ducts. Surg Gynecol Obstet. 1986;162:153–158. [PubMed] [Google Scholar]
- 47.Oberfield RA, Rossi RL. The role of chemotherapy in the treatment of bile duct cancer. World J Surg. 1988;12:105–108. doi: 10.1007/BF01658494. [DOI] [PubMed] [Google Scholar]


