Abstract
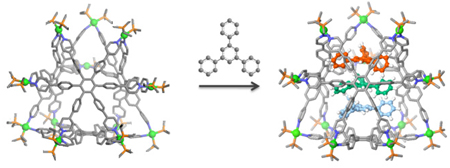
The design and synthesis of coordinative truncated tetrahedra is described. Coordination-driven self-assembly of a truncated tetrahedron was achieved using 90° organoplatinum acceptors and a hexapyridyl ligand with six-fold symmetry under mild conditions. This assembly can act as a host towards 1,3,5-triphenylbenzene. The truncated tetrahedral structures and the host-guest complex were identified using multinuclear (31P and 1H) NMR spectroscopy, electrospray ionization (ESI) mass spectrometry, X-ray crystallography, and pulsed field gradient spin-echo (PGSE) NMR, along with computational simulations.
Coordination-driven self-assembly is a successful methodology for preparing three-dimensional (3-D) supramolecular structures.1 During the past two decades, a variety of novel 3-D coordinative structures of high complexity, high symmetry, and well-defined size and shape, such as tetrahedra, cubes, double squares, cuboctahedra, adamantanoids, dodecahedra, and a sphere, have been developed.2 By virtue of their robust structural backbone and well-defined 3-D cavity, 3-D coordinative supramolecules have been used in a variety of applications such as guest encapsulation, gas storage, catalysis, and drug delivery.3 With further exo/endo functionalization,4 3-D supramolecules are endowed with a variety of novel properties, like 3-D dendrimers, nanoscale fluoro-droplet, and confined polymerization.5 Among these 3-D supramolecules, because of their unique structural features and fascinating host-guest properties, tetrahedral structures represent one of the most widely studied systems, as witnessed by the impressive results of Raymond6 and Fujita.7 However, the design of tetrahedral structures is still limited.6–8
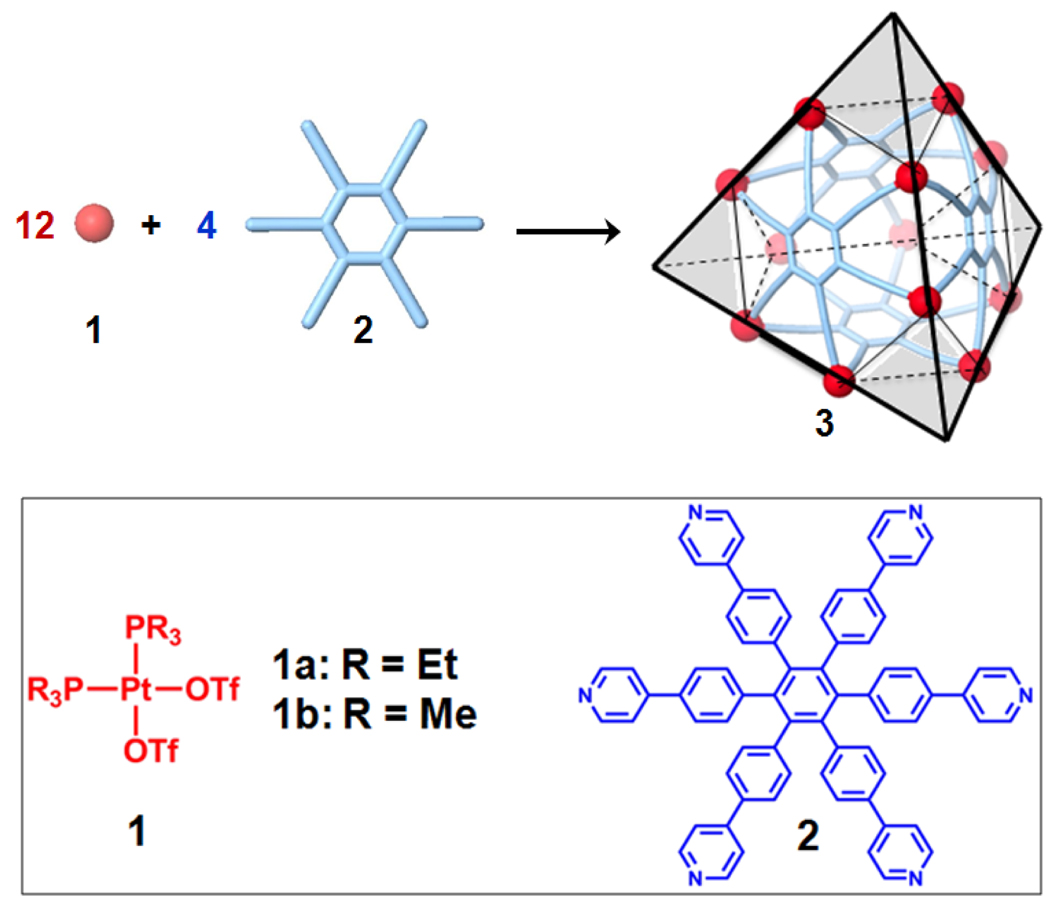
Homoleptic self-assembly of a hexapyridyl ligand and a 90° acceptor should form a tetrahedral structure, where the hexadentate ligand acts as faces and the organoplatinum acceptors are the connectors at the corners as shown in Scheme 1. We therefore carried out the self-assembly of the hexapyridyl donor 29 with the 90° organoplatinum acceptors 1, resulting in a truncated tetrahedron in quantitative yield. The structures were characterized by multinuclear (31P and 1H) NMR spectroscopy, electrospray ionization mass spectrometry (ESI-MS), pulsed field gradient spin-echo (PGSE) NMR, as well as X-ray crystallography. Furthermore, it was found that truncated tetrahedron 3b is able to encapsulate 1,3,5-triphenylbenzene in an aqueous acetone solution. This host-guest complex was characterized by NMR spectroscopy and ESI-MS as well as computational simulations.
Scheme 1.
Graphical representation of the [12 + 4] self-assembly of 90° organoplatinum acceptors 1 and hexapyridyl ligand 2 into a truncated tetrahedron 3.
By mixing 90° organoplatinum acceptor 1 and hexapyridyl donor 29 in a 3:1 ratio in an acetone-d6/CD3NO2 (v/v 7:3) solution for 3a and an aqueous acetone solution (v/v 1:1) for 3b, self-assembly of truncated tetrahedra 3 were obtained after 16 h of heating at 80 °C. The assemblies 3 can be quantitatively isolated via ion exchange with KPF6.
In the 31P{1H} NMR spectra (Figure 1) of 3, only one singlet at 0.92 ppm (3a) and −28.2 ppm (3b) with concomitant 195Pt satellites can be found, respectively, for the coordinated platinum centers. Likewise, the 1H NMR spectra (See Supporting Information) also exhibit sharp signals for the pyridyl protons of 3 (δPyα-H: 8.91 ppm and δPyβ-H: 7.44 ppm for 3a; δPyα-H: 8.68 ppm and δPyβ-H: 7.48 ppm for 3b), with approximately 0.1 ppm (HPy-β) and 0.2–4 ppm (HPy-α) downfield shifts due to the loss of electron density upon coordination with the platinum centers. Signals corresponding to the phenyl protons on the donors are split into two sets of doublets, presumably caused by the difference between the exterior and interior of the cage structure. In the ESI mass spectra of 3 (see Supporting Information), peaks corresponding to [3a – 5PF6]5+ and [3a – 6PF6]6+ can be found at m/z = 2383.1 and m/z = 1961.9, as are those for 3b at m/z = 2197.0 [3b – 5OTf]5+ and m/z = 1806.2 [3b – 6OTf]6+. These signals are isotopically resolved and in agreement with their theoretical distributions.
Figure 1.
31P{1H} NMR spectra (121.4 MHz) of truncated tetrahedra 3a in acetone-d6/CD3NO2 (a) and 3b in acetone-d6/D2O (b).
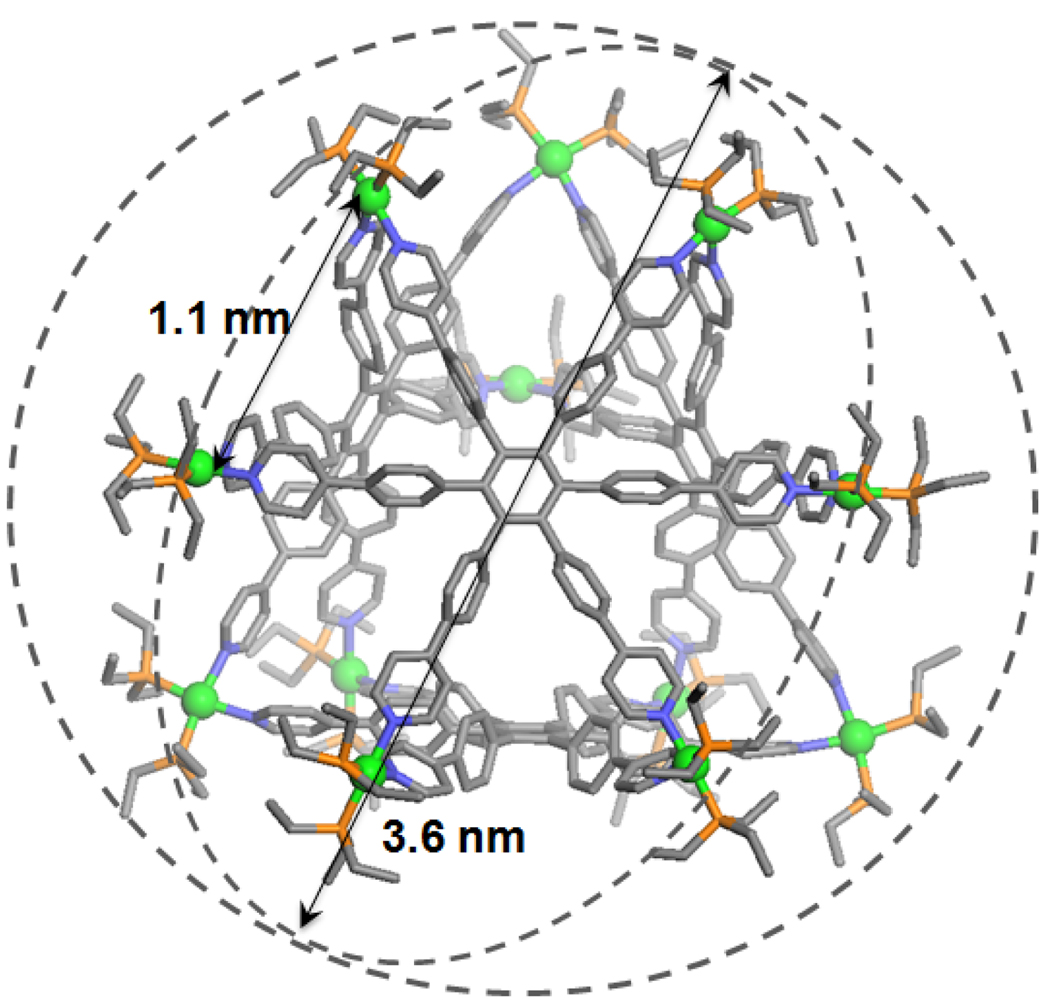
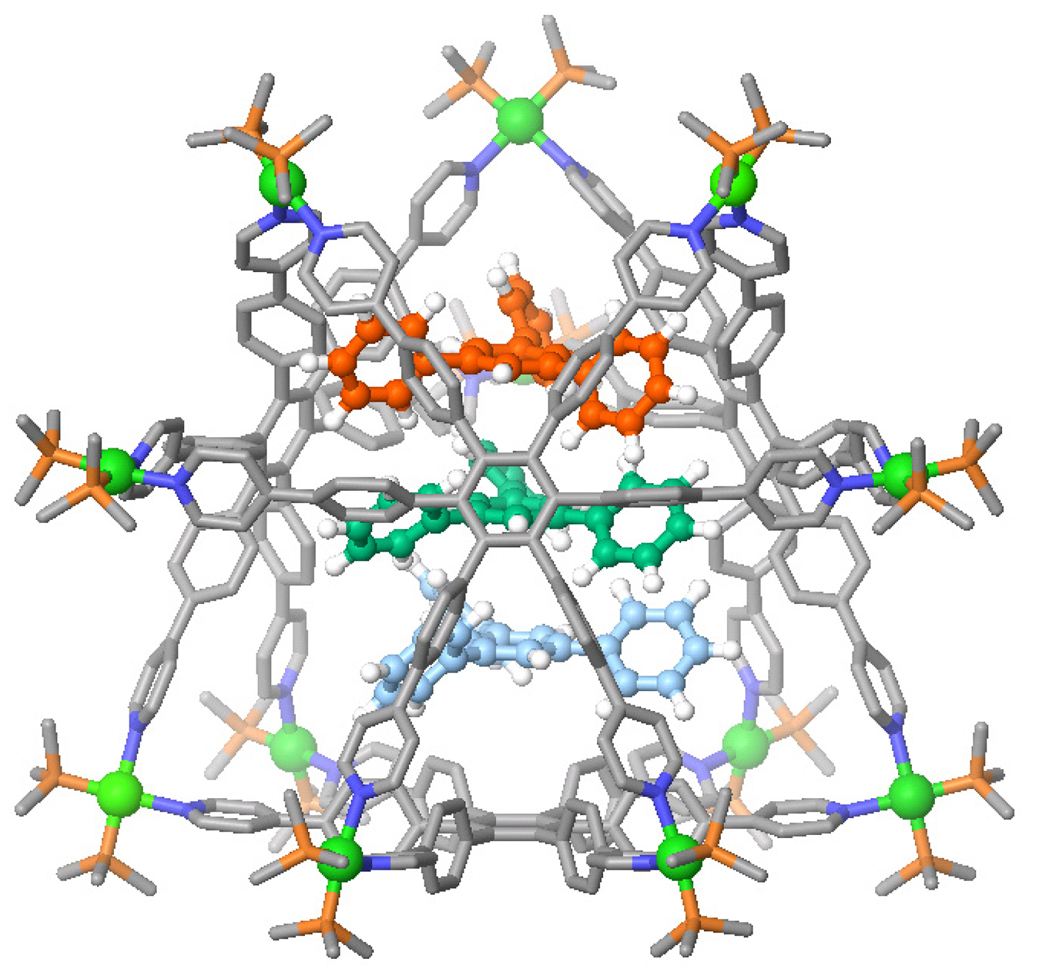
The structure of 3 was unambiguously determined by X-ray diffraction analysis using synchrotron radiation. X-ray quality crystals of 3a were obtained by slow diffusion of pentane into the acetone solution of 3a. As shown in Figure 2 and S4 in the Supporting Information, the structure has a truncated tetrahedral shape with a diameter of 3.6 nm, and bears a 1.0 nm cavity core. The shortest Pt-Pt distance is 1.1 nm. The PF6− anions are found surrounding the structure but not in the cavity, and the shortest Pt-F distance was measured to be 0.48 nm. As seen in Table S3 (see Supporting Information), the Pt atoms in the structure are coordinating with two PEt3 and two nitrogen atoms from the pyridine moieties in a distorted square planar geometry. The mean values from the square planar Pt(II) is N-Pt-N 80.6°, P-Pt-P 98.2°, N-Pt-P 90.6°, Pt-P 2.28 Å, and Pt-N 2.09 Å, respectively.
Figure 2.
Crystal structure of truncated tetrahedron 3a (Pt: green; C: grey; N: blue; P: Orange; protons, solvent, and PF6− omitted for clarity).
PGSE NMR measurements were also used to characterize the structures in solution. Using the translational self-diffusion coefficient measured by PGSE NMR in conjunction with the Stokes-Einstein equation, the “effective size” of the overall assembly in acetone-d6 was obtained: 3.35 ± 0.15 nm for 3a and 3.23 ± 0.21 nm for 3b, which are in good agreement with that of the crystal structure.
The host-guest properties of 3 were also studied. According to the symmetry and size of the host, 1,3,5-triphenylbenzene 4 was chosen for the investigation. The experiment was carried out by mixing 1b and 2 in a 3:1 ratio with excess triphenylbenzene 4 in an aqueous acetone solution (v/v 1:1). After 16 h of heating at 70 °C, the encapsulated complex 3b·43 was formed.
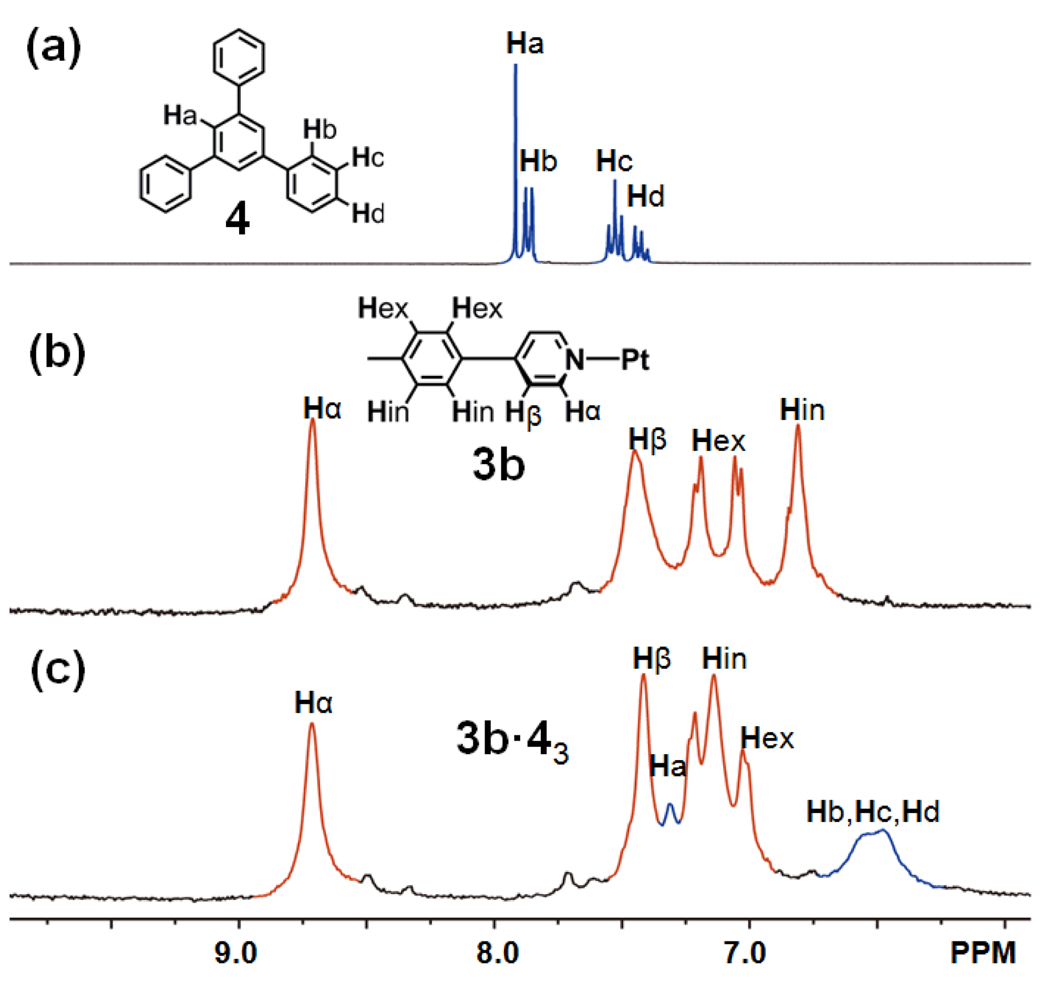
The singlet at −28.2 ppm with concomitant 195Pt satellites in the 31P{1H} NMR spectrum (see Supporting Information) and the identifiable peaks (δ = 8.76 ppm HPy-α-3b; δ = 7.36 ppm HPy-β-3b; δ = 6.94 ppm HPhenyl-3b) in the 1H NMR spectra (Figure 3b) show the formation of a truncated tetrahedron. By comparing the 1H NMR spectra of 4 (acetone-d6), 3b (acetone-d6/D2O = 1:1), and 3b·43 (acetone-d6/D2O = 1:1) in Figure 3, signals are found at 7.25 ppm (Δδ = −0.6 ppm, HPhenyl-4), 7.06 ppm (Δδ = 0.24 ppm, HPhenyl-3b), and 6.35 ppm (Δδ = −1.0–1.8 ppm, HPhenyl-4), indicating that the triphenylbenzene 4 is encapsulated in the truncated tetrahedron 3b, and integration of the peaks at 8.76 ppm (HPy-α-3b) and 6.35 ppm (HPhenyl-4) suggest that three guest molecules are encapsulated in each cage. In the ESI mass spectra (see Supporting Information), isotopically resolved signals at m/z = 2380.8 [3b·43 – 5OTf]5+ and m/z = 1959.3 [3b·43 – 6OTf]6+ confirm the complex 3b·43. Elemental analysis (see Supporting Information) of the isolated complex is also consistent with the composition of 3b·43.
Figure 3.
Partial 1H NMR spectra (300 MHz) of “free” 4 in acetone-d6 (a), 3b in acetone-d6/D2O (b), and the encapsulated complex 3b·43 in acetone-d6/D2O (c).
While X-ray quality crystals for 3b·43 were not obtained, a computational simulation was used to gain insight into the structural features of the encapsulated complex 3b·43.10 A molecular dynamics simulation using a molecular mechanics force field (MMFF), 300K, in the gas phase was used to equilibrate each supramolecule, and the output of the simulation was then minimized to full convergence. As shown in Figure 4, in the model of 3b·43, three triphenylbenzene molecules 4 are stacked within the cavity of 3b. The distance between these guests is about 4.3 Å, and that between the guest and the interior of the cage is 3.6–4.3 Å.
Figure 4.
Computational model (MMFF) of the encapsulated complex 3b·43 (For clarity, the three guest molecules are labeled as blue, green, and orange).
In conclusion, we report the facile synthesis of a new type of 3-D truncated tetrahedra via coordination-driven self-assembly, where the highly symmetrical hexapyridyl ligand acts as the faces and 90° organoplatinum acceptors are connectors at the edges. These truncated tetrahedra show a unique 3-D nanoscale pore, and preliminary studies indicate the nano-cavity is able to encapsulate 1,3,5-triphenylbenzene.
Supplementary Material
Acknowledgments
P.J.S. thanks the NIH (GM-057052) for financial support. K.W.C. and P.J.S. gratefully acknowledge the WCU program (R33-2008-000-10003-0) of the Korean Ministry of Education, Science and Technology for the support of this work. X-ray diffraction experiments using synchrotron radiation were performed at the Pohang Accelerator Laboratory supported by MOST and POSTECH in Korea.
Footnotes
Supporting Information Available: Experimental details for the synthesis and characterization of supramolecules 3 and the host-guest study. (http://pubs.acs.org/).
References
- 1.(a) Leininger S, Olenyuk B, Stang PJ. Chem. Rev. 2000;100:853. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]; (b) Seidel SR, Stang PJ. Acc. Chem. Res. 2002;35:972. doi: 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]; (c) Holliday BJ, Mirkin CA. Angew. Chem., Int. Ed. 2001;40:2022. [PubMed] [Google Scholar]; (d) Fujita M, Umemoto K, Yoshizawa M, Fujita N, Kusukawa T, Biradha K. Chem. Commun. 2001:509. [Google Scholar]; (e) Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc. Chem. Res. 2005;38:351. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]; (f) Fujita M, Tominaga M, Hori A, Therrien B. Acc. Chem. Res. 2005;38:369. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]; (g) Ruben M, Rojo J, Romero-Salguero FJ, Uppadine LH, Lehn J-M. Angew. Chem., Int. Ed. 2004;43:3644. doi: 10.1002/anie.200300636. [DOI] [PubMed] [Google Scholar]; (h) Severin K. Chem. Commun. 2006:3859. doi: 10.1039/b606632c. [DOI] [PubMed] [Google Scholar]; (i) Nitschke JR. Acc. Chem. Res. 2007;40:103. doi: 10.1021/ar068185n. [DOI] [PubMed] [Google Scholar]; (j) Oliveri CG, Ulmann PA, Wiester MJ, Mirkin CA. Acc. Chem. Res. 2008;41:1618. doi: 10.1021/ar800025w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Ward MD. Chem. Commun. 2009;30:4487. doi: 10.1039/b906726b. [DOI] [PubMed] [Google Scholar]; (l) Jin P, Dalgarno SJ, Atwood JL. Coord. Chem. Rev. 2010;254:1760. [Google Scholar]
- 2.(a) Fujita M, Oguro D, Miyazawa M, Oka H, Yamaguchi K, Ogura K. Nature. 1995;378:469. [Google Scholar]; (b) Parac TN, Caulder DL, Raymond KN. J. Am. Chem. Soc. 1998;120:8003. doi: 10.1021/ja0104507. [DOI] [PubMed] [Google Scholar]; (c) Johannessen SC, Brisbois RG, Fischer JP, Grieco PA, Counterman AE, Clemmer DE. J. Am. Chem. Soc. 2001;123:3818. doi: 10.1021/ja004007s. [DOI] [PubMed] [Google Scholar]; (d) Roche S, Haslam C, Adams H, Heath SL, Thomas JA. Chem. Commun. 1998:1681. [Google Scholar]; (e) Fujita M, Yu S-Y, Kusukawa T, Funaki H, Ogura K, Yamaguchi K. Angew, Chem. Int. Ed. Engl. 1998;37:2082. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2082::AID-ANIE2082>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]; (f) Olenyuk B, Whiteford JA, Fechtenkotter A, Stang PJ. Nature. 1999;398:796. doi: 10.1038/19740. [DOI] [PubMed] [Google Scholar]; (g) Schweiger M, Seidel SR, Schmitz M, Stang PJ. Org. Lett. 2000;2:1255. doi: 10.1021/ol005781n. [DOI] [PubMed] [Google Scholar]; (h) Olenyuk B, Levin MD, Whiteford JA, Shield JE, Stang PJ. J. Am. Chem. Soc. 1999;121:10434. [Google Scholar]; (i) Suzuki K, Kawano M, Sato S, Fujita M. J. Am. Chem. Soc. 2007;129:10652. doi: 10.1021/ja073629b. [DOI] [PubMed] [Google Scholar]; (j) Ghosh K, Hu J, White HS, Stang PJ. J. Am. Chem. Soc. 2009;131:6695. doi: 10.1021/ja902045q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Suzuki K, Tominaga M, Kawano M, Fujita M. Chem. Commun. 2009;13:1638. doi: 10.1039/b822311d. [DOI] [PubMed] [Google Scholar]; (l) Zheng Y-R, Ghosh K, Yang H-B, Stang PJ. Inorg. Chem. 2010;114:9007. doi: 10.1021/ic100330c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Sun Q-F, Iwasa J, Ogawa D, Ishido Y, Sato S, Ozeki T, Sei Y, Yamaguchi K, Fujita M. Science. 2010;328:1144. doi: 10.1126/science.1188605. [DOI] [PubMed] [Google Scholar]
- 3.(a) Rosi NL, Eckert J, Eddaoudi M, Vodak DT, Kim J, O'Keeffe M, Yaghi OM. Science. 2003;300:1127. doi: 10.1126/science.1083440. [DOI] [PubMed] [Google Scholar]; (b) Trembleau Laurent, Rebek Julius., Jr Science. 2003;301:1219. doi: 10.1126/science.1086644. [DOI] [PubMed] [Google Scholar]; (c) Yoshizawa M, Tamura M, Fujita M. Science. 2006;312:251. doi: 10.1126/science.1124985. [DOI] [PubMed] [Google Scholar]; (d) Pluth MichaelD, Bergman RobertG, Raymond KennethN. Science. 2007;316:85. doi: 10.1126/science.1138748. [DOI] [PubMed] [Google Scholar]
- 4.Northrop BH, Yang H-B, Stang PJ. Chem. Commun. 2008:5896. doi: 10.1039/b811712h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Sato S, Iida J, Suzuki K, Kawano M, Ozeki T, Fujita M. Science. 2006;313:1273. doi: 10.1126/science.1129830. [DOI] [PubMed] [Google Scholar]; (b) Kamiya N, Tominaga M, Sato S, Fujita M. J. Am. Chem. Soc. 2007;129:3816. doi: 10.1021/ja0693082. [DOI] [PubMed] [Google Scholar]; (c) Murase T, Sato S, Fujita M. Angew. Chem., Int. Ed. 2007;46:5133. doi: 10.1002/anie.200700793. [DOI] [PubMed] [Google Scholar]; (d) Zheng Y-R, Ghosh K, Yang H-B, Stang PJ. Inorg. Chem. 2010;49:4747. doi: 10.1021/ic100330c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Pluth MD, Bergman RG, Raymond KN. Acc. Chem. Res. 2009;42:1650. doi: 10.1021/ar900118t. [DOI] [PubMed] [Google Scholar]; (b) Pluth MD, Fiedler D, Mugridge JS, Bergman RG, Raymond KN. Proc. Natl. Acad. Sci. USA. 2009;106:10438. doi: 10.1073/pnas.0809806106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Brown CJ, Bergman RG, Raymond KN. J. Am. Chem. Soc. 2009;131:17530. doi: 10.1021/ja906386w. [DOI] [PubMed] [Google Scholar]; (d) Hastings CJ, Pluth MD, Bergman RG, Raymond KN. J. Am. Chem. Soc. 2010;132:6938. doi: 10.1021/ja102633e. [DOI] [PubMed] [Google Scholar]
- 7.(a) Yoshizawa M, Klosterman JK, Fujita M. Angew. Chem., Int. Ed. 2009;48:3418. doi: 10.1002/anie.200805340. [DOI] [PubMed] [Google Scholar]; (b) Murase T, Horiuchi S, Fujita M. J. Am. Chem. Soc. 2010;132:2866. doi: 10.1021/ja9107275. [DOI] [PubMed] [Google Scholar]; (c) Dolain C, Hatakeyama Y, Sawada T, Tashiro S, Fujita M. J. Am. Chem. Soc. 2010;132:5644. doi: 10.1021/ja100585w. [DOI] [PubMed] [Google Scholar]; (d) Horiuchi S, Nishioka Y, Murase T, Fujita M. Chem. Commun. 2010;46:3460. doi: 10.1039/c003191g. [DOI] [PubMed] [Google Scholar]; (e) Murase T, Otsuka K, Fujita M. J. Am. Chem. Soc. 2010;132:7864. doi: 10.1021/ja103109z. [DOI] [PubMed] [Google Scholar]
- 8.(a) Leininger S, Fan J, Schmitz M, Stang PJ. Proc. Natl. Acad. Sci. USA. 2000;97:1380. doi: 10.1073/pnas.030264697. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Schweiger M, Yamamoto T, Stang PJ, Blaeser D, Boese R. J. Org. Chem. 2005;70:4861. doi: 10.1021/jo050469i. [DOI] [PubMed] [Google Scholar]
- 9.(a) Rathore R, Burns CL, Guzei IA. J. Org. Chem. 2004;69:1524. doi: 10.1021/jo035598i. [DOI] [PubMed] [Google Scholar]; (b) Hoffmann M, Kärnbratt J, Chuan MH, Herz LM, Albinsson B, Anderson HL. Angew. Chem., Int. Ed. 2008;47:4993. doi: 10.1002/anie.200801188. [DOI] [PubMed] [Google Scholar]
- 10.Caskey DC, Yamamoto T, Addicott C, Shoemaker RK, Vacek J, Hawkridge AM, Muddiman DC, Kottas GS, Michl J, Stang PJ. J. Am. Chem. Soc. 2008;130:7620. doi: 10.1021/ja710715e. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.