Abstract
Throughout the body, the epithelial Na+ channel (ENaC) plays a critical role in salt and liquid homeostasis. In cystic fibrosis airways, for instance, improper regulation of ENaC results in hyperabsorption of sodium that causes dehydration of airway surface liquid. This dysregulation then contributes to mucus stasis and chronic lung infections. ENaC is known to undergo proteolytic cleavage, which is required for its ability to conduct Na+ ions. We have previously shown that the short, palate lung and nasal epithelial clone (SPLUNC1) binds to and inhibits ENaC in both airway epithelia and in Xenopus laevis oocytes. In this study, we found that SPLUNC1 was more potent at inhibiting ENaC than either SPLUNC2 or long PLUNC1 (LPLUNC1), two other PLUNC family proteins that are also expressed in airway epithelia. Furthermore, we were able to shed light on the potential mechanism of SPLUNC1's inhibition of ENaC. While SPLUNC1 did not inhibit proteolytic activity of trypsin, it significantly reduced ENaC currents by reducing the number of ENaCs in the plasma membrane. A better understanding of ENaC's regulation by endogenous inhibitors may aid in the development of novel therapies designed to inhibit hyperactive ENaC in cystic fibrosis epithelia.
Key words: mucociliary clearance, chronic airway disease, cystic fibrosis, protease, airway surface liquid, Na+ absorption
Introduction
ENaC is the rate-limiting step for Na+ absorption in the airways.1–3 The importance of appropriately regulated ENaC activity is illustrated by the pathogenesis that results when this channel fails to function correctly. For example, hyperabsorption of Na+ through ENaC has been proposed as an initiating event in cystic fibrosis (CF) lung disease.4,5 This likely occurs because Na+ hyperabsorption contributes to mucus dehydration and mucus stasis, which prevents clearance of inhaled pathogens.4–6 Furthermore, Na+ hyperabsorption, volume depletion, and inflammation have recently been demonstrated in transgenic mice overexpressing ENaC, thus directly linking ENaC and the initiation of chronic lung disease.7 In contrast, as ENaC is downregulated in the monogenic disorder, pseudohypoaldosteronism, it leads to an abundance of airway surface liquid and mucus transport rates that are significantly increased above normal levels.8
ENaC is regulated by intracellular second messengers including cAMP and PIP2.9,10 However, extracellular serine pro-teases also regulate ENaC.1,11 Rossier and colleagues initially identified a membrane-bound serine protease that acts as a channel activating protease (CAP) that they termed CAP1.12 The activation of ENaC by CAP1 can be mimicked by external addition of trypsin and the effects are not additive, indicating that CAP1 and trypsin act via the same pathway. Additional CAPs, termed CAP2 (TMPRSS4) and CAP3 (matriptase) have also been identified as being able to activate ENaC by increasing its open probability (Po), without any significant change in channel density or conductance.13,14 The effect of these CAPs can be blocked by the Kuitz-type serine protease inhibitor aprotinin,12,14 which prevents the cleavage of ENaC and the subsequent conduction of Na+ in both primary human bronchial epithelial cultures (HBECs) and Xenopus oocytes.15–17
The palate lung and nasal epithelial clone (PLUNC) family can be subdivided into short (SPLUNC) and long (LPLUNC) proteins that contain either one or two subdomains respectively.18 In this classification, the original protein called PLUNC is now SPLUNC1.19 SPLUNC1 is expressed in submucosal glands of normal individuals,20 and expression is increased in cystic fibrosis (CF) lungs, especially in the surface epithelia of the conducting airways.21 SPLUNC1 is an abundant protein secreted into the airway surface liquid of HBECs.22 Early characterization of SPLUNC1's sequence identity uncovered some structural homology with anti-microbial proteins and incited theories that SPLUNC1 would similarly function in a direct innate host defense capacity.23 To date, however, this theory remains uncertain. Investigators have reported minimal anti-microbial function of SPLUNC1,24 and no binding to lipopolysaccaride, as might be expected from its strong homology to lipopolysaccaride binding proteins.22 In contrast to its putative anti-microbial actions, we identified SPLUNC1 as a potent inhibitor of ENaC in both Xenopus oocytes and in human airway epithelia.25 We also found that SPLUNC1 binds to ENaC and prevents its proteolytic cleavage by serine proteases including trypsin, CAP1 and CAP2.25 Since little is known about SPLUNC1-ENaC interactions, we further explored their relationship and here we report that among the other PLUNC family members expressed in HBECs, SPLUNC1 alone significantly inhibits ENaC. We also demonstrate that SPLUNC1 exerts its effects on ENaC by lowering the number of ENaCs available for cleavage at the plasma membrane.
Results
We have previously demonstrated that SPLUNC1 is secreted into the media of SPLUNC1-injected oocytes where it binds to the extracellular loops of the α, β, γ ENaC subunits, resulting in ENaC inhibition.25 Data-mining of existing gene array data revealed that other PLUNC family members are expressed in our HBEC system, suggesting that other PLUNCs may be secreted into the airway surface liquid and could also regulate ENaC.26 Specifically, LPLUNC1 was endogenously expressed in HBECs and SPLUNC2 was not expressed under basal conditions. After exposing HBECs to supernatant of mucopurulent material (SMM), which is derived from CF airway secretions and acts as a powerful pro-inflammatory agent,27 LPLUNC1 and SPLUNC1 expression were not altered. However, SPLUNC2 expression increased by 2.4- and 1.5-fold at 6 and 24 h post-SMM addition, respectively.26 To test whether these other PLUNC family members could also affect ENaC activity, we expressed α, β, γ ENaC in Xenopus oocytes and measured their subsequent amiloride-sensitive current under control conditions and following coinjection with either SPLUNC1, SPLUNC2 or LPLUNC1. ENaC currents were reduced by ∼70% when SPLUNC1 was coinjected into the oocytes (Fig. 1). In contrast, co-injection of SPLUNC2, which has 24% amino acid homology to SPLUNC1, or LPLUNC1, which has two domains rather than the single domain of the SPLUNC subgroup, and has 30% amino acid homology to SPLUNC1, had no significant effect on ENaC activity (Fig. 1).
Figure 1.
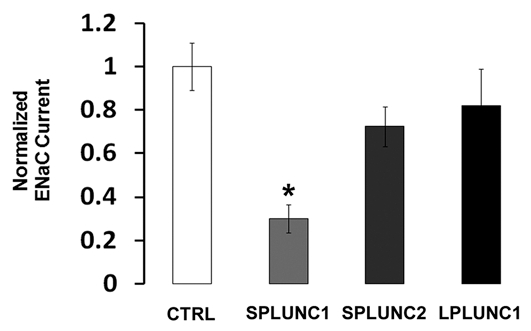
SPLUNC1, but not other PLUNC family members, inhibits ENaC activity. Current is displayed relative to amiloride-sensitive current from α, β, γENaC-expressing oocytes (ctrl, white bar; n = 18). Oocytes co-expressing SPLUNC1 showed a ∼70% reduction in ENaC current (p < 0.0001; n = 22). However, those co-expressing SPLUNC2 (n = 22) or oocytes co-expressing LPLUN C1 had no significant ENaC current reduction (n = 17). * denotes p < 0.05 different to control oocytes expressing α, β, γ ENaC.
To understand how SPLUNC1 regulated ENaC, we examined whether SPLUNC1 could alter the proteolytic activity of trypsin. We have previously observed significant inhibition of ENaC by SPLUNC1 at 50 ng/ml, which is equivalent to 1.79 nM. At this concentration, SPLUNC1 did not alter the ability of trypsin to cleave a serine protease-specific fluorogenic substrate, while the trypsin-specific inhibitor aprotinin, also administered at 1.79 nM, abolished proteolytic cleavage (Fig. 2). Thus, we conclude that the regulation of ENaC by SPLUNC1 does not result from direct protease inhibition.
Figure 2.
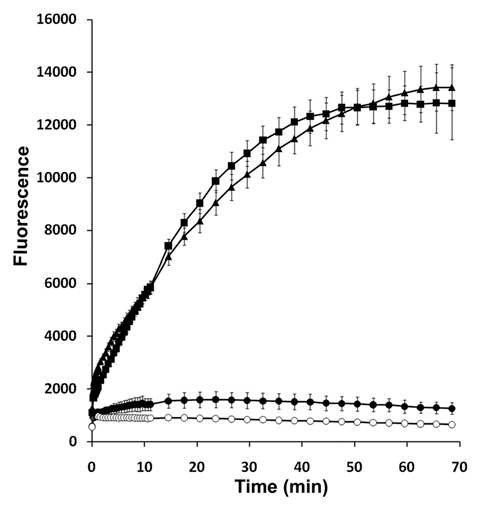
SPLUNC1 is not a protease inhibitor. The trypsin-specific fluorogenic trypsin substrate Boc-Gln-Ala-Arg-MCA, emits fluorescence upon cleavage. Trypsin (1 U/ml, ■); trypsin and aprotinin (1.79 nM, ●); trypsin and SPLUNC1 (1.79 nM, ▲). Boc-Gln-Ala-Arg-MCA alone (○). All n = 4.
We have previously shown that SPLUNC1 can inhibit both basal and trypsin-stimulated ENaC currents.25 The most straightforward explanation for this phenomenon is that that the number of ENaCs in the plasma membrane had been reduced by SPLUNC1. To test this hypothesis directly, we surface -biotinylated oocytes and then probed for αENaC surface expression levels in the presence and absence of SPLUNC1. SPLUNC1 indeed decreased the total amount of αENaC in the plasma membrane, as compared to controls that did not express SPLUNC1 (Fig. 3A). In contrast SPLUNC1 did not affect whole-cell αENaC protein levels (Fig. 3B).
Figure 3.
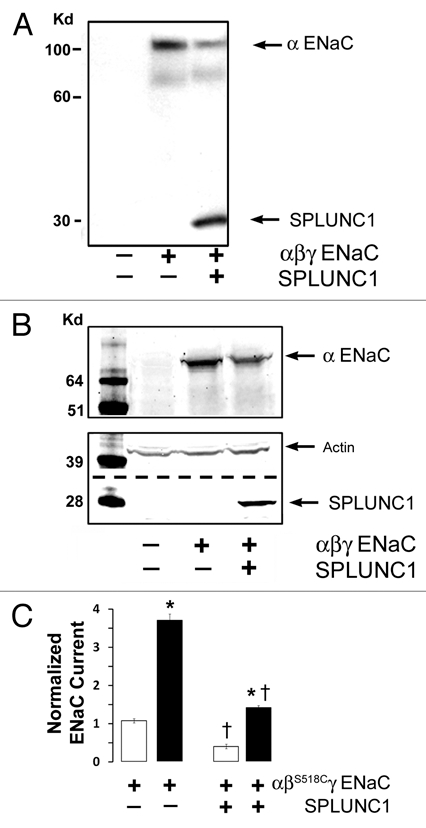
SPLUNC1 decreases the number of αENaC subunits in the plasma membrane. (A) Surface biotinylation of αENaC shows that plasma membrane ENaC is decreased following coexpression with SPLUNC1 in Xenopus oocytes. Samples were analyzed by western blot using an anti-V5 (for V5/6His-tagged SPLUNC1 and for V5-CT-tagged αENaC) monoclonal antibody Lane 1, control; Lane 2, αβγENaC; Lane 3, αβγENaC & SPLUNC1. Total lysate per lane was equivalent to 3–4 oocytes and was run on a 10% Gel. (B) Whole cell western blot of oocytes probing for αENaC (top), SPLUNC1 and actin (bottom) shows that total ENaC levels are not decreased by SPLUNC1. Total lysate per lane was equivalent to 3–4 oocytes and was run on a 12% Gel. (C) Addition of MTSET to ENaC containing the βS518C mutant increases ENaC Po to 1.0 when coexpressed in oocytes yet the overall current is still reduced by SPLUNC1 expression. Open bars, control. Closed bars, MTSET addition, n = 6 for each group. * denotes p < 0.05 different ± MTSET; † denotes p < 0.05 different ± SPLUNC1.
In order to confirm SPLUNC1's ability to reduce ENaC levels in the plasma membrane, we used the sulfhydryl-reactive reagent [2-(trimethylammonium) ethyl methanethiosulfonate, bromide] (MTSET), which locks ENaC containing a S518C mutation in the β subunit (βS518C) into the fully open position and thus yields an electrical approximation of the number of active channels in the plasma membrane.28 With this system, a reduction in α, βS518C, γ-ENaC current in the presence of MTSET would indicate fewer ENaCs in the plasma membrane, which we observed in oocytes coinjected with SPLUNC1 (Fig. 3C). Moreover, the fold-stimulation of α, βS518C, γ-ENaC currents by MTSET was 5.32 ± 0.45 while groups co-expressing SPLUNC1 had a 8.05 ± 0.48 fold increase. This indicates that ENaC resident on the cell surface in SPLUNC1 expressing oocytes reside in a low open probability state. Together with the enhanced fold-stimulation by trypsin,25 these results suggest that SPLUNC1 limits proteolysis of ENaC by reducing ENaC surface density (Fig. 3C).
Discussion
We have previously reported that SPLUNC1 inhibits ENaC, likely via direct, extracellular binding to all three ENaC subunits.25 Following on from these findings, we were curious to know whether the other PLUNC family members that are expressed in our HBEC system would produce similar inhibitory effects. While SPLUNC1 inhibits ENaC by ∼70%, SPLUNC2 and LPLUNC1 do not significantly inhibit ENaC (Fig. 1). These data are in keeping with our previous observations that the knockdown of SPLUNC1 to greater than 90% by shRNA upregulated ENaC activity in HBECs and led to airway surface liquid volume hyperabsorption, despite expression of other PLUNC family members in these epithelia.25 Interestingly, SPLUNC1 is able to reduce ENaC currents by 70% when applied at 50 ng/ml (1.79 nM) either in the Xenopus oocytes or in HBECs lacking endogenous SPLUNC1,25 while this concentration of SPLUNC1 produces negligible direct effects on the proteolytic activity of trypsin, suggesting that SPLUNC1 does not act as a protease inhibitor (Fig. 2). Thus, based on this and our previous data,25 we conclude that SPLUNC1 acts by directly interacting with ENaC and not by direct inhibition of extracellular proteases.
The mechanism of SPLUNC1's interaction with ENaC is currently unknown. However, SPLUNC1 inhibits both basal- and trypsin-activated ENaC currents,25 which may provide additional insight into its mechanism of action. The macroscopic ENaC current (I) is a product of the single channel conductance (g) multiplied by both the open probability (Po) and the number of channels in the plasma membrane (N). Proteolytically-induced changes in I have been mainly attributed to altered Po, which can be induced by trypsin exposure and rapidly reversed by aprotinin.12 In contrast, we found that acute trypsin exposure failed to fully reverse the effects of SPLUNC1,25 suggesting that SPLUNC1's actions are not exclusively limited to changes in Po. Our present data indicate that SPLUNC1 reduces the number of ENaC subunits at the plasma membrane, without affecting overall cellular ENaC levels (Fig. 3A and B). These data are consistent with the effects of SPLUNC1 on ENaC currents (Fig. 3C). For example, such a reduction in N would serve to lower basal ENaC currents in addition to preventing MTSET from further activating ENaC. While we have demonstrated this mechanism exclusively in oocytes (Fig. 3), we have previously shown that SPLUNC1 decreases the ENaC/trypsin-sensitive potential difference in HBECs, suggesting that SPLUNC1 inhibits ENaC in a similar mechanism in airway epithelia.25
ENaC is trafficked to and from the plasma membrane in a highly regulated fashion29–31 and we speculate that SPLUNC1's binding to ENaC may directly induce a conformational change in ENaC that triggers its internalization. Alternatively, SPLUNC1 has several putative adaptor protein 2 (AP2) binding domains and adaptor proteins have been identified as components in the ENaC internalization pathway.32,33 Thus, a complex containing SPLUNC1, ENaC and AP2 may facilitate ENaC's internalization. Finally, SPLUNC1 may also act as a pore blocker, in a fashion analogous to amiloride, though with less reversible binding.34 Such behavior could lead to a reduction in both basal and protease-activated ENaC currents and cannot be formerly excluded although this mode of inhibition alone is inconsistent with the observed decrease in N (Fig. 3).
In summary, we have shown that SPLUNC1, but not other PLUNC family members, induces a potent inhibition of macroscopic ENaC current. While the mechanism of SPLUNC1's inhibition of ENaC is not fully understood, we have found that SPLUNC1 is capable of reducing the number of ENaC multimers at the plasma membrane. This is the first report of an endogenously secreted protein that can facilitate removal of ENaC from the plasma membrane, and identifies a potential new avenue for therapeutic regulation of Na+ absorption.
Methods
SPLUNC1 protein purification.
V5/6His-tagged SPLUNC1 was stably expressed in HEK293 cells and purified from their media as previously described using ethanol and acetone precipitation.21 Media collected from HEK293 cells not expressing SPLUNC1 was purified using the same ethanol/acetone precipitation method and was used as a control.
Oocyte studies.
Xenopus laevis oocytes were harvested and injected as described.25 In brief, defolliculated healthy stage V–VI oocytes were injected with 0.3 ng of cRNA of each ENaC subunit or co-injected with 1 ng of SPLUNC1 cRNA. Injected oocytes were kept in modified Barth's saline (in mM: 88 NaCl, 1 KCl, 2.4 NaHCO3, 0.3 Ca(NO3)2, 0.41 CaCl2, 0.82 MgSO4 and 15 HEPES, adjusted to pH 7.35 with Tris). Oocytes were studied 24 h after injection using the two-electrode voltage clamp technique as previously described.17,25 Oocytes were clamped at a holding potential of −60 mV. The change in amiloride-sensitive whole cell current as an indicator of ENaC activity was determined by subtracting the corresponding current value measured in the presence of 10 µM amiloride from that measured before the application of amiloride. For each group, whole cell currents were presented as relative currents to control oocytes injected with ENaC and water. Thus, these control groups are assigned a relative current of 1, as units reported are nA/nA.
Western blotting.
Protein was harvested from Xenopus oocytes, resolved using SDS-PAGE and transferred to PVDF. The membrane was then probed using an anti-V5 monoclonal antibodies (Invitrogen) as previously described.25
Surface labeling.
Xenopus oocytes were injected with V5-CT(C-terminus)-tagged α, untagged-β, γ rat ENaC subunits (0.3 ng each) ± V5/6His-tagged SPLUNC1 (1 ng). After 24 h, oocytes (70 per experimental condition) were prechilled on ice for 30 minutes and labeled with 0.7 mg/ml sulfo-NHS-biotin in MBS-Ca++ (mM), 85 NaCl, 1 KCl, 2.4 NaHCO3, 0.82 MgSO4, 0.41 CaCl2, 0.33 Ca(NO3), 16.3 HEPES titrated to pH 8.0 with NaOH, while tumbling gently for 20 min at 4°C. Oocytes were washed twice with chilled MBS-Ca++ buffer and incubated in MBS-Ca++ buffer with 100 mM glycine for 10 min at 4°C to quench free biotin. Oocytes were washed again three times with chilled MBS-Ca2+ buffer. Proteins were extracted as previously described.25 Total inputs were taken from whole cell samples representing 4% of total protein. Solubilized proteins were incubated with 100 µl of neutravidin beads (Pierce) overnight while tumbling at 4°C. Samples were washed twice with (mM) 500 NaCl 50 Tris pH 7.5 buffer and once with 150 NaCl 50 Tris pH 7.5 buffer. Laemli buffer was added and samples were loaded on a 15% gradient Tris-glycine gel after incubation for 10 minutes at 96°C. Samples were transferred to PDVF membranes and western blot analysis was performed using anti-V5 (Invitrogen) and anti-actin (Chemicon International) monoclonal antibodies.
Trypsin MCA assay.
The trypsin fluorogenic substrate assay was performed using 96 well black plates (Corning Costar). Boc-Gln-Ala-Arg-MCA (100 µM; Peptides International) was added to each well in 100 µl Ringer total volume. Aprotinin (Sigma-Aldrich) or SPLUNC1 as appropriate were then combined with the substrate, and trypsin (Sigma-Aldrich) was then added at 1 U/ml to initiate the reaction. Boc-Gln-Ala-Arg-MCA was excited at 380 ± 5 nm and emission was collected at timed intervals at 460 ± 15 nm using a Tecan Infinite multi-plate reader.
Statistical analyses.
All data are presented as the mean ± SE for n experiments. Each oocyte study was repeated on three separate occasions. Differences between means were tested for statistical significance using paired or unpaired t tests or their non-parametric equivalent as appropriate to the experiment. Differences between groups were judged using ANOVA. From such comparisons, differences yielding p ≤ 0.05 were judged to be significant.
Acknowledgements
We gratefully acknowledge the kind gifts of SPLUNC1, LPLUNC1 and SPLUNC2 cDNA from Dr. Colin Bingle, University of Sheffield, UK. We appreciate Dr. Ray Caldwell's helpful insights and we also thank Mike Watson, Yan Dang and Hong He for excellent technical assistance. Funded by the CFF TARRAN07G0 and by NIH P50 HL084934, P01 HL034322 and R01 HL080561.
Abbreviations
- ENaC
epithelial sodium channel
- PLUNC
palate lung and nasal epithelial clone
- CAP
channel activating protease
- HBECs
primary human bronchial epithelial cultures
- CF
cystic fibrosis
- g
single channel conductance
- Po
open probability
- N
channel number
- MTSET
sulfhydryl-reactive reagent [2-(trimethylammonium)ethyl methaneth iosulfonate, bromide]
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/12255
References
- 1.Rossier BC, Stutts MJ. Activation of the epithelial sodium channel (ENaC) by serine proteases. Annu Rev Physiol. 2009;71:361–379. doi: 10.1146/annurev.physiol.010908.163108. [DOI] [PubMed] [Google Scholar]
- 2.Gaillard EA, Kota P, Gentzsch M, Dokholyan NV, Stutts MJ, Tarran R. Regulation of the epithelial Na+ channel and airway surface liquid volume by serine proteases. Pflugers Arch. 2010;460:1–17. doi: 10.1007/s00424-010-0827-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–289. doi: 10.1152/physrev.00026.2001. [DOI] [PubMed] [Google Scholar]
- 4.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chmiel JF, Davis PB. State of the art: why do the lungs of patients with cystic fibrosis become infected and why can't they clear the infection? Respir Res. 2003;4:8. doi: 10.1186/1465-9921-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 7.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 8.Kerem E, Bistritzer T, Hanukoglu A, Hofmann T, Zhou Z, Bennett W, et al. Pulmonary epithelial sodium-channel dysfunction and excess airway liquid in pseudohypoaldosteronism. N Engl J Med. 1999;341:156–162. doi: 10.1056/NEJM199907153410304. [DOI] [PubMed] [Google Scholar]
- 9.Ma HP, Chou CF, Wei SP, Eaton DC. Regulation of the epithelial sodium channel by phosphatidylinositides: experiments, implications and speculations. Pflugers Arch. 2007;455:169–180. doi: 10.1007/s00424-007-0294-3. [DOI] [PubMed] [Google Scholar]
- 10.Pochynyuk O, Bugaj V, Stockand JD. Physiologic regulation of the epithelial sodium channel by phosphatidylinositides. Curr Opin Nephrol Hypertens. 2008;17:533–540. doi: 10.1097/MNH.0b013e328308fff3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughey RP, Carattino MD, Kleyman TR. Role of proteolysis in the activation of epithelial sodium channels. Curr Opin Nephrol Hypertens. 2007;16:444–450. doi: 10.1097/MNH.0b013e32821f6072. [DOI] [PubMed] [Google Scholar]
- 12.Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 1997;389:607–610. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- 13.Rossier BC. The epithelial sodium channel: activation by membrane-bound serine proteases. Proc Am Thorac Soc. 2004;1:4–9. doi: 10.1513/pats.2306007. [DOI] [PubMed] [Google Scholar]
- 14.Vuagniaux G, Vallet V, Jaeger NF, Pfister C, Bens M, Farman N, et al. Activation of the amiloride-sensitive epithelial sodium channel by the serine protease mCAP1 expressed in a mouse cortical collecting duct cell line. J Am Soc Nephrol. 2000;11:828–834. doi: 10.1681/ASN.V115828. [DOI] [PubMed] [Google Scholar]
- 15.Bridges RJ, Newton BB, Pilewski JM, Devor DC, Poll CT, Hall RL. Na+ transport in normal and CF human bronchial epithelial cells is inhibited by BAY 39-9437. Am J Physiol Lung Cell Mol Physiol. 2001;281:16–23. doi: 10.1152/ajplung.2001.281.1.L16. [DOI] [PubMed] [Google Scholar]
- 16.Myerburg MM, Butterworth MB, McKenna EE, Peters KW, Frizzell RA, Kleyman TR, et al. Airway surface liquid volume regulates ENaC by altering the serine protease-protease inhibitor balance: A mechanism for sodium hypersabsorption in cystic fibrosis. J Biol Chem. 2006 doi: 10.1074/jbc.M606449200. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson SH, Hirsh A, Li DC, Holloway G, Chao J, Boucher RC, et al. Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem. 2002;277:8338–8345. doi: 10.1074/jbc.M105044200. [DOI] [PubMed] [Google Scholar]
- 18.Bingle CD, Craven CJ. PLUNC: a novel family of candidate host defence proteins expressed in the upper airways and nasopharynx. Hum Mol Genet. 2002;11:937–943. doi: 10.1093/hmg/11.8.937. [DOI] [PubMed] [Google Scholar]
- 19.Bingle CD, Gorr SU. Host defense in oral and airway epithelia: chromosome 20 contributes a new protein family. Int J Biochem Cell Biol. 2004;36:2144–2152. doi: 10.1016/j.biocel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Bingle L, Cross SS, High AS, Wallace WA, Devine DA, Havard S, et al. SPLUNC1 (PLUNC) is expressed in glandular tissues of the respiratory tract and in lung tumours with a glandular phenotype. J Pathol. 2005;205:491–497. doi: 10.1002/path.1726. [DOI] [PubMed] [Google Scholar]
- 21.Bingle L, Barnes FA, Cross SS, Rassl D, Wallace WA, Campos MA, et al. Differential epithelial expression of the putative innate immune molecule SPLUNC1 in cystic fibrosis. Respir Res. 2007;8:79. doi: 10.1186/1465-9921-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campos MA, Abreu AR, Nlend MC, Cobas MA, Conner GE, Whitney PL. Purification and characterization of PLUNC from human tracheobronchial secretions. Am J Respir Cell Mol Biol. 2004;30:184–192. doi: 10.1165/rcmb.2003-0142OC. [DOI] [PubMed] [Google Scholar]
- 23.Bingle CD, LeClair EE, Havard S, Bingle L, Gillingham P, Craven CJ. Phylogenetic and evolutionary analysis of the PLUNC gene family. Protein Sci. 2004;13:422–430. doi: 10.1110/ps.03332704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartlett JA, Hicks BJ, Schlomann JM, Ramachandran S, Nauseef WM, McCray PB., Jr PLUNC is a secreted product of neutrophil granules. J Leukoc Biol. 2008;83:1201–1206. doi: 10.1189/jlb.0507302. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Caballero A, Rasmussen JE, Gaillard E, Watson MJ, Olsen JC, Donaldson SH, et al. SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage. Proc Natl Acad Sci USA. 2009;106:11412–11417. doi: 10.1073/pnas.0903609106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribeiro CM, Hurd H, Wu Y, Martino ME, Jones L, Brighton B, et al. Azithromycin treatment alters gene expression in inflammatory, lipid metabolism and cell cycle pathways in well-differentiated human airway epithelia. PLoS One. 2009;4:5806. doi: 10.1371/journal.pone.0005806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribeiro CM, Paradiso AM, Schwab U, Perez-Vilar J, Jones L, O'Neal W, et al. Chronic airway infection/ inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J Biol Chem. 2005;280:17798–17806. doi: 10.1074/jbc.M410618200. [DOI] [PubMed] [Google Scholar]
- 28.Anantharam A, Tian Y, Palmer LG. Open probability of the epithelial sodium channel is regulated by intracellular sodium. J Physiol. 2006;574:333–347. doi: 10.1113/jphysiol.2006.109173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotin D, Kanelis V, Schild L. Trafficking and cell surface stability of ENaC. Am J Physiol Renal Physiol. 2001;281:391–399. doi: 10.1152/ajprenal.2001.281.3.F391. [DOI] [PubMed] [Google Scholar]
- 30.Butterworth MB, Edinger RS, Frizzell RA, Johnson JP. Regulation of the epithelial sodium channel by membrane trafficking. Am J Physiol Renal Physiol. 2009;296:10–24. doi: 10.1152/ajprenal.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gormley K, Dong Y, Sagnella GA. Regulation of the epithelial sodium channel by accessory proteins. Biochem J. 2003;371:1–14. doi: 10.1042/BJ20021375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimkets RA, Lifton RP, Canessa CM. The activity of the epithelial sodium channel is regulated by clathrin-mediated endocytosis. J Biol Chem. 1997;272:25537–25541. doi: 10.1074/jbc.272.41.25537. [DOI] [PubMed] [Google Scholar]
- 33.Wiemuth D, Ke Y, Rohlfs M, McDonald FJ. Epithelial sodium channel (ENaC) is multi-ubiquitinated at the cell surface. Biochem J. 2007;405:147–155. doi: 10.1042/BJ20060747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schild L, Schneeberger E, Gautschi I, Firsov D. Identification of amino acid residues in the alpha, beta and gamma subunits of the epithelial sodium channel (ENaC) involved in amiloride block and ion permeation. J Gen Physiol. 1997;109:15–26. doi: 10.1085/jgp.109.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]


