Abstract
Background & Aims
Lymphoepithelial interactions in the gut can occur in the epithelium and the sub-epithelial space. We asked whether Normal, Crohn’s Disease (CD) or Ulcerative colitis (UC) lamina propria lymphocytes (LPL) could promote intestinal epithelial cell (IEC) growth and differentiation.
Methods
T84 cells were co-cultured with freshly isolated LPL for varying periods. After removal of LPL, IECs were lysed and subjected to i) measurement of intestinal alkaline phosphatase (IAP) activity; ii) Western blot analysis for MAPK and Akt activation; and iii) Real Time-PCR to assess CDX2 mRNA levels. Tissue sections were immunostained for evidence of MAPK and PI3K activation, CDX2 and IAP; and CDX2 mRNA expression was assessed on human colonic biopsies.
Results
IAP activity was increased in T84 cells co-cultured for 8 days with Normal LPL (p<0.05), and even greater with CD LPL (p<0.001). Crypt IECs in active CD mucosa expressed IAP ex vivo. Phospho-MAPK (ERK1/2, p38, and JNK) and phospho-Akt were seen as early as 30 min after co-culture. MAPK activation was greatest in T84 cells co-cultured with CD LPL. There was a specific increase in P-p38 MAPK and P-Akt staining in the nuclei of crypt IECs in active vs inactive CD, normal mucosa and UC mucosa. CDX2 mRNA expression was increased in CD LPL co-cultured T84 cells which not correlated with the CDX2 protein localization ex vivo.
Conclusion
Our observations indicate that there is crosstalk between LPL and IECs, which leads to IEC differentiation. Moreover, in CD mucosa, the differentiation of IEC is accelerated.
INTRODUCTION
Intestinal epithelial cells (IECs) provide the first line of defense for the host by preventing the entry of potentially dangerous microorganisms into underlying lymphoid tissues. In the intestine, two major lymphocyte populations exist: the intraepithelial lymphocytes (IEL) which remain associated with the basolateral membrane of the IECs, and the LPL which localize to the subepithelial lamina propria and are in contact with IECs via basolateral projections through the semi-porous basement membrane1, 2. Previous studies from our lab suggested that IECs function as antigen-presenting cells (APCs) and, as such, can promote regulatory T-cell responses in the mucosa3-8. In a reverse interaction, studies by Chen et al. suggested that IEL could induce IEC differentiation via the production of keratinocyte growth factor (KGF)9. Thus, lymphoepithelial interactions have the potential to promote barrier function as well as regulate mucosal immune responses. The control of immune responses in the gut is critical for normal immune homeostasis in the host. Failure to control such responses has been proposed as one mechanism in the development of inflammatory bowel disease (IBD)1.
In one murine model of IBD, the Interleukin-10 (IL-10)-deficient mouse10, lymphocyte development and antibody responses are normal, but most animals are growth retarded and anemic. Profound alterations are present in the intestine of these animals, such as a chronic enterocolitis that can involve the entire intestinal tract; and is associated with either hyperregenerative or degenerative lesions of the intestinal epithelia. The typical architecture of the mucosa is disturbed by the formation of abnormal crypt and villus structures consisting of branched and fused villi, enlarged and branched crypts, and labyrinthine sheets of enterocytes on the surface10. These findings suggest that IL-10 and probably other factors involved in regulating inflammation may have a role in controlling IEC homeostasis: proliferation vs. differentiation.
Cell proliferation, lineage-specific differentiation, migration, and finally apoptosis and/or cell shedding are tightly regulated processes that are spatially and temporally regulated along the crypt/surface axis in the colon. The epithelium is characterized by its rapid and constant renewal. This process involves cell generation and migration from the stem cell populations located at the bottom of the crypt to the extrusion of terminally differentiated cells at the tip of the villus11, 12. Thus, the crypt is mainly composed of proliferative and poorly differentiated cells, whereas the villus is lined with functional absorptive, goblet, and endocrine cells13. The molecular and cellular mechanisms responsible for the fine coordination between proliferation, migration, and differentiation along the crypt-villus axis are still largely unknown.
Several studies suggest that the intestine specific, caudal-related cdx1 and cdx2 homeobox genes encode nuclear transcription factors that play a critical role in IEC proliferation and differentiation. In contrast to CDX1 which is mainly expressed in the crypt compartment (although not restricted to proliferative cells)14, the CDX2 homeoproteins are mainly expressed in differentiating enterocytes15, triggering growth retardation and cell differentiation by overexpression in several intestinal lines in vitro16. Furthermore, genes regulated by either CDX1 or CDX2 generally define a functional differentiated phenotype, such as sucrase-isomaltase16, dipeptidyl peptidase IV14, or intestinal alkaline phosphatase (IAP)15.
The mitogen-activated protein kinase (MAPK) family, such as extracellular signal-regulated kinase (ERK), Jun amino-terminal kinase (JNK), and p38 MAPK, has been shown to play various roles in regulating gene expression via transcription factor phosphorylation. This signaling pathway has been implicated in IEC differentiation. Elevated ERK1/2 activities stimulate cell cycle progression of IECs, whereas low level activities are correlated with G1 arrest and differentiation17. Moreover, p38 MAPK is rapidly activated in IECs induced to differentiate, and this activation enhances CDX2 transcriptional activity16.
Another signaling pathway, phosphatidylinositol 3-kinase/Akt (PI3K/Akt), has been implicated in cellular differentiation with conflicting reports regarding its ability to promote or inhibit IEC differentiation18-21. Laprise et al. described that PI3K is necessary for functional and morphological differentiation of IECs. The recruitment of PI3K appears to be essential for the integrity of the adherens junctions via Akt and p38 MAPK activation19. In contrast, Evers et al reported that the PI3K/Akt pathway mediates proliferative signals in IECs18, 20, 21, and, by the antagonistic effect of PTEN (phosphatase and tensin homologue deleted from chromosome 10) on the PI3K/Akt pathway, regulates the intestine-specific cdx2 homeobox gene18.
The purpose of this study was to better understand the dysregulation of IECs that occurs in the mucosa of IBD patients. To do so, the role of LPL in IEC differentiation was investigated using a new experimental model set up in our laboratory, i.e., freshly isolated LPL derived from the mucosa of normal or CD patients were co-cultured with IEC lines, such as T84. T84 cells co-cultured with CD LPL displayed a greater increase of IAP activity than with normal LPL. Both Akt and the MAPK signaling pathways were activated in T84 cells co-cultured with normal and CD LPL, but the level of CDX2 mRNA was significantly higher in T84 cells co-cultured with CD LPL. These findings were validated ex vivo using tissue sections of normal, inactive and active CD and ulcerative colitis (UC) colonic mucosa. Our findings suggest that there is crosstalk between LPL and IECs, leading to IEC differentiation and in CD mucosa this differentiation is accelerated
MATERIALS AND METHODS
Cell lines
T84, a human carcinoma cell line derived from a colon carcinoma, and Caco-2, a human colon carcinoma cell line with enterocytic properties, were obtained from the American Type Culture Collection (ATCC, Manassas, VA). T84 cells were cultured in DMEM/F12 (Invitrogen, Carlsbad, CA) /5% heat-inactivated FCS (Hyclone, Logan, UT), and Caco-2 cells in DMEM (Invitrogen) /10% heat-inactivated FCS and 1% non essential amino-acids (Invitrogen). HT29 Clone 16E, a goblet cell line and derivative of the HT29 human colonic cancer cell line was a gift of Pr. C.L. Laboisse22. This cell line was cultured in DMEM/10% heat-inactivated FCS.
Isolation of IECs and LPLs from human intestinal mucosa
Surgical specimens from patients undergoing colon resection for IBD or cancer or biopsies from patients undergoing colonoscopy at the Mount Sinai Medical Center were used as a source of IEC and LPL. The surgical specimens obtained from IBD patients were all from non inflamed areas. Isolation of IECs and LPLs was performed as previously described in our laboratory using Dispase II (Roche Diagnostics, Alameda, CA) and Collagenase treatment6. The biopsies were incubated in RPMI containing 3mg/ml Dispase II for 15-30 min, with vortexing every 5min, in a 37°C water bath. During this treatment, IECs were released from the tissues. The cell suspension resulting from the dispase treatment was washed twice, pelleted, and then, processed for Trizol extraction (Invitrogen) or further analysis.
Co-culture assay
The IEC lines were seeded in 6 or 12 well plates the night before the experiment in a non confluent state (40-50 % confluency), or on regular and inverted Transwells® with a diameter of 6.5 mm and a pore size of 3 m (Corning Inc., Corning, NY) in a confluent state. During the LPL isolation, the cells were serum-deprived. The co-cultures were set up with a ratio of 1 IEC: 1 LPL. For the Transwells® studies, the LPL were incubated on the basolateral side of the filter. In some experiments, the IEC lines were preincubated with 100nM wortmanin (Calbiochem, San Diego, CA), a PI-3K inhibitor, for 90 min prior to co-culture. The inhibitor was removed before the co-culture.
The IEC line and LPL alone served as negative controls for all studies.
Intestinal Alkaline Phosphatase activity
After one hour, 1, 2, 4 or 8 days, T84 cells were washed with cold PBS containing Ca2+ and Mg2+ in order to remove the LPL, then frozen at −20°C. The cells were lysed in 350ul of 0.5% Triton X-100, 10mM Tris-HCl [pH 8], and 150mM NaCl. 50l of each sample was mixed with 150ul of a p-nitrophenyl phosphate solution (Sigma-Aldrich, St. Louis, MO). After a 30min incubation at RT, the absorbance at 405nm was measured. The protein content of each sample was quantified using the Biorad DC kit (Biorad, Hercules, CA). Caco-2 cells exhibit a constitutively high level of IAP activity23, 24, so, we used this cell line as a positive control in our experiments.
Real-Time PCR analysis for human CDX2 mRNA expression
After 4 days, the T84 cells were washed with PBS in order to remove the LPL, and then, processed for Trizol extraction (Invitrogen). We used a previously described protocol25. Briefly, 5 μg total RNA were converted into cDNA and was used for a 40 cycle three-step PCR using an ABI Prism 7900 (PE Applied Biosystems, Foster City, CA) in 20 mM Tris, pH 8.4, 50 mM KCl, 5 mM MgCl2, 200 μM deoxynucleoside triphosphates, 0.5x SYBR Green I (Molecular Probes, Inc., Eugene, OR), 200 nM each primer, and 0.5 U Platinum Taq (Invitrogen). The number of target copies in each sample was extrapolated from its detection threshold (CT) value using a plasmid or purified PCR product standard curve included on each plate. Three different housekeeping genes were used as controls. Each transcript in each sample was assayed three times, and the median CT values were used to calculate the FOLD INCREASE IN GENE EXPRESSION as 2-ΔΔCT where the ΔΔCT corresponds to (Mean CTTARGET NORMAL- CTTARGET UC/CD)-(Mean CTCONTROL NORMAL- CTCONTROL UC/CD). By definition ΔΔCT in the control group equals 0 and 20 equals 1. With this method, we normalized our results to the reference genes and to the control group. The primers used in this study are presented in the supplementary material.
The same experimental procedure was used for real-time PCR analysis using RNA from freshly isolated IECs from biopsies.
Western Blot analysis
After 30 min to 3 hours of co-culture, the T84 cells were washed with cold PBS in order to remove the LPL, and frozen at −80°C. The cells were scraped from the plastic at 4°C into 150ul of lysis buffer (50mM Tris-HCl [pH 7.5], 150 mM NaCl, 1%NP-40, 2 mM Na3VO4, 1 mM 4-(2-aminoethyl)-benzene sulfonyl fluoride, 10 mM NaF, 5 mM NaPPi, 10 mM -glycerophosphate). The lysate was then sonicated and solubilized for 30min at 4°C, centrifuged at 14,000xg for 20min at 4°C. The protein concentration of the supernatant was determined as described above.
Equal protein concentrations of whole-cell lysates were resolved by 10% SDS-PAGE. The proteins were transferred onto a polyvinylidene fluoride membrane (Immobilon-P, Millipore, Billerica, MA) and incubated overnight at 4°C with either anti-phospho Akt, anti-phospho ERK1/2, anti-phospho p38, anti-phospho JNK, anti-Akt (Cell Signaling, Danvers, MA) or anti-ERK2 (Santa Cruz Biotechnology, Santa Cruz, CA), and HRP-conjugated anti-rabbit or anti-mouse antibodies (Cell Signaling). The binding of antibodies was revealed with an enhanced chemiluminescence detection system (ECL, Amersham Biosciences, Pittsburgh, PA).
Intestinal Alkaline Phosphatase staining
Biopsies from patients undergoing endoscopic examination (Normal, inactive and active CD and UC ) and colonic tissues from C57BL/6 (WT) and RAG1−/− mice, obtained from The Jackson Laboratory, Bar Harbor, ME., were paraffin-embedded. All mice were housed under specific-pathogen-free conditions in individually ventilated cages at the Mount Sinai School of Medicine Animal Facility. All experiments were performed following institutional guidelines. Tissue sections were dewaxed and rehydrated. IAP was stained according to the manufacturer’s protocol using the BCIP/NBT Alkaline Phosphatase Substrate Kit IV (Vector Laboratories, Burlingame, CA). The slides were then mounted with a coverslip using Vectashield Hard Set (Vector Laboratories). The slides were examined with a Zeiss Axioskop light microscope at 20X magnification.
Immunohistochemistry
For immunohistochemistry, tissue sections were dewaxed, rehydrated, and endogenous peroxidase activity was quenched with 1.5% H2O2 in methanol (15 min RT). Antigen retrieval was performed by heating for 10min at 100°C in 0.01 M sodium citrate. The stainings were done according to the manufacturer’s protocol using a rabbit or mouse Histostain-Plus kit (Invitrogen). The tissues were incubated overnight with either anti-phospho Akt, anti-phospho PTEN, anti-cyclin D1, anti-phospho ERK1/2, anti-phospho p38, anti-phospho JNK (dilution 1/50, Cell Signaling), or anti-CDX2 (Biogenex) antibodies in PBS containing 0.1% Triton-X100, or Ready-to-Use anti-CDX2 (Biogenex, San Ramon, CA). The slides were counter-stained with Mayer’s Hematoxylin Solution (Sigma-Aldrich). The slides were then mounted with a coverslip using Vectashield Hard Set (Vector Laboratories). The slides were examined with a Zeiss Axioskop light microscope at 20X and 100X magnification.
Statistical analysis
Results are presented as the mean +/− standard deviation. Because of the variability of human samples, statistical significance was determined by One-way Anova followed by post-test Newman-Keuls, t-test followed by Mann-Whitney test, or One-way Anova followed by Kruskal-Wallis test. p < 0.05 was considered significant.
Densitometric analysis
The changes in band intensity were quantified by densitometric analysis using the Scion Image program for PC. All experiments were repeated at least 3 times, and a representative result is shown for each experiment.
RESULTS
Normal LPL induce an increase of IAP activity in T84 cells
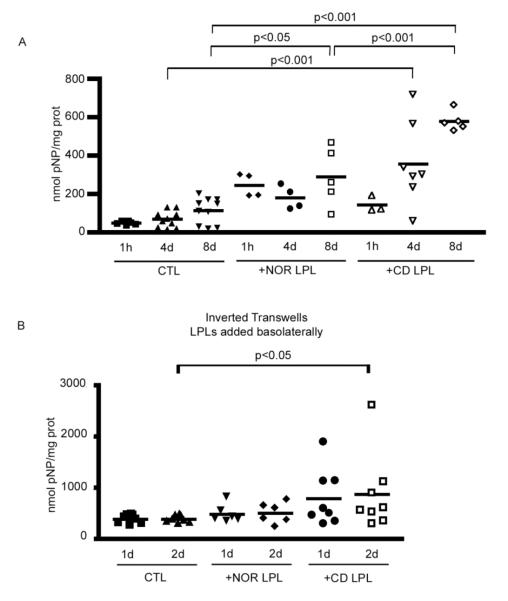
Intestinal Alkaline Phosphatase (IAP) is exclusively expressed in villus enterocytes in normal mucosa and hence serves as an excellent marker of crypt-villus differentiation23, 24. The putative effect of normal LPL on epithelial differentiation was investigated using T84, a crypt-like intestinal epithelial cell line, co-cultured with freshly isolated normal LPL for 1 hour, 4 and 8 days (Fig 1A). These different time points were based on the kinetics of T84 cell differentiation. We followed differentiation by quantifying IAP activity. Normal LPL induced a slight increase in IAP activity after 4 days of co-culture with non-polarized T84 cells (179.3+/−60.9 nmol pNP/mg prot vs. 67.2+/−41.6), and this reached significance after 8 days (287.6+/−151.8 nmol pNP/mg prot vs. 110.9+/−67.7, p<0.05), suggesting that normal LPL have an effect on IEC differentiation.
Figure 1.
A. IAP activity in T84 cells co-cultured with Nor or CD LPL for 1 hour, 4 or 8 days. Adherent cells were co-cultured with freshly isolated LPL at a ratio of 1 IEC : 1 LPL. After removing the LPL, the IEC line was lysed and IAP activity (nmol pNP/mg prot) was measured by a colorimetric enzymatic assay and normalized to the protein content in each sample. B. IAP activity in inverted polarized T84 cells co-cultured with Nor or CD LPL for 1 or 2 days. Adherent cells were co-cultured and IAP activity determiend as described in 1A.
CD LPL induce a greater increase in IAP activity than normal LPL
The colonic mucosa of IL10 KO mice has been reported to display abnormal biclonal crypts10 before the presence of active inflammation. We were interested to determine whether IBD LPL played a role in this process. T84 cells were co-cultured with freshly isolated CD or UC LPL for 1 hour, 4 and 8 days. CD LPL induced a highly significant increase in IAP activity as rapidly as after 4 days of co-culture with T84 cells (Fig 1A) (355.7+/−217.8 nmol pNP/mg prot vs. 67.2+/−41.6, p<0.001); this increase was even greater after 8 days (576.4+/−51.2 nmol pNP/mg prot vs. 110.9+/−67.7, p<0.001).
We performed this assay using Caco-2 and HT29 Cl16E cells (data not shown) and each cell line showed a different pattern of differentiation in the presence of LPL, particularly with CD LPL. The T84 cell line was the most responsive in terms of an increase in IAP activity. This cell line mimicks an intestinal crypt cell that can potentially explain its susceptibility to differentiate. Non-polarized Caco-2 cells are known to be highly differentiated (baseline IAP activity was already quite high), so further differentiation was difficult to detect. The HT29 Cl16E cell line is a goblet like cell line, and IAP activity is not an accurate marker of differentiation.
Freshly isolated LPL from UC patients had a cytotoxic effect on the IEC lines so these results are not shown. We are currently trying to better understand this phenomenon.
Taken together, these findings are consistent with enhanced differentiation of intestinal crypt cells induced by CD LPL.
IAP induction due to CD LPL is contact dependent
In order to determine whether IEC differentiation in the presence of CD LPL was mediated by soluble factors or required cell contact, T84 cells were seeded on conventional or inverted Transwells® the night before the experiment. The inverted Transwells® were flipped the morning of the LPL isolation. LPL were added to the basolateral side of the IECs allowing contacts between T84 cells and LPL while assessing the involvement of soluble factors in conventional Transwells®. IAP activity was assessed after 1 or 2 days of co-culture (Fig 1B). In T84 cells co-cultured with either Nor or CD LPL in conventional Transwells®, the IAP activity was comparable to the control (data not shown). In T84 cells co-cultured with. When T84 cells were co-cultured with CD LPL in inverted Transwells®, IAP activity was increased after 1 day (781.3+/−550 nmol pNP/mg prot vs. 377+/−85.7), and this induction was significant after 2 days of co-culture (858+/−753.9 nmol pNP/mg prot vs. 377+/−69.9, p<0.05). In contrast, with Nor LPL the IAP activity was comparable to controls
These findings strongly suggest that the enhancement of IEC differentiation by CD LPL is contact dependent.
Normal, CD and UC LPL induce PI3K dependent Akt activation in T84
The PI3K/Akt pathway has been shown to affect proliferation, differentiation, and apoptosis in a number of cells26, including IECs. PI3K was shown to modulate enterocyte-like differentiation in HT29 and Caco-2 cells, as well as goblet cell differentiation18, 20, 21, 27. Therefore, the activation of Akt was assessed in T84 cells co-cultured with normal, CD or UC LPL for 30 min to 3 hours. WB analysis was performed using an antibody directed against the phosphorylated (activated) form of Akt (Fig 2). Normal, CD and UC LPL induced the phosphorylation of Akt in T84 cells within 30 min of co-culture (Fig 2A) and this lasted at least 3 hours. WB assessing total Akt revealed the presence of comparable total Akt protein in the analyzed samples. Freshly isolated LPL, a control for the presence of residual LPL in the co-cultures., did not demonstrate Akt phosphorylation (data not shown), suggesting that the Akt activation seen in T84 cells was specifically induced by the LPL. In a preliminary experiment, we analyzed the phosphorylation of Akt at varying ratios of IEC:LPL (1:1 or 1:2), and showed that the ratio of 1:2 led to a more robust, but not earlier, response (data not shown). However, since the differences were not dramatic and given the presence of limiting cell numbers, we used a 1:1 ratio for subsequent experiments.
Figure 2.
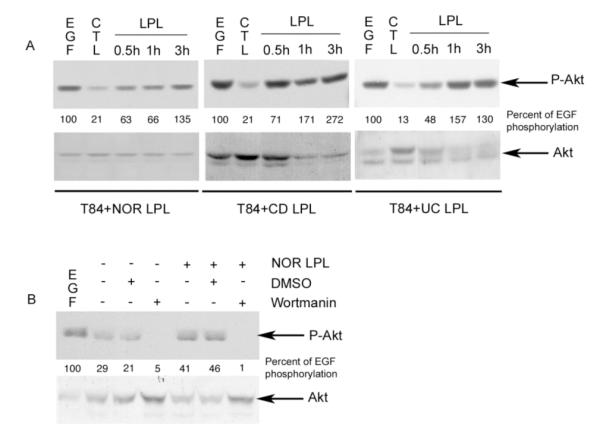
PI3K activation in T84 cells co-cultured with LPL. A. Immunoblotting for P-Akt and Akt in lysates obtained from T84 cells co-cultured with freshly isolated Nor, CD or UC LPL for 30 min to 3 hours. The CTL (control) lane contains T84 alone. EGF (10 nM; 15min) was used as a positive control. B. Immunoblotting for P-Akt and Akt in lysates obtained from T84 cells pre-treated for 90 min with DMSO or Wortmannin (100 nM), washed out and co-cultured with freshly isolated Nor LPL for 1 hour. The CTL lanes are as described in 2A. (Representative of 3 experiments). The percentage of Akt activity related to EGF induced activity was quantified by densitometric analysis.
In order to confirm that the Akt activation was PI3K dependent, pharmacological inhibition of this pathway was performed. T84 cells were pretreated for 90 min with either DMSO (negative control) or Wortmanin (100nM), washed with PBS before the co-culture in order to remove the inhibitor, and then co-cultured for 3 hours with normal LPL. Wortmanin treatment did not affect the viability of T84 cells. The effect of wortmanin on phospho-Akt induction was assessed by WB analysis (Fig 2B). PI3K inhibition totally abrogated Akt phosphorylation in T84 cells co-cultured with normal LPL. Thus, PI3K and subsequently Akt are activated when T84 cells are co-cultured with freshly isolated Nor, CD or UC LPL.
Normal, CD and UC LPL activate the MAPK pathway in T84 cells
The MAPK pathway is also involved in intestinal epithelial cell proliferation and differentiation. ERK1/2 has been shown to modulate the microvillus architecture of IECs and p38 MAPK has been shown to be involved in adherens junction assembly, and expression of several differentiation markers including IAP17, 19. Therefore, the activation of the three MAPK members (ERK1/2, p38 MAPK, and JNK) was assessed in T84 cells co-cultured with normal, CD or UC LPL for 30 min to 3 hours. We performed a WB analysis using specific antibodies directed against the phosphorylated forms of ERK1/2, p38 MAPK, and JNK (supplementary data). Normal, and to a greater extent CD and UC, LPL led to ERK1/2, p38 MAPK, and JNK activation within 30 min of co-culture. WB using an antibody recognizing all ERK2 proteins confirmed equivalent loading of protein.
Thus, the MAPK pathway is activated when IEC are co-cultured with freshly isolated LPL as well, however, IBD LPL (both CD and UC) induced a stronger activation of the MAPK pathway.
CD LPL induce an increase in the expression of CDX-2 in colonocyte-like cells
p38 MAPK and PI3K/Akt pathways are thought to play an important role in intestinal epithelial cell differentiation via activation of the intestine-specific transcription factor CDX216, 18, 19. Moreover, it was recently shown that CDX2 alters the proliferation of intestinal crypt cells and stimulates the expression of distinct intestinal differentiation markers14, 15. We therefore examined CDX2 mRNA by Real-Time PCR in T84 cells co-cultured with normal, CD or UC LPL for 4 days (Fig 3). The level of CDX2 mRNA in T84 cells co-cultured with Nor LPL was significantly increased (2.28+/−1.10.58 fold increase vs. 0.80+/−0.515 in control cells, p<0.005) (Fig 3), and this was further increased when the T84 cells were co-cultured with CD LPL (3.98+/−2.91.45 fold increase, p<0.005). As mentioned above, freshly isolated LPL from UC patients had a cytotoxic effect on the IEC lines at 4 days.
Figure 3.
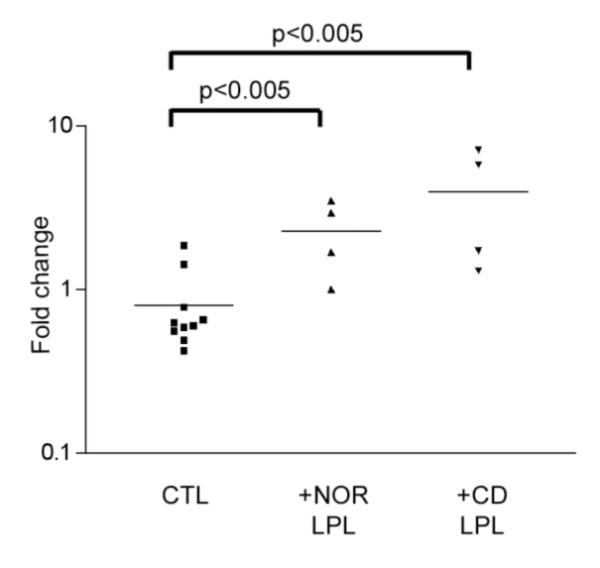
CDX2 mRNA expression in T84 cells co-cultured with Nor or CD LPL for 4 days. Adherent cells were co-cultured as described in 1A. After removing the LPL, T84 mRNA was extracted and quantified by Real Time-PCR. The fold increase in CDX2 mRNA expression was calculated as described in Materials and Methods.
Preliminary studies using supernatants from LPL cultured for 2 days and co-cultured with the IEC line failed to induce CDX2 mRNA expression (data not shown), suggesting that CDX2 expression may be contact dependent.
Ex vivo histological correlation of in vitro findings: IAP staining
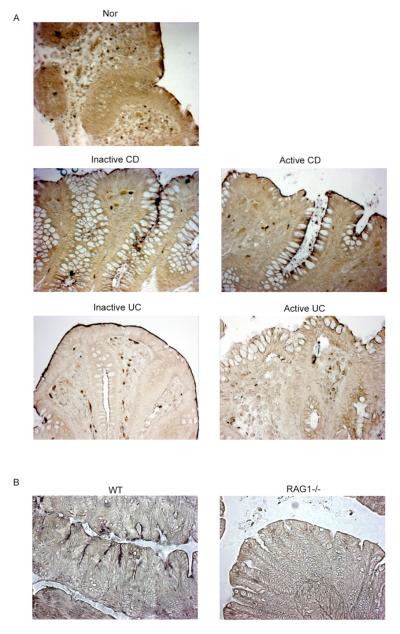
To further investigate whether the in vitro findings, described above, correlated with changes ex vivo, we performed a series of histological analyses. We first assessed IAP staining in colonic mucosa of normal, inactive and active CD and UC patients (Fig 4A). In normal mucosa, IAP staining was restricted to the apical surface of the surface epithelium, as reported. In contrast, IAP expression extended down the crypt-surface axis in inactive CD mucosa, with greater and more extensive expression in active CD mucosa. In inactive UC mucosa, the staining was restricted to the apical surface of the epithelium (similar to normal), and this staining was almost undetectable in active UC mucosa.
Figure 4.
A. IAP staining of paraffin embedded colonic tissue of Nor, inactive and active CD, and inactive and active UC. The slides were examined with a Zeiss Axioskop light microscope at 20X magnification. These data are representative of 4 experiments. B. IAP staining of paraffin embedded colonic tissue from WT and RAG1−/− mice (20X magnification). (Representative of 4 experiments).
These data are consistent with the in vitro findings of enhanced IAP expression induced by CD LPL and suggest that the changes seen are not just related to the presence of inflammation.
If LPLs are involved in normal IEC differentiation, one would predict that IEC differentiation would be altered in the absence of LPL. To evaluate this in vivo, we analyzed IAP expression in the colons of RAG1−/− mice. As seen in figure 4B, IAP staining was absent in colonocytes in RAG1−/− mice while expressed (albeit in a patchy manner) in WT mice.
These data support the crosstalk between IEC and LPL which leads to IEC differentiation.
Anti-phospho Akt and anti-phospho PTEN staining of colonic biopsies show a different localization pattern in normal vs. inactive and active CD and UC mucosa
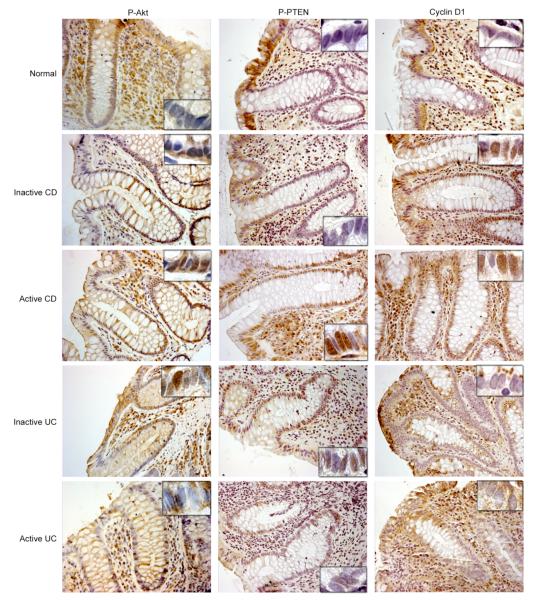
We next analyzed phospho-Akt expression by immunohistochemistry (Fig 5). The localization of phospho-Akt in normal mucosa revealed basolateral expression in the upper 2/3 of the surface IEC. In contrast, expression extended into the nuclei of the crypt in inactive and active CD and inactive UC mucosa, but to a lesser extent in active UC mucosa. The phospho-Akt staining exhibited a nuclear pattern in both CD and UC, suggesting that this effect may be inflammation related.
Figure 5.
Nor, inactive and active CD, and inactive and active UC colonic tissue sections were immunostained using anti-phospho Akt, anti-phospho PTEN, and anti-Cyclin D1 antibodies. The slides were counter-stained with Mayer’s Hematoxylin solution, and examined with a Zeiss Axioskop light microscope at 20X magnification. The insets represent 100X magnification of the crypt epithelial cells to document nuclear localization. (Representative of 4 experiments).
To determine the factors regulating phospho-Akt, we analyzed the expression of phosphatase and tensin homologue, PTEN. Since PTEN, antagonizes the effects of PI3K on phosphoinositide phosphorylation, the loss of PTEN permits the constitutive activation of signaling downstream from PI3K18. The phosphorylated (inactive) form of PTEN was analyzed in colonic biopsies by immunohistochemistry (Fig 5). The pattern of phospho-PTEN expression was similar to that seen for phospho-Akt, with the most striking observation being the abundance of the nuclear staining along the crypt-surface axis in active CD mucosa. This was less remarkable in active UC mucosa. As the PI3K/PTEN pathway seemed dysregulated in active CD mucosa, we investigated one of the nuclear targets downstream of Akt, cyclin D1. This protein is involved in the balance between cell cycle and cell arrest. Interestingly, normal mucosa exhibited surface epithelial staining (Fig 5). This staining tended to become more nuclear in the crypt of inactive and active CD. In contrast to CD mucosa, the staining of UC mucosa was almost the same as normal mucosa.
Thus, the PI3K pathway appears to be much more activated in epithelial cells from CD mucosa than UC mucosa. Crypt cells, which are undifferentiated in the normal state, were the most dysregulated cell type in CD.
Nuclear phospho-MAPK staining is increased in active CD mucosa vs. normal mucosa
The MAPK pathway was also analyzed by immunohistochemistry ex vivo (supplementary data). Normal and inactive CD mucosal epithelium revealed almost the same phospho-ERK1/2 staining, i.e. cytoplasmic expression in surface epithelium, while active CD mucosa exhibited largely nuclear staining of the active forms of ERK1/2 all through the crypt-surface axis. This staining was much more remarkable in UC mucosa, suggesting that this effect might be inflammation related.
Phospho-p38 MAPK staining revealed a localization mostly in the surface epithelium and some sporadic positive nuclei along the crypt-surface axis in normal and inactive CD mucosa. In active CD mucosa, almost all the epithelium were extensively stained, including expression in the nuclei of surface and crypt IEC. Interestingly, inactive and active UC mucosa did not reveal the presence of any phospho-p38 MAPK along the crypt, suggesting that the translocation of Phospho-p38 MAPK to the nuclei of crypt IEC might be specific to CD.
Phospho-JNK staining was barely detectable in the basolateral side of the surface epithelium in normals and was more nuclear in the crypt of inactive CD and UC, and active CD mucosa. In contrast, active UC mucosa showed greater expression of the active form in the surface epithelium. Thus, p38 MAPK, like PI3K, seems to be altered in crypt epithelial cells in CD mucosa.
CDX2 protein expression is increased in crypt epithelial cells of IBD mucosa
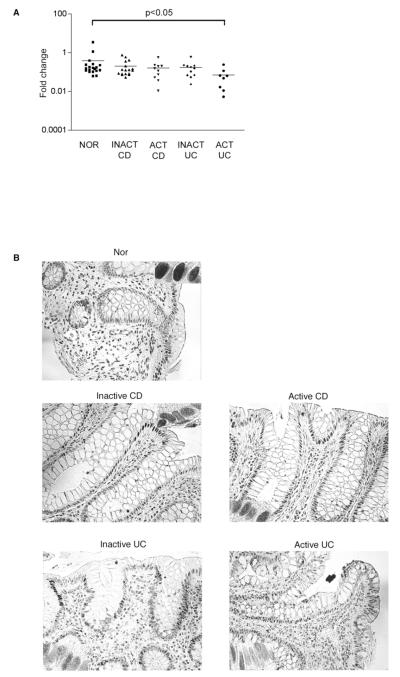
In order to confirm the involvement of CDX2 in the dysregulated differentiation of IECs in CD mucosa, surface colonic IECs derived from biopsies were isolated and analyzed for their CDX2 mRNA expression level by real-time PCR. There was large variability from one sample to another within the same disease type (Fig 6A). In surface IECs from active UC biopsies, the expression level of CDX2 mRNA was significantly decreased compared to normal IECs (0.07+/−0.08 fold increase vs 0.39+/−0.79, p<0.05), potentially accounting for the decrease in IAP staining (Fig 4A). This last result suggests that there is a strong correlation between the absence of IAP staining and the decrease in CDX2 mRNA expression, potentially explaining the decrease in IEC maturity in UC patients.
Figure 6.
A. CDX2 mRNA expression in surface IECs from Nor, inactive and active CD, and inactive and active UC colonic biopsies. The IECs were isolated, mRNA extracted and quantified by Real Time-PCR. The fold increase of CDX2 mRNA expression was calculated as described in Materials and Methods. B. Colonic tissue sections were immunostained and analyzed as described in Fig 5 using anti-CDX2 antibodies. The insets represent 100X magnification of the surface epithelial cells. (Representative of 4 experiments).
In order to correlate the mRNA expression with the protein localization, we stained colonic tissue sections for CDX2 (Fig 6B). CDX2 expression is localized in the nuclei of surface IECs in Nor and CD. CDX2 is also expressed in the nuclei of crypt IEC in CD and UC. However, there was a decrease in CDX2 expression in surface epithelial cells in UC. The crypt staining in UC might be due to a differently dysregulated pathway.
Taken together these data fit with the in vitro effect of CD LPL on CDX2 expression in T84 cells and the ex vivo IAP staining of crypt IECs, i.e. CDX2 is involved in the dysregulation of IEC differentiation in IBD.
DISCUSSION
Defects in epithelial barrier function have been described in IBD (CD, UC). Blair et al. described that MLCK upregulation is connected with barrier dysfunction and IBD pathogenesis28; however, the pathophysiologic mechanisms leading to this dysfunction are not well defined. Prior studies from our laboratory have demonstrated interactions between IECs and lymphocytes leading to lymphocyte activation. In this manuscript, we asked whether lymphocytes can affect epithelial cell differentiation and whether this effect is altered in IBD. Epithelial regeneration was reportedly found to be accelerated in UC. UC is a preneoplastic disease characterized by chronic epithelial damage and ulceration resulting in epithelial regeneration and loss of IEC differentiation due to high turnover. The normal differentiation pattern of colonic epithelial cells is characterized by growth in the crypts with maturation of cells as they move to the surface. In UC, the regenerative zone has been shown to extend into the upper part of the crypt. This process is exaggerated in the presence of active inflammation.
Based on the immunologic phenotypes of CD and UC, we asked whether CD mucosa displayed the same epithelial dysregulation and whether this could be driven by interactions with LPL.
In this study, we showed that Nor LPL promoted T84 cell differentiation. Using both in vitro and ex vivo systems (supplementary Table 2 summarizes the in vitro and ex vivo findings), we documented an increase in crypt epithelial cell maturation in colonic CD mucosa driven by LPL, and this pattern was restricted to CD. Inflamed UC epithelium did not show evidence of enhanced colonocyte differentiation. The enhanced IEC differentiation in CD correlated with the severity of disease but was not purely inflammation related as it did not occur in UC mucosa and was cell contact dependent as i) IAP was only induced in inverted T84 cell polarized monolayers co-cultured with CD LPL in the basolateral compartment, and ii) supernatants from CD LPL did not promote similar changes. These findings are consistent with other data from our group showing the nuclear localization of SOX9 and β-catenin in crypt epithelial cells in colonic CD mucosa, i.e. the dysregulation and acceleration of crypt epithelial cell differentiation in CD (unpublished data). Söderholm et al. reported that increased endosomal uptake of the protein antigen horseradish peroxidase (HRP) into enterocytes was correlated with the expression of TNF mRNA in histologically unaffected ileal mucosa of CD patients29. The process of endocytosis is believed to occur preferentially in highly differentiated enterocytes, providing further indirect evidence of enhanced differentiation. In our study, we showed that IAP is upregulated in crypt epithelial cells of CD but not in UC mucosa, suggesting that these IECs are more mature than normal crypt IECs. The enhancement of maturation of IECs in CD may contribute to the process of mucosal inflammation. Since CD IECs are more mature, transcytosis of intact proteins or presentation of antigen fragments by MHC complexes on enterocytes to lymphocytes30 would be increased, promoting inflammation.
Kernéis et al. documented the ability of Peyer’s patch lymphocytes to induce the differentiation of Caco-2 cells into M cells31, and showed that this was cell contact dependent, supporting the hypothesis that lymphocytes affect IEC differentiation. Chen et al. evaluated the role of IELs in the dextran sodium sulfate (DSS) mouse colitis model 9. IEL from DSS treated mice expressed KGF, a potent IEC mitogen. T cell (TCR−/) and KGF-dual deficient mice (KGF−/−), but not T cell-deficient mice (TCR−/−), were more prone than WT mice to DSS-induced mucosal injury and demonstrated delayed tissue repair. Termination of DSS treatment resulted in vigorous IEC proliferation in WT mice but not in TCR−/− mice or KGF−/− mice, suggesting that IEL help preserve the integrity of damaged epithelial surfaces by providing the localized delivery of an epithelial cell growth factor.
In order to understand the mechanisms involved in the accelerated maturation process, we studied signaling pathways known to participate in IEC differentiation. CD LPL activated PI3K and MAPK pathways in IEC lines in T84 cells, while UC LPL activated MAPK pathways. These data were correlated ex vivo: activation of p38 MAPK and PI3K pathways were predominantly restricted to crypt epithelial cells in CD mucosa and were not detected in UC mucosa. The differences seen between the in vitro and the ex vivo studies might be due to the fact that we are dealing with a malignant cell line, which may respond differently to various stimuli. Several studies have highlighted the p38 MAPK pathway, especially c-Raf, as a potential target for therapy in CD32-34. In those studies, the authors correlated inhibition of the MAPK pathway in the CD lamina propria with clinical remission. Based upon our study, activation of these signaling pathways in IECs correlates with disease severity. Furthermore, to our knowledge, no report has noted the involvement of the PI3K pathway in IBD. In view of these results, the PI3K pathway could be a new target for therapy.
This manuscript provides a relatively unique model assessing the communication between the epithelium and mucosal lymphocytes. Dysregulation of IECs in CD is driven by alterations in the crosstalk between LPL and IECs leading to an acceleration of differentiation. This alteration seems to involve the transcription factor CDX2 via the activation of PI3K and p38 MAPK pathways, providing new insights into the dysfunction of the epithelial barrier in CD vs. UC.
Supplementary Material
Table 1: Primers used for Real Time PCR
Table 2: Correlation between in vitro and ex vivo findings
Figure 1: MAPK pathway activation in T84 cells co-cultured with LPL. Immunoblotting for PERK1/ 2, P-p38 MAPK, P-JNK, and ERK2 in lysates obtained from T84 cells co-cultured with freshly isolated Nor, CD or UC LPL for 30 min to 3 hours. The CTL lane contains T84 alone. EGF (10 nM; 15min) was used as a positive control. These data are representative of 3 experiments. The percentage of MAPK activity related to the activity induced by EGF was quantified after densitometric analysis.
Figure 2: Nor, inactive and active CD, and inactive and active UC colonic tissue sections were immunostained using anti-phospho ERK1/2, anti-phospho p38 MAPK, and anti-phospho JNK antibodies. The slides were counter-stained with Mayer’s Hematoxylin solution, and examined with a Zeiss Axioskop Light Microscope at 20X magnification. The insets represent 100X magnification of the crypt epithelial cells to document nuclear localization. These data are representative of 4 experiments.
Acknowledgments
Grant support: This work was supported by NIH grants AI23504, DK58288, and AI44236. The light microscopy was performed at the MSSM-Microscopy Shared Resource Facility, supported with funding from NIH-NCI shared resources grant (5R24 CA095823-04), NSF Major Research Instrumentation grant (DBI-9724504) and NIH shared instrumentation grant (1 S10 RR0 9145-01).
Abbreviations
- IECs
intestinal epithelial cells
- Nor
normal
- CD
Crohn’s disease
- UC
Ulcerative colitis
- LPL
lamina propria lymphocyte
- IAP
intestinal alkaline phosphatase
- MAPK
mitogen-activated protein kinase
- CDX2
caudal-related homeoprotein 2
- PI3K
phosphatidylinositol 3-kinase
- APCs
antigen-presenting cells
- IEL
intraepithelial lymphocyte
- KGF
keratinocyte growth factor
- IBD
inflammatory bowel disease
- IL-10
interleukin-10
- PTEN
phosphatase and tensin homologue deleted from chromosome 10
- HRP
horseradish peroxidase
- FAE
follicle associated epithelium
- DSS
dextran sodium sulfate
- TCR
T cell antigen receptor
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There is no conflict of interest to disclose.
BIBLIOGRAPHY
- 1.Hershberg RM, Mayer LF. Antigen processing and presentation by intestinal epithelial cells - polarity and complexity. Immunol Today. 2000;21:123–8. doi: 10.1016/s0167-5699(99)01575-3. [DOI] [PubMed] [Google Scholar]
- 2.Dahan S, Roth-Walter F, Arnaboldi P, Agarwal S, Mayer L. Epithelia: lymphocyte interactions in the gut. Immunol Rev. 2007;215:243–53. doi: 10.1111/j.1600-065X.2006.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allez M, Brimnes J, Dotan I, Mayer L. Expansion of CD8+ T cells with regulatory function after interaction with intestinal epithelial cells. Gastroenterology. 2002;123:1516–26. doi: 10.1053/gast.2002.36588. [DOI] [PubMed] [Google Scholar]
- 4.Allez M, Brimnes J, Shao L, Dotan I, Nakazawa A, Mayer L. Activation of a unique population of CD8(+) T cells by intestinal epithelial cells. Ann N Y Acad Sci. 2004;1029:22–35. doi: 10.1196/annals.1309.004. [DOI] [PubMed] [Google Scholar]
- 5.Allez M, Mayer L. Regulatory T cells: peace keepers in the gut. Inflamm Bowel Dis. 2004;10:666–76. doi: 10.1097/00054725-200409000-00027. [DOI] [PubMed] [Google Scholar]
- 6.Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol. 2005;174:5814–22. doi: 10.4049/jimmunol.174.9.5814. [DOI] [PubMed] [Google Scholar]
- 7.Nakazawa A, Dotan I, Brimnes J, Allez M, Shao L, Tsushima F, Azuma M, Mayer L. The expression and function of costimulatory molecules B7H and B7-H1 on colonic epithelial cells. Gastroenterology. 2004;126:1347–57. doi: 10.1053/j.gastro.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Perera L, Shao L, Patel A, Evans K, Meresse B, Blumberg R, Geraghty D, Groh V, Spies T, Jabri B, Mayer L. Expression of nonclassical class I molecules by intestinal epithelial cells. Inflamm Bowel Dis. 2007;13:298–307. doi: 10.1002/ibd.20026. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A. 2002;99:14338–43. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 11.Grossmann J, Mohr S, Lapentina EG, Fiocchi C, Levine AD. Sequential and rapid activation of select caspases during apoptosis of normal intestinal epithelial cells. Am J Physiol. 1998;274:G1117–24. doi: 10.1152/ajpgi.1998.274.6.G1117. [DOI] [PubMed] [Google Scholar]
- 12.Mariadason JM, Nicholas C, L’Italien KE, Zhuang M, Smartt HJ, Heerdt BG, Yang W, Corner GA, Wilson AJ, Klampfer L, Arango D, Augenlicht LH. Gene expression profiling of intestinal epithelial cell maturation along the crypt-villus axis. Gastroenterology. 2005;128:1081–8. doi: 10.1053/j.gastro.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 13.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–59. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 14.Escaffit F, Pare F, Gauthier R, Rivard N, Boudreau F, Beaulieu JF. Cdx2 modulates proliferation in normal human intestinal epithelial crypt cells. Biochem Biophys Res Commun. 2006;342:66–72. doi: 10.1016/j.bbrc.2006.01.128. [DOI] [PubMed] [Google Scholar]
- 15.Alkhoury F, Malo MS, Mozumder M, Mostafa G, Hodin RA. Differential regulation of intestinal alkaline phosphatase gene expression by Cdx1 and Cdx2. Am J Physiol Gastrointest Liver Physiol. 2005;289:G285–90. doi: 10.1152/ajpgi.00037.2005. [DOI] [PubMed] [Google Scholar]
- 16.Houde M, Laprise P, Jean D, Blais M, Asselin C, Rivard N. Intestinal epithelial cell differentiation involves activation of p38 mitogen-activated protein kinase that regulates the homeobox transcription factor CDX2. J Biol Chem. 2001;276:21885–94. doi: 10.1074/jbc.M100236200. [DOI] [PubMed] [Google Scholar]
- 17.Boucher MJ, Rivard N. Regulation and role of brush border-associated ERK1/2 in intestinal epithelial cells. Biochem Biophys Res Commun. 2003;311:121–8. doi: 10.1016/j.bbrc.2003.09.172. [DOI] [PubMed] [Google Scholar]
- 18.Kim S, Domon-Dell C, Wang Q, Chung DH, Di Cristofano A, Pandolfi PP, Freund JN, Evers BM. PTEN and TNF-alpha regulation of the intestinal-specific Cdx-2 homeobox gene through a PI3K, PKB/Akt, and NF-kappaB-dependent pathway. Gastroenterology. 2002;123:1163–78. doi: 10.1053/gast.2002.36043. [DOI] [PubMed] [Google Scholar]
- 19.Laprise P, Chailler P, Houde M, Beaulieu JF, Boucher MJ, Rivard N. Phosphatidylinositol 3-kinase controls human intestinal epithelial cell differentiation by promoting adherens junction assembly and p38 MAPK activation. J Biol Chem. 2002;277:8226–34. doi: 10.1074/jbc.M110235200. [DOI] [PubMed] [Google Scholar]
- 20.Sheng H, Shao J, Townsend CM, Jr., Evers BM. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut. 2003;52:1472–8. doi: 10.1136/gut.52.10.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Wang X, Hernandez A, Kim S, Evers BM. Inhibition of the phosphatidylinositol 3-kinase pathway contributes to HT29 and Caco-2 intestinal cell differentiation. Gastroenterology. 2001;120:1381–92. doi: 10.1053/gast.2001.24044. [DOI] [PubMed] [Google Scholar]
- 22.Augeron C, Laboisse CL. Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res. 1984;44:3961–9. [PubMed] [Google Scholar]
- 23.Jumarie C, Malo C. Caco-2 cells cultured in serum-free medium as a model for the study of enterocytic differentiation in vitro. J Cell Physiol. 1991;149:24–33. doi: 10.1002/jcp.1041490105. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto H, Erickson RH, Gum JR, Yoshioka M, Gum E, Kim YS. Biosynthesis of alkaline phosphatase during differentiation of the human colon cancer cell line Caco-2. Gastroenterology. 1990;98:1199–207. doi: 10.1016/0016-5085(90)90334-w. [DOI] [PubMed] [Google Scholar]
- 25.Yuen T, Zhang W, Ebersole BJ, Sealfon SC. Monitoring G-protein-coupled receptor signaling with DNA microarrays and real-time polymerase chain reaction. Methods Enzymol. 2002;345:556–69. doi: 10.1016/s0076-6879(02)45047-1. [DOI] [PubMed] [Google Scholar]
- 26.Garcia Z, Kumar A, Marques M, Cortes I, Carrera AC. Phosphoinositide 3-kinase controls early and late events in mammalian cell division. Embo J. 2006;25:655–61. doi: 10.1038/sj.emboj.7600967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durual S, Blanchard C, Estienne M, Jacquier MF, Cuber JC, Perrot V, Laboisse C, Cuber JC. Expression of human TFF3 in relation to growth of HT-29 cell subpopulations: involvement of PI3-K but not STAT6. Differentiation. 2005;73:36–44. doi: 10.1111/j.1432-0436.2005.07301006.x. [DOI] [PubMed] [Google Scholar]
- 28.Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest. 2006;86:191–201. doi: 10.1038/labinvest.3700373. [DOI] [PubMed] [Google Scholar]
- 29.Soderholm JD, Streutker C, Yang PC, Paterson C, Singh PK, McKay DM, Sherman PM, Croitoru K, Perdue MH. Increased epithelial uptake of protein antigens in the ileum of Crohn’s disease mediated by tumour necrosis factor alpha. Gut. 2004;53:1817–24. doi: 10.1136/gut.2004.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer L. Current concepts in mucosal immunity. I. Antigen presentation in the intestine: new rules and regulations. Am J Physiol. 1998;274:G7–9. doi: 10.1152/ajpgi.1998.274.1.G7. [DOI] [PubMed] [Google Scholar]
- 31.Kerneis S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949–52. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- 32.Hommes D, van den Blink B, Plasse T, Bartelsman J, Xu C, Macpherson B, Tytgat G, Peppelenbosch M, Van Deventer S. Inhibition of stress-activated MAP kinases induces clinical improvement in moderate to severe Crohn’s disease. Gastroenterology. 2002;122:7–14. doi: 10.1053/gast.2002.30770. [DOI] [PubMed] [Google Scholar]
- 33.Lowenberg M, Verhaar A, van den Blink B, Kate ten F, van Deventer S, Peppelenbosch M, Hommes D. Specific inhibition of c-Raf activity by semapimod induces clinical remission in severe Crohn’s disease. J Immunol. 2005;175:2293–300. doi: 10.4049/jimmunol.175.4.2293. [DOI] [PubMed] [Google Scholar]
- 34.Waetzig GH, Seegert D, Rosenstiel P, Nikolaus S, Schreiber S. p38 mitogen-activated protein kinase is activated and linked to TNF-alpha signaling in inflammatory bowel disease. J Immunol. 2002;168:5342–51. doi: 10.4049/jimmunol.168.10.5342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1: Primers used for Real Time PCR
Table 2: Correlation between in vitro and ex vivo findings
Figure 1: MAPK pathway activation in T84 cells co-cultured with LPL. Immunoblotting for PERK1/ 2, P-p38 MAPK, P-JNK, and ERK2 in lysates obtained from T84 cells co-cultured with freshly isolated Nor, CD or UC LPL for 30 min to 3 hours. The CTL lane contains T84 alone. EGF (10 nM; 15min) was used as a positive control. These data are representative of 3 experiments. The percentage of MAPK activity related to the activity induced by EGF was quantified after densitometric analysis.
Figure 2: Nor, inactive and active CD, and inactive and active UC colonic tissue sections were immunostained using anti-phospho ERK1/2, anti-phospho p38 MAPK, and anti-phospho JNK antibodies. The slides were counter-stained with Mayer’s Hematoxylin solution, and examined with a Zeiss Axioskop Light Microscope at 20X magnification. The insets represent 100X magnification of the crypt epithelial cells to document nuclear localization. These data are representative of 4 experiments.