Abstract
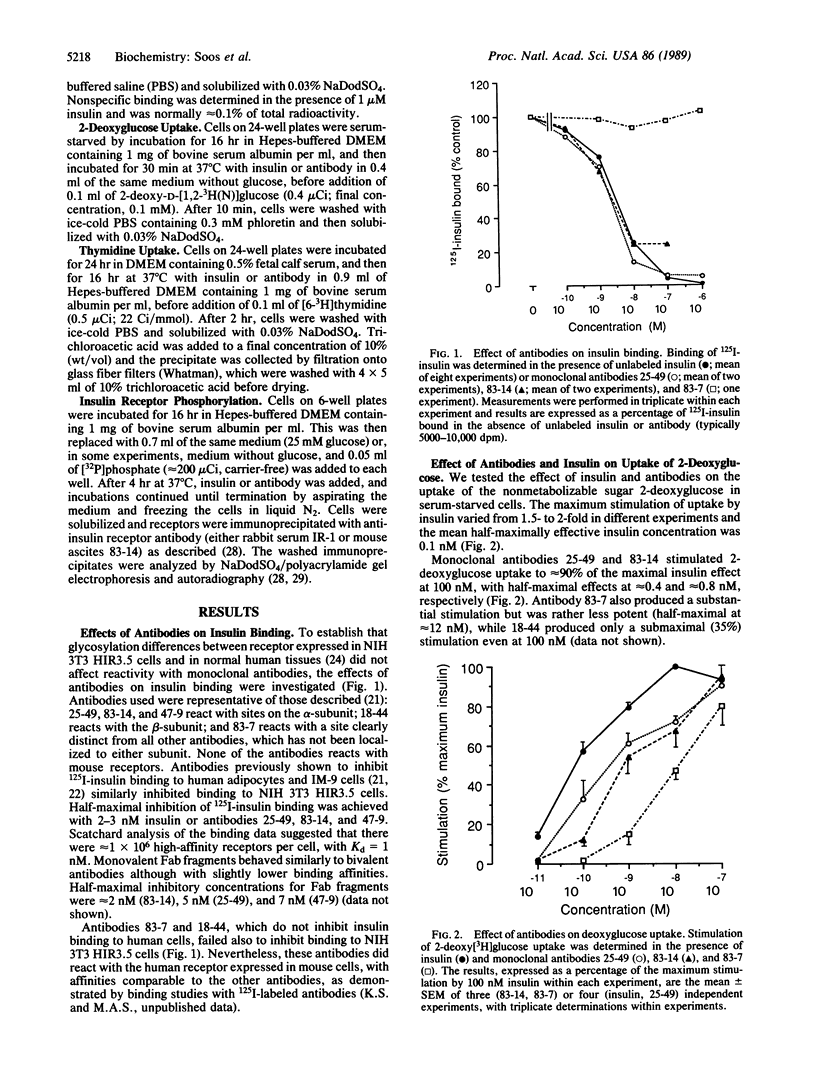
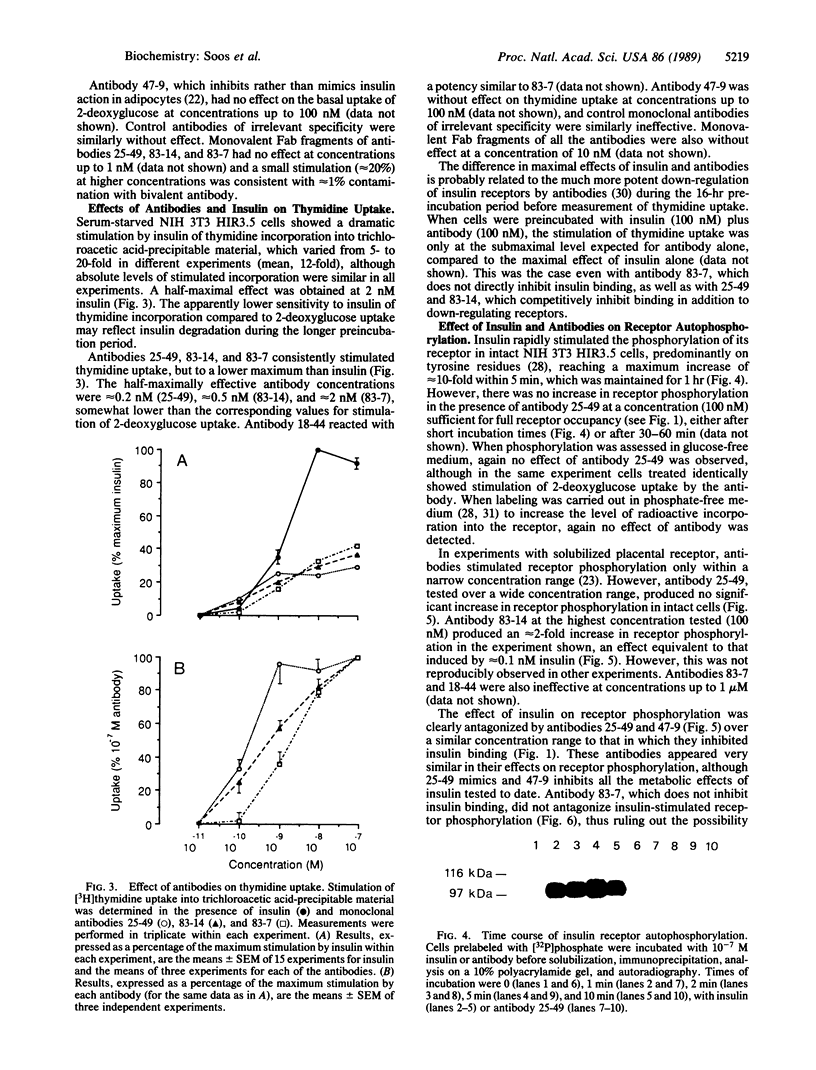
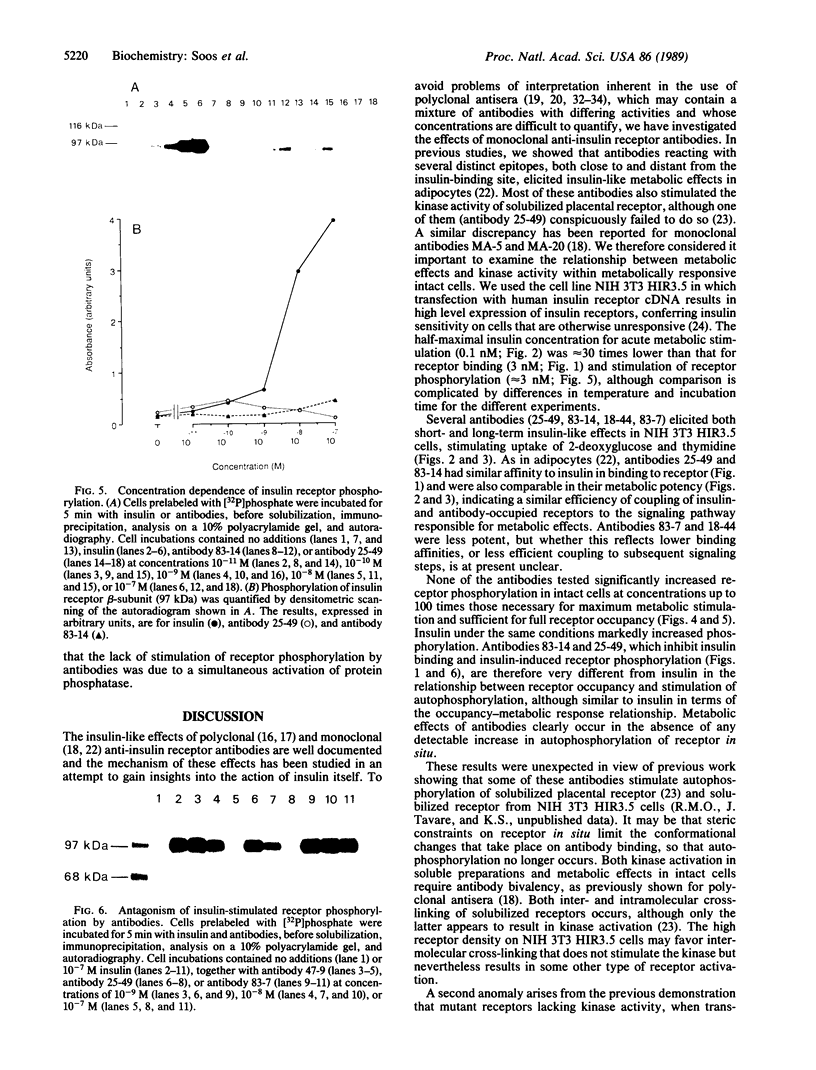
The metabolic actions of insulin and anti-insulin receptor monoclonal antibodies were compared with their effects on insulin receptor phosphorylation in mouse NIH 3T3 fibroblasts transfected with human insulin receptor cDNA. In serum-starved NIH 3T3 HIR3.5 cells, uptake of 2-deoxy-[3H]glucose was stimulated up to 2-fold after 30 min with insulin, with a half-maximal effect at 0.1 nM insulin. Incorporation of [3H]thymidine was stimulated approximately 12-fold after a 16-hr preincubation with insulin, with a half-maximal effect at 2 nM insulin. Phosphorylation of insulin receptor beta-subunit in cells prelabeled with [32P]phosphate was increased 10- to 20-fold within 5 min of adding insulin, with a half-maximal effect at approximately 3 nM insulin. Monoclonal antibodies reacting with four different epitopes on the insulin receptor mimicked the effect of insulin on 2-deoxyglucose uptake. These antibodies also stimulated thymidine incorporation, although the maximum stimulation was only approximately 30% that of insulin. Two antibodies (25-49 and 83-14) showed a similar concentration dependence to insulin in their metabolic effects and in the inhibition of 125I-labeled insulin binding to cells. The other two antibodies (83-7 and 18-44) were somewhat less potent and did not inhibit insulin binding. None of the antibodies significantly increased insulin receptor phosphorylation at concentrations up to 100 nM, which at least in the case of 25-49 and 83-14 was sufficient for full receptor occupancy. It is concluded that the insulin-like metabolic effects of antibodies involve a mechanism of receptor activation that is independent of autophosphorylation and hence that receptor autophosphorylation is not an essential step in triggering at least some events in the insulin signaling pathway.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernier M., Laird D. M., Lane M. D. Insulin-activated tyrosine phosphorylation of a 15-kilodalton protein in intact 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1844–1848. doi: 10.1073/pnas.84.7.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Czech M. P. The nature and regulation of the insulin receptor: structure and function. Annu Rev Physiol. 1985;47:357–381. doi: 10.1146/annurev.ph.47.030185.002041. [DOI] [PubMed] [Google Scholar]
- Denton R. M. Early events in insulin actions. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:293–341. [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Forsayeth J. R., Caro J. F., Sinha M. K., Maddux B. A., Goldfine I. D. Monoclonal antibodies to the human insulin receptor that activate glucose transport but not insulin receptor kinase activity. Proc Natl Acad Sci U S A. 1987 May;84(10):3448–3451. doi: 10.1073/pnas.84.10.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammeltoft S., Van Obberghen E. Protein kinase activity of the insulin receptor. Biochem J. 1986 Apr 1;235(1):1–11. doi: 10.1042/bj2350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherzi R., Russell D. S., Taylor S. I., Rosen O. M. Reevaluation of the evidence that an antibody to the insulin receptor is insulinmimetic without activating the protein tyrosine kinase activity of the receptor. J Biol Chem. 1987 Dec 15;262(35):16900–16905. [PubMed] [Google Scholar]
- Hawley D. M., Maddux B. A., Patel R. G., Wong K. Y., Mamula P. W., Firestone G. L., Brunetti A., Verspohl E., Goldfine I. D. Insulin receptor monoclonal antibodies that mimic insulin action without activating tyrosine kinase. J Biol Chem. 1989 Feb 15;264(5):2438–2444. [PubMed] [Google Scholar]
- Jacobs S., Chang K. J., Cuatrecasas P. Antibodies to purified insulin receptor have insulin-like activity. Science. 1978 Jun 16;200(4347):1283–1284. doi: 10.1126/science.663609. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Flier J. S., Grunfeld C., Harmon J. T., Harrison L. C., Karlsson F. A., Kasuga M., King G. L., Lang U. C. Insulin receptors, receptor antibodies, and the mechanism of insulin action. Recent Prog Horm Res. 1981;37:477–538. doi: 10.1016/b978-0-12-571137-1.50015-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamoyi E. Preparation of F(ab')2 fragments from mouse IgG of various subclasses. Methods Enzymol. 1986;121:652–663. doi: 10.1016/0076-6879(86)21064-2. [DOI] [PubMed] [Google Scholar]
- Linde S., Hansen B., Sonne O., Holst J. J., Gliemann J. Tyrosine A14[125I]monoiodoinsulin: Preparation, Biologic Properties, and long-term stability. Diabetes. 1981 Jan;30(1):1–8. doi: 10.2337/diab.30.1.1. [DOI] [PubMed] [Google Scholar]
- McClain D. A., Maegawa H., Lee J., Dull T. J., Ulrich A., Olefsky J. M. A mutant insulin receptor with defective tyrosine kinase displays no biologic activity and does not undergo endocytosis. J Biol Chem. 1987 Oct 25;262(30):14663–14671. [PubMed] [Google Scholar]
- O'Brien R. M., Houslay M. D., Milligan G., Siddle K. The insulin receptor tyrosyl kinase phosphorylates holomeric forms of the guanine nucleotide regulatory proteins Gi and Go. FEBS Lett. 1987 Feb 23;212(2):281–288. doi: 10.1016/0014-5793(87)81361-3. [DOI] [PubMed] [Google Scholar]
- O'Brien R. M., Soos M. A., Siddle K. Monoclonal antibodies to the insulin receptor stimulate the intrinsic tyrosine kinase activity by cross-linking receptor molecules. EMBO J. 1987 Dec 20;6(13):4003–4010. doi: 10.1002/j.1460-2075.1987.tb02743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti N., Accili D., Marcus-Samuels B., Rees-Jones R. W., Taylor S. I. Insulin stimulates phosphorylation of a 120-kDa glycoprotein substrate (pp120) for the receptor-associated protein kinase in intact H-35 hepatoma cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3137–3140. doi: 10.1073/pnas.84.10.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzio G., Dolais-Kitabgi J., Louvard D., Gautier N., Rossi B. Insulin and rabbit anti-insulin receptor antibodies stimulate additively the intrinsic receptor kinase activity. EMBO J. 1987 Feb;6(2):333–340. doi: 10.1002/j.1460-2075.1987.tb04759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J., Maddux B. A., Goldfine I. D. Regulation of insulin receptor kinase activity by insulin mimickers and an insulin antagonist. Biochem Biophys Res Commun. 1983 Aug 30;115(1):245–252. doi: 10.1016/0006-291x(83)90996-8. [DOI] [PubMed] [Google Scholar]
- Sacks D. B., McDonald J. M. Insulin-stimulated phosphorylation of calmodulin by rat liver insulin receptor preparations. J Biol Chem. 1988 Feb 15;263(5):2377–2383. [PubMed] [Google Scholar]
- Simpson I. A., Hedo J. A. Insulin receptor phosphorylation may not be a prerequisite for acute insulin action. Science. 1984 Mar 23;223(4642):1301–1304. doi: 10.1126/science.6367041. [DOI] [PubMed] [Google Scholar]
- Soos M. A., Siddle K., Baron M. D., Heward J. M., Luzio J. P., Bellatin J., Lennox E. S. Monoclonal antibodies reacting with multiple epitopes on the human insulin receptor. Biochem J. 1986 Apr 1;235(1):199–208. doi: 10.1042/bj2350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanker L. H., Vanderlaan M., Juarez-Salinas H. One-step purification of mouse monoclonal antibodies from ascites fluid by hydroxylapatite chromatography. J Immunol Methods. 1985 Jan 21;76(1):157–169. doi: 10.1016/0022-1759(85)90488-0. [DOI] [PubMed] [Google Scholar]
- Tavaré J. M., O'Brien R. M., Siddle K., Denton R. M. Analysis of insulin-receptor phosphorylation sites in intact cells by two-dimensional phosphopeptide mapping. Biochem J. 1988 Aug 1;253(3):783–788. doi: 10.1042/bj2530783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R., Soos M. A., Wells A., Argyraki M., Siddle K. Insulin-like and insulin-inhibitory effects of monoclonal antibodies for different epitopes on the human insulin receptor. Biochem J. 1987 Feb 15;242(1):123–129. doi: 10.1042/bj2420123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornqvist H. E., Gunsalus J. R., Nemenoff R. A., Frackelton A. R., Pierce M. W., Avruch J. Identification of the insulin receptor tyrosine residues undergoing insulin-stimulated phosphorylation in intact rat hepatoma cells. J Biol Chem. 1988 Jan 5;263(1):350–359. [PubMed] [Google Scholar]
- White M. F., Shoelson S. E., Keutmann H., Kahn C. R. A cascade of tyrosine autophosphorylation in the beta-subunit activates the phosphotransferase of the insulin receptor. J Biol Chem. 1988 Feb 25;263(6):2969–2980. [PubMed] [Google Scholar]
- White M. F., Stegmann E. W., Dull T. J., Ullrich A., Kahn C. R. Characterization of an endogenous substrate of the insulin receptor in cultured cells. J Biol Chem. 1987 Jul 15;262(20):9769–9777. [PubMed] [Google Scholar]
- Whittaker J., Okamoto A. K., Thys R., Bell G. I., Steiner D. F., Hofmann C. A. High-level expression of human insulin receptor cDNA in mouse NIH 3T3 cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5237–5241. doi: 10.1073/pnas.84.15.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor-associated tyrosine kinase activity. J Biol Chem. 1984 Apr 25;259(8):5277–5286. [PubMed] [Google Scholar]
- Zick Y., Rees-Jones R. W., Taylor S. I., Gorden P., Roth J. The role of antireceptor antibodies in stimulating phosphorylation of the insulin receptor. J Biol Chem. 1984 Apr 10;259(7):4396–4400. [PubMed] [Google Scholar]