Abstract
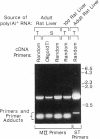
A clone encoding Golgi mannosidase II (MII; GlcNAc-transferase I-dependent alpha 1,3(alpha 1,6) mannosidase), an enzyme involved in asparagine-linked oligosaccharide processing, was isolated from a rat liver lambda gt11 cDNA library by a method that employs a modification of the polymerase chain reaction. Specific oligonucleotide primers were designed from two regions of protein sequence and were combined in an amplification reaction with a single-stranded cDNA preparation derived from rat liver poly(A)+ RNA. Based upon mapping of the protein sequences 42 kDa apart on the MII polypeptide, the procedure was expected to generate an approximately 1150-base-pair amplification product representing a segment of the MII gene between the two primer regions. The size of the amplified product (1170 base pairs) was in close agreement with this predicted fragment size. The authenticity of the amplified fragment was confirmed by the agreement of the DNA sequence with additional protein sequence data. A 1474-base-pair clone was isolated from a cDNA library by plaque hybridization using the amplification fragment as a radiolabeled probe. The nucleotide sequence of this clone predicts a single continuous open reading frame and, based upon a polypeptide molecular mass of 117 kDa for the enzyme subunit, is consistent with the clone representing approximately 50% of the coding sequence of MII. Both the clone and the amplification product hybridized to a rat liver mRNA of approximately 8 kilobases, a message size approximately 4.7 kilobases larger than the size of the predicted open reading frame. This extensive non-coding information on the MII message is a feature common to two other Golgi processing enzymes, both of which contain most of the non-coding information on the 3' end of their messages. The function of these disproportionately large untranslated regions is not clear.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kraus J. P., Rosenberg L. E. Purification of low-abundance messenger RNAs from rat liver by polysome immunoadsorption. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4015–4019. doi: 10.1073/pnas.79.13.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. C., Wu X. W., Gibbs R. A., Cook R. G., Muzny D. M., Caskey C. T. Generation of cDNA probes directed by amino acid sequence: cloning of urate oxidase. Science. 1988 Mar 11;239(4845):1288–1291. doi: 10.1126/science.3344434. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Moremen K. W., Touster O. Topology of mannosidase II in rat liver Golgi membranes and release of the catalytic domain by selective proteolysis. J Biol Chem. 1986 Aug 15;261(23):10945–10951. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shaper N. L., Hollis G. F., Douglas J. G., Kirsch I. R., Shaper J. H. Characterization of the full length cDNA for murine beta-1,4-galactosyltransferase. Novel features at the 5'-end predict two translational start sites at two in-frame AUGs. J Biol Chem. 1988 Jul 25;263(21):10420–10428. [PubMed] [Google Scholar]
- Shaper N. L., Shaper J. H., Meuth J. L., Fox J. L., Chang H., Kirsch I. R., Hollis G. F. Bovine galactosyltransferase: identification of a clone by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1573–1577. doi: 10.1073/pnas.83.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Kato K., Hayashizaki Y., Wakabayashi T., Ohtsuka E., Matsuki S., Ikehara M., Matsubara K. Molecular cloning of the human cholecystokinin gene by use of a synthetic probe containing deoxyinosine. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1931–1935. doi: 10.1073/pnas.82.7.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindall K. R., Kunkel T. A. Fidelity of DNA synthesis by the Thermus aquaticus DNA polymerase. Biochemistry. 1988 Aug 9;27(16):6008–6013. doi: 10.1021/bi00416a027. [DOI] [PubMed] [Google Scholar]
- Tulsiani D. R., Touster O. Swainsonine causes the production of hybrid glycoproteins by human skin fibroblasts and rat liver Golgi preparations. J Biol Chem. 1983 Jun 25;258(12):7578–7585. [PubMed] [Google Scholar]
- Weinstein J., Lee E. U., McEntee K., Lai P. H., Paulson J. C. Primary structure of beta-galactoside alpha 2,6-sialyltransferase. Conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J Biol Chem. 1987 Dec 25;262(36):17735–17743. [PubMed] [Google Scholar]