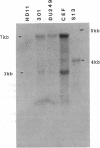
Abstract
The cloning and sequencing of the oncogene of the avian erythroblastosis virus S13 is described. The oncogene, termed v-sea, was found to be another member of the protein-tyrosine kinase gene family. The oncogene was fused in frame with the retrovirus S13 envelope gene, thus generating a fusion protein with a structure resembling that of a growth factor receptor. Sequence comparisons revealed that the v-sea gene was most closely related to the insulin receptor family of protein-tyrosine kinases, the greatest similarity being with the human MET oncogene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedict S. H., Maki Y., Vogt P. K. Avian retrovirus S13: properties of the genome and of the transformation-specific protein. Virology. 1985 Aug;145(1):154–164. doi: 10.1016/0042-6822(85)90210-7. [DOI] [PubMed] [Google Scholar]
- Besmer P., Murphy J. E., George P. C., Qiu F. H., Bergold P. J., Lederman L., Snyder H. W., Jr, Brodeur D., Zuckerman E. E., Hardy W. D. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature. 1986 Apr 3;320(6061):415–421. doi: 10.1038/320415a0. [DOI] [PubMed] [Google Scholar]
- Beug H., Hayman M. J., Graf T., Benedict S. H., Wallbank A. M., Vogt P. K. S13, a rapidly oncogenic replication-defective avian retrovirus. Virology. 1985 Aug;145(1):141–153. doi: 10.1016/0042-6822(85)90209-0. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Kitchener G., Vogt P. K., Beug H. The putative transforming protein of S13 avian erythroblastosis virus is a transmembrane glycoprotein with an associated protein kinase activity. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8237–8241. doi: 10.1073/pnas.82.23.8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman M. J. The sea oncogene of the avian erythroblastosis virus S13. Pathol Immunopathol Res. 1987;6(5-6):390–399. doi: 10.1159/000157065. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Greenhouse J. J., Petropoulos C. J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987 Oct;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Knight J., Zenke M., Disela C., Kowenz E., Vogt P., Engel J. D., Hayman M. J., Beug H. Temperature-sensitive v-sea transformed erythroblasts: a model system to study gene expression during erythroid differentiation. Genes Dev. 1988 Feb;2(2):247–258. doi: 10.1101/gad.2.2.247. [DOI] [PubMed] [Google Scholar]
- Langlois A. J., Lapis K., Ishizaki R., Beard J. W., Bolognesi D. P. Isolation of a transplantable cell line induced by the MC29 avian leukosis virus. Cancer Res. 1974 Jun;34(6):1457–1464. [PubMed] [Google Scholar]
- Leutz A., Beug H., Walter C., Graf T. Hematopoietic growth factor glycosylation. Multiple forms of chicken myelomonocytic growth factor. J Biol Chem. 1988 Mar 15;263(8):3905–3911. [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke M., Kahn P., Disela C., Vennström B., Leutz A., Keegan K., Hayman M. J., Choi H. R., Yew N., Engel J. D. v-erbA specifically suppresses transcription of the avian erythrocyte anion transporter (band 3) gene. Cell. 1988 Jan 15;52(1):107–119. doi: 10.1016/0092-8674(88)90535-1. [DOI] [PubMed] [Google Scholar]