Abstract
Aspergillus fumigatus sterol 14α-demethylase isoenzymes CYP51A and CYP51B were heterologously expressed in a Saccharomyces cerevisiae mutant (YUG37-erg11), wherein native ERG11/CYP51 expression is controlled using a doxycycline-regulatable promoter. When cultured in the presence of doxycycline, recombinant YUG37-pcyp51A and YUG37-pcyp51B yeasts were able to synthesize ergosterol and grow; a control strain harboring reverse-oriented cyp51A could not. YUG37-pcyp51A and YUG37-pcyp51B constructs showed identical sensitivity to itraconazole, posaconazole, clotrimazole, and voriconazole. Conversely, YUG37-pcyp51A withstood 16-fold-higher concentrations of fluconazole than YUG37-pcyp51B (8 and 0.5 μg ml−1, respectively).
Azoles are used for treatment of Aspergillus infections (11, 13) and also in prophylactic drug regimens for immunocompromised patients (8). The emergence (4, 14, 30, 31, 32) and potential for spread (2) of azole-resistant Aspergillus (hereafter focusing on Aspergillus fumigatus) have highlighted the need to develop diagnostic tools (6, 9) and novel antifungal agents (15). These requirements demand better understanding of the mechanisms that mediate azole resistance in Aspergillus.
Cytochrome P450 (CYP) was first investigated in A. fumigatus in 1990 (1); genome sequencing has revealed approximately 70 genes from this superfamily (29) that have not been fully annotated (manually verified) as for the 111 members of the Aspergillus nidulans cytochrome P450 complement (CYPome) (16). Given their importance in other pathogenic fungi (e.g., Candida albicans [18, 19, 21, 36]), the significance of mutations in A. fumigatus sterol 14α-demethylase, the CYP51 protein target of azoles, has attracted particular attention. Since the discovery that A. fumigatus possesses two genes (cyp51A and cyp51B) encoding sterol 14α-demethylase-like enzymes (26), it has been reasoned that the relative importance of each for ergosterol biosynthesis and/or resistance phenotypes observed in the clinic might differ. To date, the most prevalent mechanism of azole resistance in A. fumigatus appears to be the modification of CYP51A (5, 22, 25, 27, 28). Missense mutations in cyp51A are associated with cross-resistance, elevated MICs to azoles, and increased CYP51A expression (25, 27).
Research has demonstrated the essentiality of the erg11 gene family (cyp51A and cyp51B) in A. fumigatus despite neither member being essential individually (15). It has also been postulated that CYP51A might provide the major 14α-demethylase activity required for growth in A. fumigatus and that CYP51B may serve a redundant or alternative function under certain growth conditions (28). However, despite the research interest surrounding A. fumigatus, it has not yet been shown that cyp51A and cyp51B both encode functional sterol 14α-demethylase. We investigated the use of a doxycycline-regulated Saccharomyces cerevisiae erg11/cyp51 (sterol 14α-demethylase) mutant to heterologously express A. fumigatus CYP51A and CYP51B in order to demonstrate complementation for ergosterol biosynthesis. The azole sensitivity of yeast transformants expressing A. fumigatus CYP51A and CYP51B was then screened.
Plasmid and strain construction.
Genes encoding A. fumigatus isoenzymes CYP51A and CYP51B (EXPASY accession no. Q4WNT5 and Q96W81) were synthesized without introns as previously described (34). The following gene-specific forward (F) and reverse (R) primers for cyp51A and cyp51B were used to amplify both genes for direct T/A ligation into the S. cerevisiae yeast expression vector pYES2.1 TOPO (Invitrogen): cyp51AF (5′-ATGGTCCCGATGCTGTG-3′), cyp51AR (5′-CTATTTGGAAGTGTTCTTGG-3′), cyp51BF (5′-ATGGGTCTGATCGCCTT-3′), and cyp51BR (5′-CTACGCTTTAGTCGC-3′). DNA polymerase with proofreading capacity (High Fidelity Expand; Roche) was used for all PCRs. The S. cerevisiae host (YUG37-erg11), wherein native erg11/cyp51 expression is controlled using a doxycycline-regulatable promoter (10, 33), was first transformed with pYES2.1 vector containing a reverse-oriented cyp51A gene insertion to create the control strain (YUG37-pCTRL). Experimental yeast transformants harboring cyp51A and cyp51B plasmid DNA (hereafter YUG37-pcyp51A and YUG37-pcyp51B) and YUG37-pCTRL were all selected and maintained using glucose-based yeast minimal (glcYM−dox) medium (Difco) containing 1.34% yeast nitrogen base without amino acids, 2% glucose, leucine and tryptophan (both 100 mg liter−1), and 2% agarose (as required) (wt/vol).
Heterologous expression.
For complementation experiments (Fig. 1), medium to induce plasmid expression (gal/rafYM+dox) was prepared as above except for the replacement of glucose with galactose and raffinose (2%) and the addition of 5 μg ml−1 doxycycline (Sigma-Aldrich). Single colonies from YUG37-pcyp51A, YUG37-pcyp51B, and YUG37-pCTRL transformation plates (all constructs in triplicate) were used to inoculate 15-ml volumes of gal/rafYM+dox; the resulting cultures were incubated for 72 h (30°C, 180 rpm) prior to checks for cell growth and subsequent sterol analyses. Sterol analysis of the YUG37-pCTRL construct cultured using gal/rafYM without doxycycline (gal/rafYM−dox) was also undertaken.
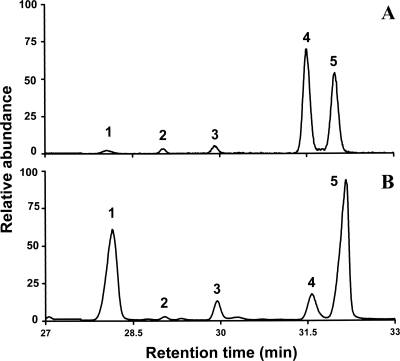
FIG. 1.
Example GC-MS chromatograms for the YUG37-pCTRL construct (A) and the complementing YUG37-pcyp51A construct (B) cultured using gal/rafYM+dox induction medium. 1, ergosterol; 2, 14α-methyl fecosterol; 3, 4,14α-dimethyl cholesta 8,24-dienol; 4, 14α-methyl ergosta 8,24(28) dien-3β-6α-diol; 5, lanosterol and/or obtusifoliol.
Sterol analysis.
The sterol composition of YUG37-pcyp51A, YUG37-pcyp51B, and YUG37-pCTRL constructs cultured using gal/rafYM medium (Table 1) was determined by gas chromatography mass spectrometry (GC-MS) as previously described (23). Trimethylsilyl (TMS)-derivatized sterols were identified with reference to retention times and fragmentation spectra for known standards. GC-MS data files were analyzed using Agilent software (MSD enhanced ChemStation, Agilent Technologies Inc.) for derivation of integrated peak areas.
TABLE 1.
Heterologous expression of A. fumigatus isoenzymes CYP51A and CYP51B in an S. cerevisiae ERG11/CYP51 (sterol 14α-demethylase) mutant
| Construct | Medium | Mean % of sterol (SD) in indicated constructa |
MIC (μg ml−1)b |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ergosterol | Other 14α- demethylated sterolsc | 14α-Methyl fecosterol | 4,14α-Dimethyl cholesta 8,24-dienol | 14α-Methyl ergosta 8,24 (28) dien-3β-6α-diol | Lanosterol/ obtusifoliold | Fluconazole | Clotrimazole | Voriconazole | Posaconazole | Itraconazole | ||
| YUG37-pcyp51A | gal/rafYM+dox | 40.8 (2.0) | 2.0 (1.3) | 2.6 (1.7) | 20.5 (3.1) | 34.1 (4.4) | 8 | 0.016 | 0.004 | 0.063 | 0.125 | |
| YUG37-pcyp51B | gal/rafYM+dox | 39.5 (3.1) | 1.7 (0.9) | 2.9 (1.3) | 10.5 (5.6) | 45.4 (3.8) | 0.5 | 0.016 | 0.004 | 0.063 | 0.125 | |
| YUG37-pCTRL | gal/rafYM+dox | 4.0 (2.2) | 1.9 (2.2) | 3.3 (1.1) | 51.1 (3.3) | 40.6 (4.8) | — | — | — | — | — | |
| YUG37-pCTRLe | gal/rafYM−dox | 80 (3.3) | 15.5 (1.1) | 4.0 (1.3) | 0.25 | 0.016 | 0.004 | 0.063 | 0.031 | |||
Mean percentage ± SD of sterol composition of experimental constructs.
MICs recorded in azole sensitivity assays. —, insufficient growth for MIC testing.
Sum of all 14α-demethylated sterols (except ergosterol).
14α-Methylated sterols with identical molecular weights and GC-MS retention times.
YUG37-pCTRL strain cultured in the absence of doxycycline.
Azole sensitivity assays.
The sensitivity of YUG37-pcyp51A and YUG37-pcyp51B constructs to selected azoles was assayed using standard CLSI M27-A2 broth dilution methodology, except for the use of gal/rafYM+dox induction medium, initial inoculums equivalent to 2.5 × 103 cells ml−1, and final azole concentrations of fluconazole (0.031 to 16 μg ml−1), clotrimazole, itraconazole, and posaconazole (0.004 to 2.0 μg ml−1), and voriconazole (0.0005 to 0.25). Owing to its inability to grow in gal/rafYM+dox medium, azole MIC values for the YUG37-pCTRL construct were determined using gal/rafYM−dox. Microtiter plates were incubated at 30°C, and MIC values (Table 1) were scored after 72 h. Azole MICs were determined as the minimum drug concentration yielding at least 80% inhibition of growth compared with growth in control wells.
The YUG37-pcyp51A and YUG37-pcyp51B constructs were both culturable using gal/rafYM+dox. The ergosterol content of each (Table 1) indicates that A. fumigatus CYP51A and CYP51B both complemented S. cerevisiae sterol 14α-demethylase function with comparable efficiency. YUG37-pCTRL cultures did not grow in gal/rafYM+dox medium, as evidenced by GC-MS chromatograms (Fig. 1A). Briefly, 14α-methylated sterols comprised >95% of the total sterol fraction in YUG37-pCTRL as a result of downregulation of the endogenous S. cerevisiae cyp51. That the fungistatic sterol 14α-methyl ergosta 8,24(28)-dien-3β-6α-diol (17, 35) comprised >50% of gal/rafYM+dox-cultured YUG37-pCTRL (Table 1) is consistent with the failure of the reverse-oriented cyp51A gene to complement and accounts for its inability to grow. The sterol (specifically high ergosterol) content of the YUG37-pCTRL construct cultured in the absence of doxycycline is typical of wild-type S. cerevisiae.
MIC values from azole sensitivity assays with YUG37-pcyp51A and YUG37-pcyp51B (Table 1) agree with literature regarding the efficacy of azoles for general treatment of A. fumigatus infections. Specifically, the potency of voriconazole and posaconazole (in this study, MIC values of 0.004 and 0.063 μg ml−1, respectively) is well documented (8, 11, 13, 24). That both YUG37-pcyp51A and YUG37-pcyp51B withstood comparatively higher concentrations of fluconazole (Table 1) is in agreement with the intrinsic resistance of A. fumigatus to this azole (12). It is noteworthy that YUG37-pcyp51A cultures withstood 16-fold-higher concentrations of fluconazole than YUG37-pcyp51B (MIC values of 8 and 0.5 μg ml−1, respectively). This is consistent with the results of Mellado et al. (28) and indicates that the expression and properties of CYP51A may be central to fluconazole resistance in A. fumigatus. The MIC values for the YUG37-pCTRL construct cultured using gal/rafYM−dox (Table 1) demonstrate the susceptibility of the endogenous yeast CYP51 to all azoles; they also indicate the potential importance of A. fumigatus CYP51A and CYP51B for resistance to both fluconazole (12) and itraconazole (4, 7).
It is possible that, besides variation in the structural properties of A. fumigatus CYP51A and CYP51B, differences in gene expression could contribute to the altered fluconazole susceptibility of the YUG37-pcyp51A and YUG37-pcyp51B constructs. However, the consistency and value of this yeast expression system for evaluating mutations in CYP51 from the fungal wheat pathogen Mycosphaerella graminicola has already been demonstrated (3). It is also significant that previous experimental work with Candida albicans CYP51 has indicated that expression levels in transformants differing by more than 1,000-fold do not alter azole MICs more than 5-fold (20). Hence, differences in the expression of CYP51A and CYP51B are unlikely to be responsible for azole MIC values observed in the present study.
Results from this study unequivocally demonstrate that A. fumigatus cyp51A and cyp51B both encode functional sterol 14α-demethylase. Given the complicating presence of both cyp51A and cyp51B in A. fumigatus and (owing to the efficiency of A. fumigatus DNA repair mechanisms) the challenge of creating stable gene knockout strains, use of the nonpathogenic S. cerevisiae sterol 14α-demethylase mutant to complement and assay the individual azole sensitivity of CYP51A and CYP51B constitutes a model system through which the screening of novel azole antifungals might be undertaken in the future.
Acknowledgments
This research was supported by the EU FP6 project EURESFUN (European Resistance Fungal Network) and analytical facilities provided by the EPSRC National Mass Spectrometry Service Centre (Swansea University, United Kingdom).
We thank Schering-Plough Ltd. (Welwyn Garden City, United Kingdom) for the kind gift of the posaconazole used in this study.
Footnotes
Published ahead of print on 23 August 2010.
REFERENCES
- 1.Ballard, S. A., S. L. Kelly, S. W. Ellis, and P. F. Troke. 1990. Interaction of microsomal cytochrome-P450 isolated from Aspergillus fumigatus with fluconazole and itraconazole. J. Med. Vet. Mycol. 28:327-334. [PubMed] [Google Scholar]
- 2.Beernaert, L. A., F. Pasmans, L. Van Waeyenberghe, G. M. Dorrestein, F. Verstappen, F. Vercammen, F. Haesebrouck, and A. Martel. 2009. Avian Aspergillus fumigatus strains resistant to both itraconazole and voriconazole. Antimicrob. Agents Chemother. 53:2199-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cools, H. J., J. E. Parker, D. E. Kelly, J. A. Lucas, B. A. Fraaije, and S. L. Kelly. 2010. Heterologous expression of mutated eburicol 14α-demethylase (CYP51) proteins of Mycosphaerella graminicola to assess effects on azole fungicide sensitivity and intrinsic protein function. Appl. Environ. Microbiol. 76:2866-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denning, D. W., K. Venkateswarlu, K. L. Oakley, M. J. Anderson, N. J. Manning, D. A. Stevens, D. W. Warnock, and S. L. Kelly. 1997. Itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 41:1364-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz-Guerra, T. M., E. Mellado, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2003. A point mutation in the 14 alpha-sterol demethylase gene CYP51A contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 47:1120-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erjavec, Z., H. Kluin-Nelemans, and P. E. Verweij. 2009. Trends in invasive fungal infections, with emphasis on invasive aspergillosis. Clin. Microbiol. Infect. 15:625-633. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira, M. E. D., J. L. Capellaro, E. D. Marques, I. Malavazi, D. Perlin, S. Park, J. B. Anderson, A. L. Colombo, B. A. Arthington-Skaggs, M. H. S. Goldman, and G. H. Goldman. 2004. In vitro evolution of itraconazole resistance in Aspergillus fumigatus involves multiple mechanisms of resistance. Antimicrob. Agents Chemother. 48:4405-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frampton, J. E., and L. J. Scott. 2008. Posaconazole: a review of its use in the prophylaxis of invasive fungal infections. Drugs 68:993-1016. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Effron, G., A. Dilger, L. Alcazar-Fuoli, S. Park, E. Mellado, and D. S. Perlin. 2008. Rapid detection of triazole antifungal resistance in Aspergillus fumigatus. J. Clin. Microbiol. 46:1200-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groeneveld, P., N. Rolley, D. B. Kell, S. L. Kelly, and D. E. Kelly. 2002. Metabolic control analysis and engineering of the yeast sterol biosynthetic pathway. Mol. Biol. Rep. 29:27-29. [DOI] [PubMed] [Google Scholar]
- 11.Guinea, J., S. Recio, T. Pelaz, M. Torres-Narbona, and E. Bouza. 2008. Clinical isolates of Aspergillus species remain fully susceptible to voriconazole in the post-voriconazole era. Antimicrob. Agents Chemother. 52:3444-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helmerhorst, E. J., I. M. Reijnders, W. van't Hof, I. Simoons-Smit, E. C. I. Veerman, and A. V. N. Amerongen. 1999. Amphotericin B- and fluconazole-resistant Candida spp., Aspergillus fumigatus, and other newly emerging pathogenic fungi are susceptible to basic antifungal peptides. Antimicrob. Agents Chemother. 43:702-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodiamont, C. J., K. M. Dolman, I. J. M. Ten Berge, W. J. G. Melchers, P. E. Verweij, and D. Pajkrt. 2009. Multiple-azole-resistant Aspergillus fumigatus osteomyelitis in a patient with chronic granulomatous disease successfully treated with long-term oral posaconazole and surgery. Med. Mycol. 47:217-220. [DOI] [PubMed] [Google Scholar]
- 14.Howard, S. J., D. Cerar, M. J. Anderson, A. Albarrag, M. C. Fisher, A. C. Pasqualotto, M. Laverdiere, M. C. Arendrup, D. S. Perlin, and D. W. Denning. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg. Infect. Dis. 15:1068-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu, W. Q., S. Sillaots, S. Lemieux, J. Davison, S. Kauffman, A. Breton, A. Linteau, C. L. Xin, J. Bowman, J. Becker, B. Jiang, and T. Roemer. 2007. Essential gene identification and drug target prioritization in Aspergillus fumigatus. PLoS Pathog. 3:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly, D. E., N. Krasevec, J. Mullins, and D. R. Nelson. 2009. The CYPome (cytochrome P450 complement) of Aspergillus nidulans. Fungal Genet. Biol. 46:S53-S61. [DOI] [PubMed] [Google Scholar]
- 17.Kelly, S. L., D. C. Lamb, A. J. Corran, B. C. Baldwin, and D. E. Kelly. 1995. Mode of action and resistance to azole antifungals associated with the formation of 14-alpha-methylergosta-8,24(28)-dien-3-beta,6-alpha-diol. Biochem. Biophys. Res. Commun. 207:910-915. [DOI] [PubMed] [Google Scholar]
- 18.Kelly, S. L., D. C. Lamb, and D. E. Kelly. 1999. Y132H substitution in Candida albicans sterol 14 alpha-demethylase confers fluconazole resistance by preventing binding to haem. FEMS Microbiol. Lett. 180:171-175. [DOI] [PubMed] [Google Scholar]
- 19.Kelly, S. L., D. C. Lamb, J. Loeffler, H. Einsele, and D. E. Kelly. 1999. The G464S amino acid substitution in Candida albicans sterol 14 alpha-demethylase causes fluconazole resistance in the clinic through reduced affinity. Biochem. Biophys. Res. Commun. 262:174-179. [DOI] [PubMed] [Google Scholar]
- 20.Lamb, D. C., D. E. Kelly, W. H. Schunck, A. Z. Shyadehi, M. Akhtar, D. J. Lowe, B. C. Baldwin, and S. L. Kelly. 1997. The mutation T315A in Candida albicans sterol 14α-demethylase causes reduced enzyme activity and fluconazole resistance through reduced affinity. J. Biol. Chem. 272:5682-5688. [DOI] [PubMed] [Google Scholar]
- 21.Loeffler, J., S. L. Kelly, H. Hebart, U. Schumacher, C. Lass-Floerl, and H. Einsele. 1997. Molecular analysis of CYP51 from fluconazole-resistant Candida albicans strains. FEMS Microbiol. Lett. 151:263-268. [DOI] [PubMed] [Google Scholar]
- 22.Mann, P. A., R. M. Parmegiani, S. Q. Wei, C. A. Mendrick, X. Li, D. Loebenberg, B. DiDomenico, R. S. Hare, S. S. Walker, and P. A. McNicholas. 2003. Mutations in Aspergillus fumigatus resulting in reduced susceptibility to posaconazole appear to be restricted to a single amino acid in the cytochrome p450 14 alpha-demethylase. Antimicrob. Agents Chemother. 47:577-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martel, C. M., J. E. Parker, O. Bader, M. Weig, U. Gross, A. G. S. Warrilow, D. E. Kelly, and S. L. Kelly. 2010. A clinical isolate of Candida albicans with mutations in ERG11 (encoding sterol 14α-demethylase) and ERG5 (encoding C22-desaturase) is cross-resistant to azoles and amphotericin B. Antimicrob. Agents Chemother. 54:3578-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mavridou, E., R. J. M. Brueggemann, W. J. G. Melchers, J. W. Mouton, and P. E. Verweij.2010. Efficacy of posaconazole against three clinical Aspergillus fumigatus isolates with mutations in the cyp51A gene. Antimicrob. Agents Chemother. 54:860-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mellado, E., L. Alcazar-Fuoli, G. Garcia-Effron, A. Alastruey-Izquierdo, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2006. New resistance mechanisms to azole drugs in Aspergillus fumigatus and emergence of antifungal drug-resistant A. fumigatus atypical strains. Med. Mycol. 44:S367-S371. [DOI] [PubMed] [Google Scholar]
- 26.Mellado, E., T. M. Diaz-Guerra, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2001. Identification of two different 14-alpha sterol demethylase-related genes (cyp51A and cyp51B) in Aspergillus fumigatus and other aspergillus species. J. Clin. Microbiol. 39:2431-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mellado, E., G. Garcia-Effron, L. Alcazar-Fuoli, W. J. G. Melchers, P. E. Verweij, A. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2007. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob. Agents Chemother. 51:1897-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mellado, E., G. Garcia-Effron, M. J. Buitrago, L. Alcazar-Fuoli, A. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2005. Targeted gene disruption of the 14-alpha sterol demethylase (cyp51A) in Aspergillus fumigatus and its role in azole drug susceptibility. Antimicrob. Agents Chemother. 49:2536-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park, J., S. Lee, J. Choi, K. Ahn, B. Park, J. Park, S. Kang, and Y. H. Lee. 2008. Fungal cytochrome p450 database. BMC Genomics 9:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snelders, E., H. A. L. van der Lee, J. Kuijpers, A. Rijs, J. Varga, R. A. Samson, E. Mellado, A. R. T. Donders, W. J. G. Melchers, and P. E. Verweij. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 5:1629-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snelders, E., R. Veld, A. Rijs, G. H. J. Kema, W. J. G. Melchers, and P. E. Verweij. 2009. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl. Environ. Microbiol. 75:4053-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verweij, P. E., E. Snelders, G. H. J. Kema, E. Mellado, and W. J. G. Melchers. 2009. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect. Dis. 9:789-795. [DOI] [PubMed] [Google Scholar]
- 33.Warrilow, A. G. S., C. Ugochukwu, D. Lamb, D. Kelly, and S. Kelly. 2008. Expression and characterization of CYP51, the ancient sterol 14-demethylase activity for cytochromes P450 (CYP), in the white-rot fungus Phanerochaete chrysosporium. Lipids 43:1143-1153. [DOI] [PubMed] [Google Scholar]
- 34.Warrilow, A. G. S., N. Melo, C. M. Martel, J. E. Parker, W. D. Nes, S. L. Kelly, and D. E. Kelly. 2010. Expression, purification and characterization of Aspergillus fumigatus sterol 14α-demethylase (CYP51) isoenzymes A and B. Antimicrob. Agents Chemother. [Epub ahead of print.] doi: 10.1128/AAC.00316-10. [DOI] [PMC free article] [PubMed]
- 35.Watson, P. F., M. E. Rose, S. W. Ellis, H. England, and S. L. Kelly. 1989. Defective sterol C5-6 desaturation and azole resistance—a new hypothesis for the mode of action of azole antifungals. Biochem. Biophys. Res. Commun. 164:1170-1175. [DOI] [PubMed] [Google Scholar]
- 36.White, T. C. 1997. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14 alpha-demethylase in Candida albicans. Antimicrob. Agents Chemother. 41:1488-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]