Abstract
The probiotic approach represents an alternative strategy in the prevention and treatment of infectious diseases, not only at the intestinal level but also at other sites of the body where the microbiota plays a role in the maintenance of physiological homeostasis. In this context, we evaluated in vitro the potential abilities of probiotic and dairy bacteria in controlling Streptococcus pyogenes infections at the pharyngeal level. Initially, we analyzed bacterial adhesion to FaDu hypopharyngeal carcinoma cells and the ability to antagonize S. pyogenes on FaDu cell layers and HaCat keratinocytes. Due to its promising adhesive and antagonistic features, we studied the dairy strain Lactobacillus helveticus MIMLh5, also through in vitro immunological experiments. First, we performed quantification of several cytokines and measurement of NF-κB activation in FaDu cells. MIMLh5 efficiently reduced the induction of interleukin-6 (IL-6), IL-8, and tumor necrosis factor alpha (TNF-α), in a dose-dependent manner. After stimulation of cells with IL-1β, active NF-κB was still markedly lowered. Nevertheless, we observed an increased secretion of IL-6, gamma interferon (IFN-γ), and granulocyte-macrophage colony-stimulating factor (GM-CSF) under these conditions. These effects were associated with the ability of MIMLh5 to enhance the expression of the heat shock protein coding gene hsp70. In addition, MIMLh5 increased the GM-CSF/G-CSF ratio. This is compatible with a switch of the immune response toward a TH1 pathway, as supported by our observation that MIMLh5, once in contact with bone marrow-derived dendritic cells, triggered the secretion of TNF-α and IL-2. In conclusion, we propose MIMLh5 as a potential probiotic bacterium for the human pharynx, with promising antagonistic and immunomodulatory properties.
Over 100 years ago, the Nobel laureate Ilya Ilyich Metchnikoff stated that “the dependence of the intestinal microbes on the food makes it possible to adopt measures to modify the flora in our bodies and to replace the harmful microbes by useful microbes” (35). Metchnikoff, who proposed a link between lactic acid bacteria ingested with food and human health, laid the foundations for what was later named the probiotic approach.
In 2002, a joint FAO/WHO working group recommended the adoption of the definition of probiotics as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (16). Today, probiotics have become a multimillion dollar business, involving markets all over the Western world, especially in Japan, Europe, and the United States (28). Most of the probiotic products commercialized to date have been intended for the gastrointestinal tract and the gut microbiota. Nevertheless, more recently, new categories of probiotic products have been developed, targeting different body compartments, such as the vaginal mucosa, skin, or oral cavity (9, 31, 42).
The pharyngeal tract is a primary way of entrance into the human body for many bacteria and viruses. The host can manage the presence of potentially harmful microorganisms in the oropharyngeal cavity by means of the local immune system and through the presence of the autochthonous microbiota. The alteration of a proper human-microbiota mutualism in the oral and pharyngeal mucosa can facilitate the onset of local infections (pharyngotonsillitis or rhinitis) or systemic diseases (pulmonary and cardiac infections, rheumatic fever, septicemia, or meningitis). The consumption of products containing probiotic bacteria has been proposed as a strategy for preventing oral pathogen colonization and proliferation. Probiotic lactic acid bacteria, especially lactobacilli, are in fact known to play an important role in the maintenance of human health at the mucosal level, by stimulating the natural immunity and contributing to the balance of the microbiota through competitive exclusion of virulent microorganisms (13, 14, 28). Accordingly, it has been demonstrated that the administration of lactobacilli can reduce the oral carriage of potentially harmful streptococci (1, 11). Furthermore, probiotics can prevent the occurrence or decrease the severity of inflammatory diseases through interaction with the host's immune system cells. As a matter of fact, pathogenic and nonpathogenic microorganisms activate different immunological pathways and intracellular signal transmission. In particular, commensal and probiotic bacteria can modulate the activation of important transmission factors, such as nuclear factor κB (NF-κB), and the production of different cytokines (12, 14, 46).
In the oropharyngeal tract as well as in the intestine, lymphoid tissue is copiously present and forms the mucosa- associated lymphoreticular tissue (MALT). The two main components of MALT, known as GALT (gut-associated lymphoreticular tissue) and NALT (nasopharynx-associated lymphoreticular tissue), are strictly linked, so the reciprocal exchange of immune system cells and molecules through blood and lymph is possible. Therefore, the administration of selected probiotic microorganisms can potentially affect host immune responses at local and systemic levels, representing a milder alternative to anti-inflammatory pharmaceutical therapies.
In this study, we investigated dairy and probiotic bacteria in vitro for their potential as probiotics for the pharyngeal mucosa. As a consequence of the experiments carried out during this research, we selected a bacterial strain, Lactobacillus helveticus MIMLh5, which exhibits noticeable adhesion and antagonistic activities on the FaDu human pharyngeal cell line and shows promising immunomodulatory properties.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in this study are listed in Table 1. Lactobacilli and Bacillus coagulans were cultivated in MRS broth (Sharlau, Barcelona, Spain) supplemented with 0.05% l-cysteine-HCl (cMRS) and incubated at 37°C overnight. Oral streptococci and Streptococcus thermophilus were routinely grown overnight in M17 broth (Sharlau, Barcelona, Spain) supplemented with 2% lactose (LM17). Lactococci were grown overnight in LM17 at 30°C. Bifidobacterium animalis subsp. lactis Bb12 was grown under anaerobic conditions (Anaerocult A system; Merck, Darmstadt, Germany) at 37°C in prereduced cMRS. Streptococcus pyogenes C11 (emm type 77; clinically isolated from a pharyngitis patient) was grown overnight at 37°C in brain heart infusion (BHI) broth (Difco, Detroit, MI) supplemented with 0.3% yeast extract.
TABLE 1.
Bacteria included in the study
| Species | Strain | Source | Reference |
|---|---|---|---|
| Bacillus coagulans | NF4 | Probiotic commercial strain | |
| Bifidobacterium animalis subsp. lactis | Bb12 | Probiotic commercial strain | 25 |
| Lactobacillus acidophilus | La-5 | Probiotic commercial strain | 25 |
| Lactobacillus casei | Shirota | Probiotic commercial strain | 25 |
| Lactobacillus helveticus | MIMLh5 | Dairy natural starter | 21 |
| Lactobacillus rhamnosus | GG | Probiotic commercial strain | 25 |
| Lactococcus lactis subsp. cremoris | Viili | Finnish fermented milk | 27 |
| Streptococcus oralis | ST4 | Human pharynx | 23 |
| Streptococcus pyogenes | C11 | Human pharyngeal pathogen | |
| Streptococcus salivarius | K12 | BLIS K12 Throat Guard | 47 |
| ST3 | Human pharynx | 23 | |
| Streptococcus thermophilus | DSM20617T | Yogurt (species type strain) | 2 |
Bacterial adhesion to FaDu cell layer.
FaDu cells were grown in 3-cm petri plates on microscope cover glasses until confluence was reached, as described previously (23). Afterward, adhesion assays were performed as previously illustrated (22, 23).
Determination of antagonistic activity against Streptococcus pyogenes.
Bacterial antagonism was studied against the recombinant Streptococcus pyogenes strain C11Luc, harboring the bioluminescence reporter vector pCSS945 (34). Antagonism experiments were performed through exclusion and competition assays according to the methods of Guglielmetti and collaborators (23), using two different cell lines: FaDu (human pharynx carcinoma cell line; ATCC HTB-43) and HaCat (human keratinocytes from a spontaneously immortalized, nontumorigenic cell line). Cell lines were routinely grown until confluence was reached, as described previously (23). The bacterial cell concentration of an overnight culture was determined microscopically by use of a Neubauer Improved counting chamber (Marienfeld GmbH, Lauda-Königshofen, Germany). Bacterial cells were washed with phosphate-buffered saline (PBS) and resuspended in the same buffer at the concentrations reported below. In the exclusion assay, the cell layer was preincubated with 1 ml of a tester strain suspension (5 × 108 cells ml−1), followed by incubation with the indicator strain (2 × 108 cells ml−1 of S. pyogenes C11Luc). In exclusion experiments, we used a concentration of 5 × 108 tester cells ml−1 because this matched the plateau of a dose-response curve prepared during the setup of the experiment by measuring the antagonistic activity as a function of tester cell concentration (data not shown). Competition consisted of coincubation of the same number of tester and indicator cells (2 × 108 cells ml−1). At the end of the incubation, the pH was monitored in order to detect possible acidification. Bioluminescence was measured with a Victor 3 luminometer (PerkinElmer, Monza, Italy). Each tester strain was analyzed in triplicate in at least two independent experiments. Unpaired Student's t tests were run to determine statistically significant differences.
Enzyme-linked immunosorbent assay (ELISA) measurement of cytokine production by FaDu cells.
Human pharyngeal carcinoma cells (FaDu) were grown in 24-well plates until confluence. Bacterial cells were resuspended in fresh antibiotic-free Eagle's minimum essential medium (EMEM) containing 100 mM HEPES (pH 7.4) and added to monolayers at a multiplicity of infection (MOI) of about 1,000. EMEM-HEPES medium without bacterial cells was used as a control. The same samples were also prepared by incubating bacteria and FaDu cells in the presence of 2 ng ml−1 of interleukin-1β (IL-1β). After overnight incubation at 37°C in the presence of 5% carbon dioxide, the supernatants were collected and kept at −80°C. Finally, concentrations of different cytokines in the supernatants were determined on a Bioplex array reader (Luminex 100; Bio-Rad Laboratories, Hercules, CA), using a Bio-Plex human cytokine 17-plex panel (Bio-Rad) according to the Bio-Plex human cytokine panel assay protocol (Bio-Rad). The tested cytokines and corresponding detection limits were as follows: IL-1β, 0.3 pg ml−1; IL-2, 0.2 pg ml−1; IL-4, 0.1 pg ml−1; IL-5, 0.3 pg ml−1; IL-6, 0.2 pg ml−1; IL-7, 0.3 pg ml−1; IL-8, 0.3 pg ml−1; IL-10, 0.2 pg ml−1; IL-12 (p70), 0.4 pg ml−1; IL-13, 0.3 pg ml−1; IL-17, 0.5 pg ml−1; granulocyte colony-stimulating factor (G-CSF), 0.2 pg ml−1; granulocyte-macrophage colony-stimulating factor (GM-CSF), 1.1 pg ml−1; gamma interferon (IFN-γ), 2.6 pg ml−1; monocyte chemotactic protein 1 (MCP-1), 0.8 pg ml−1; macrophage inflammatory protein 1β (MIP-1β), 0.6 pg ml−1; and tumor necrosis factor alpha (TNF-α), 0.6 pg ml−1.
Measurement of NF-κB activation in reporter FaDu cells.
The FaDu cell line was stably transfected with the luciferase reporter plasmid pNiFty2-Luc (InvivoGen, LaboGen, Rho, Italy), as described previously (23). In pNiFty2-Luc, the expression of an insect luciferase gene is induced by the active NF-κB molecules present in the cell. Recombinant FaDu cells were cultured using the same protocol as that for nontransfected FaDu cells, in the presence of 50 μg ml−1 of zeocin. In order to study the effect of bacterial cells on NF-κB activation, recombinant FaDu cells were incubated for 4 h with bacteria resuspended in EMEM broth supplemented with 100 mM HEPES (pH 7.4). After incubation, the samples were treated as described by Guglielmetti et al. (23). Finally, ATP and d-luciferin were added to the samples, and the emitted bioluminescence was immediately recorded with a Victor 3 luminometer (PerkinElmer, Monza, Italy). In a different set of experiments, recombinant FaDu cells were simultaneously stimulated with IL-1β (2 ng ml−1). All strains were analyzed in duplicate in at least three independent experiments for each MOI. Unpaired Student's t test was run to determine statistically significant differences.
Transcriptional analysis of heat shock genes in FaDu cells.
FaDu cells were cultured until confluence, using the same protocol as that described above. After growth, the FaDu cell layer was detached by trypsinization, and cells were resuspended in Dulbecco's modified Eagle's medium (DMEM) at a concentration of 250,000 cells ml−1 in the presence of 100 mM HEPES (pH 7.4). Subsequently, 50 μl of tester bacterial suspension, containing 2 × 109 or 2 × 108 cells, was added to 450 μl of FaDu cell suspension, resulting in an MOI of about 1,000 or 100, respectively. The analysis was performed at baseline and after the addition of 2 ng ml−1 of IL-1β. After incubation at 37°C for 4 h, cells were recovered by centrifugation (2,000 × g for 4 min at 4°C), and the supernatant was discarded. Total RNA was isolated from a FaDu cellular pellet by use of iScript RT-qPCR sample preparation reagent (Bio-Rad Laboratories, Milano, Italy) following the manufacturer's protocol. RNA was used for reverse transcription with an iScript cDNA synthesis kit (Bio-Rad Laboratories) according to the manufacturer's instructions. Real-time quantitative PCR (qPCR) was carried out using EvaGreen PCR master mix (Bio-Rad Laboratories) and 6.5 μl of 1:40-diluted cDNA reaction mix. The qPCRs were performed in triplicate and run on a CFX96 thermocycler (Bio-Rad Laboratories). Data were recorded as threshold cycles (CT) and expressed as means ± standard deviations, computed using Bio-Rad CFX Manager software and expressed as normalized expression (ΔΔCT) ± standard errors of the means. The following primers (5) were used in the qPCRs: hsp27 forward, 5′-CCCACCCTCTATCACGGCTAC-3′; hsp27 reverse, 5′-GGGCTCAACTCTGGCTATCTC-3′; hsp70 forward, 5′-GCGACCTGAACAAGAGCATC-3′; and hsp70 reverse, 5′-GAGCTTGCCCTTGAGACCC-3′. The expression levels of the heat shock genes hsp27 and hsp70 were normalized using genes coding for β-actin (ACTB) (forward primer, 5′-CTGGAACGGTGAAGGTGACA-3′; and reverse primer, 5′-AAGGGACTTCCTGTAACAATGCA-3′) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward primer, 5′-GGAGTCCACTGGCGTCTTCAC-3′; and reverse primer, 5′-GAGGCATTGCTGATGATCTTGAGG-3′) as reference housekeeping genes, because these genes were recently shown to be the most stable in an epithelial cell line (40). The qPCR cycle program used was the following: initial denaturation at 95°C for 3 min, followed by 40 cycles of denaturation at 95°C for 10 s, annealing at 55.9°C for 30 s, and extension at 72°C for 40 s. Finally, melting curve analysis from 65 to 95°C was also performed. For each condition, the measurements were carried out in triplicate with cDNAs synthesized from two independent RNA samples.
Study of activation of BMDCs.
Dendritic cells were obtained in vitro from bone marrow hematopoietic precursors isolated from C57BL/6 mouse femurs as described previously (18). In brief, hematopoietic precursors were recovered from mouse femoral bone marrow and resuspended in conditioned medium (complete medium supplemented with 10% of the growth supernatant of GM-CSF-transduced B16 cells). About 7 × 106 cells were plated in 100-mm suspension plates. The proportion of CD11c+ cells (corresponding to dendritic cells) was monitored periodically by flow cytometry until it reached 90% (ca. 10 days). The bone marrow-derived dendritic cells (BMDCs) were then used for bacterial activation assays. On the day of bacterial infection, BMDCs were plated at a concentration of 0.5 million per ml in 96-well plates (105 cells/well). After 1 h, BMDCs were incubated with four different concentrations of bacterial cells for 2 h, washed with saline, resuspended in a culture medium containing penicillin G, streptomycin, tetracycline, and gentamicin, and incubated overnight. Finally, IL-2 and TNF-α in the supernatant were quantified by ELISA, using DuoSet kits (R&D Systems, Minneapolis, MN).
Antibiotic susceptibility of Lactobacillus helveticus MIMLh5.
The inhibitory concentrations of antimicrobial agents were determined by broth microdilution assay as described elsewhere (23), using the following concentration ranges: for ampicillin, chloramphenicol, erythromycin, oxytetracycline, and vancomycin, 1 to 16 μg ml−1; for gentamicin, 8 to 64 μg ml−1; and for kanamycin and streptomycin, 16 to 128 μg ml−1. All antibiotics were from Sigma-Aldrich (Deisenhofen, Germany). The experiments were performed by cultivating strain MIMLh5 in cMRS broth.
RESULTS
Dairy and probiotic strains adhere differently to FaDu human pharyngeal cell line.
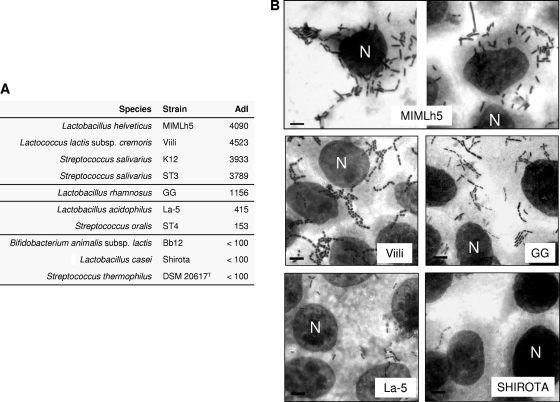
Lactic acid bacterial strains were tested for the ability to adhere to a FaDu epithelial cell layer. Two dairy strains, Lactobacillus helveticus MIMLh5 and Lactococcus lactis Viili, displayed adhesion properties comparable to those of the oral bacterial strains Streptococcus salivarius K12 and ST3, which were previously shown to adhere strongly to FaDu cells (23). These four strains resulted in an adhesion index (AdI; number of bacterial cells per 100 FaDu cells) of >2,500 (Fig. 1). Among probiotic bacteria, only Lactobacillus rhamnosus GG displayed a good adhesion ability (AdI between 500 and 2,500). The other intestinal probiotics adhered poorly (Lactobacillus acidophilus La-5) or negligibly (Bifidobacterium animalis Bb12 and Lactobacillus casei Shirota).
FIG. 1.
Bacterial adhesion to FaDu epithelial cell layer. (A) Adhesion indexes (AdI; number of bacteria/100 FaDu cells). Adhesion values are the means for two independent experiments conducted in duplicate. The variation between the replicates was less than 15%. (B) Cell layers observed after Giemsa staining, using light microscopy. Bar, 8 μm. N, FaDu cell nucleus, indicated once for each layer.
Adhesive bacteria can efficiently antagonize Streptococcus pyogenes on human epithelial cells.
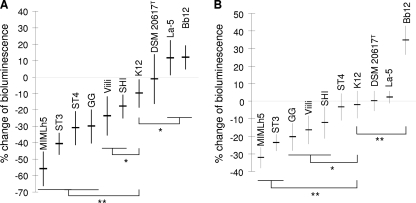
The ability of lactic acid bacteria to antagonize S. pyogenes was investigated in vitro by means of a three-component system consisting of the epithelial cell layer, the indicator bioluminescent strain S. pyogenes C11Luc, and the tester bacterium. In these experiments, we measured the reduction of bioluminescence produced by S. pyogenes C11Luc as an indication of the antagonistic activity exerted by the tester strains.
Antagonism through exclusion was tested on two different human cell lines, FaDu and HaCaT. Significant differences in antagonistic exclusion were observed among the strains. Roughly, with only a few exceptions, strains displaying good adhesion properties antagonized S. pyogenes more efficiently on human epithelial cells (Fig. 2). For instance, the nonadhesive strains Bifidobacterium animalis Bb12 and Streptococcus thermophilus DSM 20617T did not reduce the ability of recombinant S. pyogenes to produce light. In contrast, the strongly adhesive strain Lactobacillus helveticus MIMLh5 was the most active antagonist on both cell lines, with an average reduction of bioluminescence of 55% on FaDu cells and 32% on HaCaT cells (Fig. 2). Furthermore, strain MIMLh5 exhibited antagonistic exclusion comparable to that of the oral isolate Streptococcus salivarius ST3 (40% reduction of bioluminescence on FaDu cells and 24% reduction on HaCaT cells), which was demonstrated in a previous study to be the best antagonist of S. pyogenes among several oral bacteria, on the same epithelial layers (23). In addition to MIMLh5 and ST3, the probiotic strains L. rhamnosus GG and L. casei Shirota and the dairy strain L. lactis subsp. cremoris Viili also displayed antagonistic exclusion significantly stronger than that of the reference oral probiotic strain S. salivarius K12 (Fig. 2).
FIG. 2.
Antagonistic exclusion activities of dairy and probiotic bacteria against bioluminescent Streptococcus pyogenes C11Luc on FaDu hypopharyngeal carcinoma cells (A) and HaCaT keratinocytes (B). Data are reported as percentages of variation in light emission relative to the cell layer treated with PBS buffer only before incubation with S. pyogenes. Numerical results are given as arithmetic means ± standard deviations. Each sample was processed in triplicate in at least two independent experiments. Statistically significant differences compared to strain K12 were determined by unpaired Student's t test. **, P < 0.001; *, P < 0.05.
Antagonism by competition was tested only on the FaDu cell layer. In this experiment, all tested strains markedly inhibited S. pyogenes bioluminescence (>50% reduction), particularly L. helveticus MIMLh5 (78% reduction) and S. salivarius strains (ca. 80% reduction) (data not shown).
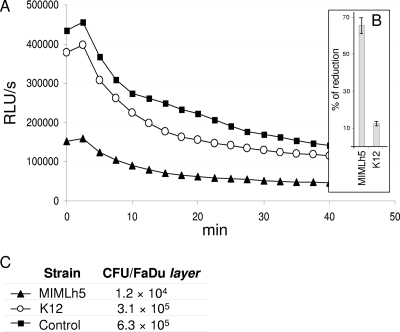
In successive experiments performed with strain MIMLh5, we noted a possible correlation between the number of S. pyogenes cells that adhered to the epithelial cell layer (determined by means of agar plate counts) and the bioluminescence emitted by the sample (Fig. 3). This result suggests that a determinant of the modification of light emission could be the number of recombinant S. pyogenes cells adhering to the epithelial layer.
FIG. 3.
Antagonistic exclusion activity against bioluminescent Streptococcus pyogenes C11Luc on FaDu hypopharyngeal carcinoma cells. (A) Light emission curves registered after addition of d-luciferin. RLU, relative luminescence units. (B) Percentage variation in light emission relative to the cell layer treated with PBS buffer only before incubation with S. pyogenes. Results are given as arithmetic means ± standard deviations calculated for three replicates. (C) Agar plate counts of S. pyogenes cells that remained adhered after treatment of the FaDu cell layer with MIMLh5, K12, or PBS (control).
Immune responses by FaDu cells are importantly modulated by L. helveticus MIMLh5.
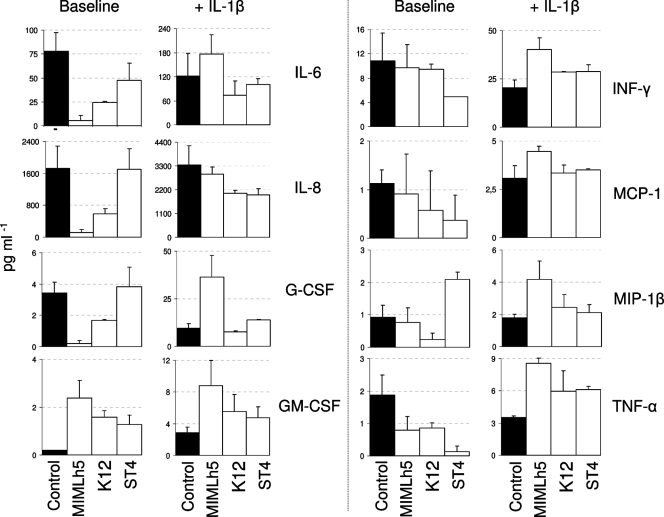
We carried out an investigation of the potential immunomodulatory properties of the strain L. helveticus MIMLh5 in comparison with the oral bacteria S. salivarius K12 and Streptococcus oralis ST4. After overnight incubation of a FaDu cell monolayer with selected bacteria, we performed a quantification of 17 different secreted cytokines by means of a Bio-Plex human cytokine 17-plex array system. All three bacterial strains induced a modification of the cytokine production profile compared to that for FaDu cells incubated under the same conditions without bacteria (Fig. 4). Under baseline conditions, Streptococcus oralis ST4 was the only strain to increase the secretion of IL-1β and to reduce that of IFN-γ. It also reduced TNF-α and increased GM-CSF levels. The oral probiotic bacterium S. salivarius K12 predominantly decreased the secretion of IL-6, IL-8, TNF-α, and G-CSF, while it increased GM-CSF secretion (Fig. 4). Finally, Lactobacillus helveticus MIMLh5 displayed the strongest ability to inhibit the secretion of IL-6 (from 78.1 to 5.5 pg ml−1), IL-8 (from 1,721 to 111 pg ml−1), and G-CSF (from 3.5 to 0.2 pg ml−1). Moreover, the strain MIMLh5 decreased TNF-α and enhanced GM-CSF levels (Fig. 4).
FIG. 4.
Histograms showing selected cytokines whose secretion changed after overnight incubation of the FaDu cell layer with bacterial cells (2 × 108 cells ml−1), as determined using Bio-Plex assay. The values are the means for two independent experiments conducted in duplicate. The error bars indicate standard deviations.
We also performed the same experiments by stimulating FaDu cells with 2 ng ml−1 of the proinflammatory cytokine IL-1β (Fig. 4). With respect to baseline, the presence of IL-1β induced a drastic change in cytokine profile for strain MIMLh5. In fact, several cytokines which were considerably reduced by L. helveticus MIMLh5 at baseline showed increased secretion when FaDu cells were incubated with MIMLh5 in the presence of IL-1β. The modification of MIMLh5's effect in the presence of IL-1β was particularly evident for the cytokines IL-6, IL-8, G-CSF, IFN-γ, MIP-1β, and TNF-α. This trend also emerged for the other two tested strains, but to a much lesser extent. Specifically, the presence of IL-1β increased the secretion of MCP-1, MIP-1β, and TNF-α for S. salivarius K12 and that of IFN-γ, MCP-1, and TNF-α for S. oralis ST4 (Fig. 4).
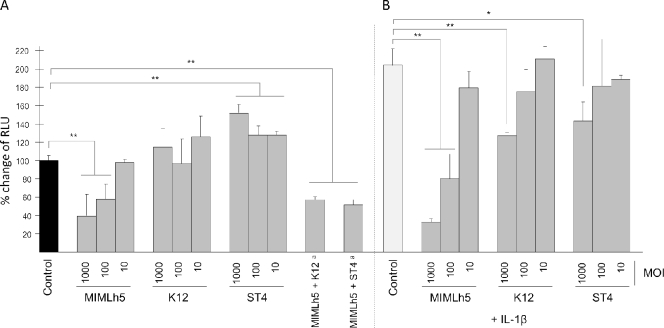
Afterward, we explored the immunomodulatory properties of selected lactic acid bacteria by testing the effect of the microorganisms on NF-κB activation. This study was done through the use of a reporter cell line obtained by transfecting FaDu cells with a luciferase reporter vector induced by active NF-κB. At baseline, L. helveticus MIMLh5 was the only strain that decreased the NF-κB-dependent production of bioluminescence. In contrast, S. oralis ST4 showed a stimulatory effect, while S. salivarius K12 did not significantly affect NF-κB activation (Fig. 5A). Furthermore, we found that the inhibitory effect of strain MIMLh5 on NF-κB activation was not significantly affected by the presence of an equal number of S. salivarius K12 or S. oralis ST4 cells during incubation with FaDu cells (Fig. 5A).
FIG. 5.
Modulation of light emission expressed by FaDu cells stably transfected with an NF-κB/luciferase reporter vector and incubated with selected bacterial strains, at baseline (A) or stimulated with 2 ng ml−1 of IL-1β (B). Luciferase activity is expressed as the percent change in light emission (relative light units [RLU]), assuming the control without IL-1β to be 100%. Control, FaDu cell layers incubated without bacterial cells. Footnote a, bacterial strains employed at an MOI of 1,000 each (number of bacterial cells per FaDu cell). The values are the means (+ standard deviations) for at least three independent experiments conducted in duplicate. Asterisks indicate statistically significant differences compared to the corresponding control (**, P < 0.001; *, P < 0.05).
The effect of bacterial strains on NF-κB was also assessed during stimulation of FaDu cells with IL-1β. The addition of 2 ng ml−1 of IL-1β to recombinant FaDu cells induced an approximately 2-fold increase in bioluminescence, indicating a higher level of activation of NF-κB. S. salivarius K12 and S. oralis ST4 partially inhibited the IL-1β-dependent increase in bioluminescence, but only at the highest MOI tested (39% and 30% reductions, respectively). Nevertheless, only strain L. helveticus MIMLh5 strongly inhibited NF-κB activation in the presence of IL-1β in a concentration-dependent manner (84% reduction at an MOI of 1,000 and 61% reduction at an MOI of 100) (Fig. 5B).
L. helveticus MIMLh5 triggers a heat shock response in IL-1β-treated FaDu cells.
According to the literature, the upregulation of proinflammatory cytokines in FaDu cells treated with L. helveticus MIMLh5 in the presence of IL-1β can be explained by the induction of a heat shock response (43). For this reason, reverse transcription-qPCR was used to assess the effect of MIMLh5 on the expression by FaDu cells of two genes involved in the heat shock response (hsp27 and hsp70). At baseline, mRNA levels of hsp27 and hsp70 did not change significantly when MIMLh5 was coincubated with FaDu cells at MOIs of 100 and 1,000. The same experiment was also performed after addition of 2 ng ml−1 of IL-1β before the incubation of FaDu cells with MIMLh5. Under this condition, the expression of hsp27 also stayed unaltered. In contrast, coincubation of MIMLh5 with IL-1β-stimulated FaDu cells showed 4.3- and 4.2-fold higher hsp70 mRNA expression at MOIs of 100 and 1,000, respectively (data not shown).
L. helveticus MIMLh5 can trigger the secretion of IL-2 by DCs in vitro.
Dendritic cells (DCs) are known to be key regulators of the immune system. Their capacities to recognize microorganisms and to start an appropriate immune response are broadly described. Once activated, DCs produce many soluble and cell surface molecules able to initiate and direct the activity of innate and adaptive lymphocytes. Among the molecules released by DCs upon their activation, proinflammatory (such as TNF-α and IL-6) and immunomodulatory (such as IL-12, IL-2, and transforming growth factor beta [TGF-β]) cytokines play a key role in this process.
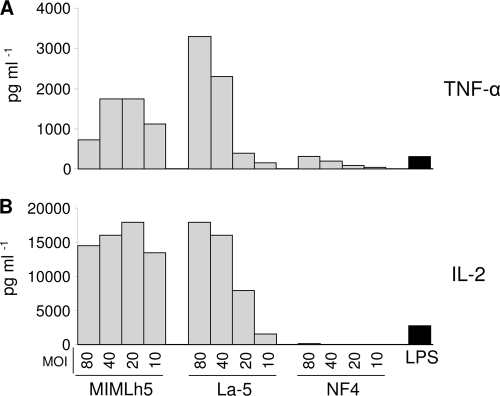
To evaluate the capacity of the selected bacteria to interact with and activate DCs, BMDCs were incubated with different concentrations of L. helveticus MIMLh5, L. acidophilus La-5, and Bacillus coagulans NF4. Strain La-5 was introduced in this experiment as a positive control, because it has been shown that L. acidophilus can significantly upregulate activating surface markers on DCs (53). Furthermore, Escherichia coli-derived lipopolysaccharide (LPS), since previously described to be a potent regulator of DC immunoregulatory functions, was added as a positive control (52). On the other hand, we used Bacillus coagulans NF4 as a negative control, because this strain did not show any immunomodulatory properties in previous experiments carried out in our laboratory (unpublished data). Supernatants from activated BMDCs were collected and analyzed by ELISA for the presence of TNF-α and IL-2 24 h after incubation with bacterial cells. Strains MIMLh5 and La-5 effectively induced the production of both cytokines at all MOIs tested, while NF4, as expected, did not (Fig. 6). In particular, while the effect of La-5 on IL-2 and TNF-α secretion was strictly dose dependent, the production of these cytokines by DCs stimulated with MIMLh5 was already high at the lowest MOI (Fig. 6).
FIG. 6.
Production of TNF-α and IL-2 by BMDCs after 24 h of stimulation with the indicated bacteria. MOI, multiplicity of infection (number of bacterial cells per BMDC); LPS, lipopolysaccharide from Escherichia coli (positive control); MIMLh5, Lactobacillus helveticus MIMLh5; La-5, Lactobacillus acidophilus La-5; NF4, Bacillus coagulans NF4 (negative control).
Lactobacillus helveticus MIMLh5 is susceptible to most antibiotics.
Bearing in mind the possible use of Lactobacillus helveticus MIMLh5 as a probiotic, we preliminarily assessed the safety of this strain by studying its antibiotic resistance with reference to the European Food Safety Authority (EFSA) breakpoints for Lactobacillus helveticus (15). MIMLh5 was found to be sensitive to all tested antibiotics except for kanamycin (Table 2). However, it appears reasonable to consider the resistance to kanamycin as intrinsic and not related to a specific gene. It is known, in fact, that several lactobacilli have a high level of natural resistance to kanamycin and other aminoglycosidic antibiotics (7).
TABLE 2.
Antibiotic sensitivities of Lactobacillus helveticus MIMLh5
DISCUSSION
In the current study, we characterized the in vitro properties of lactic acid bacteria in order to establish their possible use as probiotics for the pharyngeal mucosa. Among the features required for a potential probiotic, the guidelines proposed by the Food and Agricultural Organization (FAO) emphasize the need for a correct taxonomic identification and safety status of the microorganism (16). For this reason, we focused our attention on bacterial strains of dairy and probiotic origin already present on the market, which in general can be deemed safe. In detail, strains MIMLh5, Viili, and DSM 20617T are used as starters in the production of Grana Padano cheese, Finnish fermented milk, and yogurt, respectively. The strains Bifidobacterium animalis subsp. lactis Bb12, Lactobacillus acidophilus La-5, Lactobacillus rhamnosus GG, and Lactobacillus casei Shirota are among the most widespread probiotic strains in Western countries. Certain properties of these strains have been compared to those of Streptococcus salivarius K12, the only probiotic bacterium specifically commercialized as a probiotic for the pharyngeal mucosa to date (BLIS K12 Throat Guard [47]). Finally, we also included in the study two strains, Streptococcus salivarius ST3 and Streptococcus oralis ST4, isolated from human pharyngeal mucosa and recently studied in a project focusing on the selection of oral bacteria for use as probiotics (23).
The first probiotic property that we considered was adhesion to the host's target epithelium. The ability of commensals and probiotics to bind human mucosa has pivotal importance because it can promote colonization and sustain host epithelium-bacterium cross talk (33). Our experiment showed that two dairy strains, L. helveticus MIMLh5 and L. lactis Viili, can adhere to the FaDu pharyngeal epithelial cell line comparably to the oral probiotic bacterium S. salivarius K12, which is known to be able to colonize the oropharyngeal mucosa after oral administration (24).
Streptococcus pyogenes (group A streptococci [GAS]) causes approximately 15 to 30% of pediatric sore throat cases (8), and it is the etiological agent of skin and soft tissue infections, glomerulonephritis, and acute rheumatic fever. The oral administration of probiotic bacteria could be a prophylactic strategy effective to reduce the transmission of GAS in the community (49). For this reason, we tested the ability of probiotic bacteria to antagonize GAS on in vitro epithelial layers. Since the oral cavity contains several types of surface epithelia, including keratinized and nonkeratinized epithelia, we also included in the experimentation, besides FaDu cells, the HaCat cell line, which resembles many characteristics of human keratinocytes (10). The dairy strain L. helveticus MIMLh5, which adhered as well as or better than all the other bacteria on the FaDu cell layer, displayed the best antagonizing activity against GAS on both cell lines. Microscopic observation (Fig. 1) and plate counts of adhered GAS after antagonism assays (Fig. 3) seemed to suggest that a main mechanism of antagonism of MIMLh5 against GAS on human in vitro epithelia could be competition for the adhesion sites.
Due to its promising properties, L. helveticus MIMLh5 was chosen for the following experiments focusing on the in vitro characterization of the host-bacterium cross talk from an immunological point of view. Many beneficial claims have been attributed to probiotic supplementation for human health, especially for effects on the systemic and mucosal immunity. It has in fact been demonstrated that probiotics, particularly lactobacilli, can modulate the innate or adaptive immune response (17, 29).
In our study, we investigated the immunomodulatory properties of the selected strain, L. helveticus MIMLh5, in contact with FaDu cells by the quantification of 17 different secreted cytokines and through the measurement of NF-κB activation. We also included in the study strain S. oralis ST4, a characteristic oral bacterium, and S. salivarius K12, a commercial oral probiotic which was recently demonstrated to induce in vitro anti-inflammatory responses in epithelial cells (14).
Under baseline conditions, we found that strains K12 and MIMLh5 reduced IL-8, IL-6, and TNF-α secretion. The reduced secretion of these cytokines promoted by strain MIMLh5 in FaDu cells can potentially be explained by the concomitant inhibition of NF-κB activation found in our experiments. In fact, the transcriptional regulator NF-κB is a transcriptional inducer of IL-8 and several other cytokines and is a therapeutic target in a wide range of human (auto)inflammatory diseases (51). It is well known that the mechanism of action of many anti-inflammatory compounds, including salicylate and corticosteroids, involves NF-κB inhibition (4, 30). Furthermore, intestinal and/or probiotic microorganisms have also been proposed to exert their immunomodulatory activity through the modulation of NF-κB signaling and the subsequent reduction of IL-8 production (46).
Interestingly, the cell concentration-dependent ability of L. helveticus MIMLh5 to reduce NF-κB activation was particularly significant after stimulation by the proinflammatory cytokine IL-1β. Nevertheless, under this condition, we observed a simultaneous increase of the secretion of several cytokines, such as IL-6, G-CSF, GM-CSF, IFN-γ, MCP-1, MIP-1β, and TNF-α. A possible explanation can be found in a recent study in which Lactobacillus paracasei was shown to potentiate the effect of IL-1β on IL-6 production in cultured enterocytes by inducing a heat shock response (43). Accordingly, in our study, coincubation of FaDu cells with L. helveticus MIMLh5 increased the expression level of the heat shock protein coding gene hsp70 about 4-fold when IL-1β was present. The induction of heat shock proteins such as HSP27 and HSP70 has an inhibitory effect on the degradation of the inhibitory protein IκB and, consequently, attenuates NF-κB activation (5). Simultaneously, it is also known that the heat shock response upregulates basal and IL-1β-stimulated IL-6 production in human enterocytes (39, 41). It can be hypothesized, therefore, that transcription factors other than NF-κB become important for the regulation of several cytokine genes during the heat shock response potentially induced by MIMLh5. In addition, previous studies suggested that heat shock proteins may protect mRNAs from degradation (26, 32, 36, 48). We can speculate that a similar mechanism can protect the transcripts of cytokines from degradation as well, resulting in their enhanced synthesis and secretion.
Particular attention should be given to IL-6, a pleiotropic cytokine abundantly produced at the mucosal level, which is generally associated with proinflammatory effects (38) but can also have anti-inflammatory properties (50). IL-6, in fact, has been shown to exert protective effects during inflammation caused by injury and sepsis (6, 45). In our experiments, MIMLh5 modulated IL-6 secretion differently, depending on the context: IL-6 secretion was drastically inhibited at baseline, while it was enhanced in the presence of IL-1β. In light of the multiple important biological roles of IL-6, locally in the mucosa and systemically (38, 50), the ability of MIMLh5 to modulate mucosal IL-6 production may have important clinical implications.
We found that MIMLh5 at baseline can inhibit G-CSF secretion, concomitantly with the stimulation of GM-CSF. Myeloid DCs, which drive the T-cell response to a TH1 type, require the presence of GM-CSF for survival, in contrast to lymphoid DCs, which induce a TH2 response and are mobilized by G-CSF (3, 37, 44). A systemic and local increase of the GM-CSF/G-CSF ratio, for instance, has been correlated with a TH1-dominating response and good clinical status in cystic fibrosis patients (37). In order to ascertain whether MIMLh5 can switch the immune response to a TH1 response, we tested the ability of this bacterium to activate DCs. DCs are special leukocytes particularly frequent in tissues forming an interface with the external environment, for instance, in mucosal lymphoid organs and tonsil crypts, where they perform a sentinel function and can recruit and activate cells of the innate immune system, such as natural killer (NK) cells (20).
Activated DCs are required for NK cell priming. It has been demonstrated that the capacity of NK cells to produce IFN-γ, which is necessary for TH1 polarization, is strictly dependent upon IL-2 derived from DCs (19, 20). In our study, the stimulation of mouse bone marrow-derived DCs with MIMLh5 resulted in an enhanced production of IL-2 and TNF-α, confirming that this bacterium could potentially skew the immune response toward the TH1 phenotype, thus having a potential immunomodulatory role at the systemic level (Fig. 6).
In conclusion, during this research we evaluated in vitro the potential health-promoting properties at the pharyngeal level of some of the most common commercial probiotic bacteria, including two oral streptococci and three dairy microorganisms. According to this study, we propose a dairy bacterium, L. helveticus MIMLh5, as a potential pharyngeal probiotic because of its ability to adhere to human epithelial cell lines and to efficiently antagonize GAS on these cells. Furthermore, L. helveticus MIMLh5 appears to be a promising probable modulator of the immune system which is able to reduce NF-κB activation, to influence cytokine secretion at the epithelial level, and potentially to skew the immune system toward a TH1 response. A new study is now in progress to identify the bacterial cell components involved in the immunomodulatory properties of L. helveticus MIMLh5.
Acknowledgments
We thank Roberto Gualandris for his invaluable technical support.
Part of this study was financed by PUR 2008.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 23 August 2010.
REFERENCES
- 1.Ahola, A. J., H. Yli-Knuuttila, T. Suomalainen, T. Poussa, A. Ahlstrom, J. H. Meurman, and R. Korpela. 2002. Short-term consumption of probiotic-containing cheese and its effect on dental caries risk factors. Arch. Oral Biol. 47:799-804. [DOI] [PubMed] [Google Scholar]
- 2.Arioli, S., C. Monnet, S. Guglielmetti, C. Parini, I. De Noni, J. Hogenboom, P. M. Halami, and D. Mora. 2007. Aspartate biosynthesis is essential for the growth of Streptococcus thermophilus in milk, and aspartate availability modulates the level of urease activity. Appl. Environ. Microbiol. 73:5789-5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arpinati, M., C. L. Green, S. Heimfeld, J. E. Heuser, and C. Anasetti. 2000. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood 95:2484-2490. [PubMed] [Google Scholar]
- 4.Auphan, N., J. A. DiDonato, C. Rosette, A. Helmberg, and M. Karin. 1995. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 270:286-290. [DOI] [PubMed] [Google Scholar]
- 5.Bao, X. Q., and G. T. Liu. 2009. Induction of overexpression of the 27- and 70-kDa heat shock proteins by bicyclol attenuates concanavalin A-induced liver injury through suppression of nuclear factor-kappaB in mice. Mol. Pharmacol. 75:1180-1188. [DOI] [PubMed] [Google Scholar]
- 6.Barton, B. E., and J. V. Jackson. 1993. Protective role of interleukin 6 in the lipopolysaccharide-galactosamine septic shock model. Infect. Immun. 61:1496-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernardeau, M., J. P. Vernoux, S. Henri-Dubernet, and M. Gueguen. 2008. Safety assessment of dairy microorganisms: the Lactobacillus genus. Int. J. Food Microbiol. 126:278-285. [DOI] [PubMed] [Google Scholar]
- 8.Bisno, A. L. 1996. Acute pharyngitis: etiology and diagnosis. Pediatrics 97:949-954. [PubMed] [Google Scholar]
- 9.Bonifait, L., F. Chandad, and D. Grenier. 2009. Probiotics for oral health: myth or reality? J. Can. Dent. Assoc. 75:585-590. [PubMed] [Google Scholar]
- 10.Boukamp, P., R. T. Petrussevska, D. Breitkreutz, J. Hornung, A. Markham, and N. E. Fusenig. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106:761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caglar, E., S. K. Cildir, S. Ergeneli, N. Sandalli, and S. Twetman. 2006. Salivary mutans streptococci and lactobacilli levels after ingestion of the probiotic bacterium Lactobacillus reuteri ATCC 55730 by straws or tablets. Acta Odontol. Scand. 64:314-318. [DOI] [PubMed] [Google Scholar]
- 12.Clarke, J. O., and G. E. Mullin. 2008. A review of complementary and alternative approaches to immunomodulation. Nutr. Clin. Pract. 23:49-62. [DOI] [PubMed] [Google Scholar]
- 13.Corr, S. C., C. Hill, and C. G. Gahan. 2009. Understanding the mechanisms by which probiotics inhibit gastrointestinal pathogens. Adv. Food Nutr. Res. 56:1-15. [DOI] [PubMed] [Google Scholar]
- 14.Cosseau, C., D. A. Devine, E. Dullaghan, J. L. Gardy, A. Chikatamarla, S. Gellatly, L. L. Yu, J. Pistolic, R. Falsafi, J. Tagg, and R. E. Hancock. 2008. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect. Immun. 76:4163-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.EFSA. 2008. Update of the criteria used in the assessment of bacterial resistance to antibiotics of human or veterinary importance. Prepared by the Panel on Additives and Products or Substances used in Animal Feed. EFSA J. 732:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FAO/WHO. 2002. Report of a joint FAO/WHO expert consultation on guidelines for the evaluation of probiotics in food. World Health Organization and Food and Agriculture Organization of the United Nations, London, Ontario, Canada.
- 17.Galdeano, C. M., A. de Moreno de LeBlanc, G. Vinderola, M. E. Bonet, and G. Perdigon. 2007. Proposed model: mechanisms of immunomodulation induced by probiotic bacteria. Clin. Vaccine Immunol. 14:485-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granucci, F., C. Vizzardelli, N. Pavelka, S. Feau, M. Persico, E. Virzi, M. Rescigno, G. Moro, and P. Ricciardi-Castagnoli. 2001. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat. Immunol. 2:882-888. [DOI] [PubMed] [Google Scholar]
- 19.Granucci, F., I. Zanoni, S. Feau, and P. Ricciardi-Castagnoli. 2003. Dendritic cell regulation of immune responses: a new role for interleukin 2 at the intersection of innate and adaptive immunity. EMBO J. 22:2546-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granucci, F., I. Zanoni, and P. Ricciardi-Castagnoli. 2008. Central role of dendritic cells in the regulation and deregulation of immune responses. Cell. Mol. Life Sci. 65:1683-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guglielmetti, S., I. De Noni, F. Caracciolo, F. Molinari, C. Parini, and D. Mora. 2008. Bacterial cinnamoyl esterase activity screening for the production of a novel functional food product. Appl. Environ. Microbiol. 74:1284-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guglielmetti, S., I. Tamagnini, D. Mora, M. Minuzzo, A. Scarafoni, S. Arioli, J. Hellman, M. Karp, and C. Parini. 2008. Implication of an outer surface lipoprotein in adhesion of Bifidobacterium bifidum to Caco-2 cells. Appl. Environ. Microbiol. 74:4695-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guglielmetti, S., V. Taverniti, M. Minuzzo, S. Arioli, M. Stuknyte, M. Karp, and D. Mora. 23 April 2010, posting date. Oral bacteria as potential probiotic for the pharyngeal mucosa. Appl. Environ. Microbiol. doi: 10.1128/AEM.00109-10. [DOI] [PMC free article] [PubMed]
- 24.Horz, H. P., A. Meinelt, B. Houben, and G. Conrads. 2007. Distribution and persistence of probiotic Streptococcus salivarius K12 in the human oral cavity as determined by real-time quantitative polymerase chain reaction. Oral Microbiol. Immunol. 22:126-130. [DOI] [PubMed] [Google Scholar]
- 25.Juntunen, M., P. V. Kirjavainen, A. C. Ouwehand, S. J. Salminen, and E. Isolauri. 2001. Adherence of probiotic bacteria to human intestinal mucus in healthy infants and during rotavirus infection. Clin. Diagn. Lab. Immunol. 8:293-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaarniranta, K., M. Elo, R. Sironen, M. J. Lammi, M. B. Goldring, J. E. Eriksson, L. Sistonen, and H. J. Helminen. 1998. Hsp70 accumulation in chondrocytic cells exposed to high continuous hydrostatic pressure coincides with mRNA stabilization rather than transcriptional activation. Proc. Natl. Acad. Sci. U. S. A. 95:2319-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahala, M., M. Maki, A. Lehtovaara, J. M. Tapanainen, R. Katiska, M. Juuruskorpi, J. Juhola, and V. Joutsjoki. 2008. Characterization of starter lactic acid bacteria from the Finnish fermented milk product viili. J. Appl. Microbiol. 105:1929-1938. [DOI] [PubMed] [Google Scholar]
- 28.Kleerebezem, M., and E. E. Vaughan. 2009. Probiotic and gut lactobacilli and bifidobacteria: molecular approaches to study diversity and activity. Annu. Rev. Microbiol. 63:269-290. [DOI] [PubMed] [Google Scholar]
- 29.Konstantinov, S. R., H. Smidt, W. M. de Vos, S. C. Bruijns, S. K. Singh, F. Valence, D. Molle, S. Lortal, E. Altermann, T. R. Klaenhammer, and Y. van Kooyk. 2008. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc. Natl. Acad. Sci. U. S. A. 105:19474-19479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopp, E., and S. Ghosh. 1994. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 265:956-959. [DOI] [PubMed] [Google Scholar]
- 31.Krutmann, J. 2009. Pre- and probiotics for human skin. J. Dermatol. Sci. 54:1-5. [DOI] [PubMed] [Google Scholar]
- 32.Laroia, G., R. Cuesta, G. Brewer, and R. J. Schneider. 1999. Control of mRNA decay by heat shock-ubiquitin-proteasome pathway. Science 284:499-502. [DOI] [PubMed] [Google Scholar]
- 33.Lee, Y. K., K. Nomoto, S. Salminen, and S. L. Gorbach. 1999. Introduction, p. 24. In Handbook of probiotics. John Wiley & Sons, Inc., New York, NY.
- 34.Loimaranta, V., J. Tenovuo, L. Koivisto, and M. Karp. 1998. Generation of bioluminescent Streptococcus mutans and its usage in rapid analysis of the efficacy of antimicrobial compounds. Antimicrob. Agents Chemother. 42:1906-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metchnikoff, E. 1907. The prolongation of life. Optimistic studies. Translated and edited by P. Chalmers Mitchell. Heinemann, London, United Kingdom.
- 36.Moon, R., T. A. Pritts, A. A. Parikh, J. E. Fischer, A. L. Salzman, M. Ryan, H. R. Wong, and P. O. Hasselgren. 1999. Stress response decreases the interleukin-1beta-induced production of complement component C3 in human intestinal epithelial cells. Clin. Sci. (London) 97:331-337. [PubMed] [Google Scholar]
- 37.Moser, C., P. O. Jensen, T. Pressler, B. Frederiksen, S. Lanng, A. Kharazmi, C. Koch, and N. Hoiby. 2005. Serum concentrations of GM-CSF and G-CSF correlate with the Th1/Th2 cytokine response in cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. APMIS 113:400-409. [DOI] [PubMed] [Google Scholar]
- 38.Papanicolaou, D. A., R. L. Wilder, S. C. Manolagas, and G. P. Chrousos. 1998. The pathophysiologic roles of interleukin-6 in human disease. Ann. Intern. Med. 128:127-137. [DOI] [PubMed] [Google Scholar]
- 39.Parikh, A. A., M. R. Moon, C. D. Kane, A. L. Salzman, J. E. Fischer, and P. O. Hasselgren. 1998. Interleukin-6 production in human intestinal epithelial cells increases in association with the heat shock response. J. Surg. Res. 77:40-44. [DOI] [PubMed] [Google Scholar]
- 40.Piana, C., M. Wirth, S. Gerbes, H. Viernstein, F. Gabor, and S. Toegel. 2008. Validation of reference genes for qPCR studies on Caco-2 cell differentiation. Eur. J. Pharm. Biopharm. 69:1187-1192. [DOI] [PubMed] [Google Scholar]
- 41.Pritts, T. A., E. S. Hungness, D. D. Hershko, B. W. Robb, X. Sun, G. J. Luo, J. E. Fischer, H. R. Wong, and P. O. Hasselgren. 2002. Proteasome inhibitors induce heat shock response and increase IL-6 expression in human intestinal epithelial cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282:R1016-R1026. [DOI] [PubMed] [Google Scholar]
- 42.Reid, G., J. Dols, and W. Miller. 2009. Targeting the vaginal microbiota with probiotics as a means to counteract infections. Curr. Opin. Clin. Nutr. Metab. Care 12:583-587. [DOI] [PubMed] [Google Scholar]
- 43.Reilly, N., V. Poylin, M. Menconi, A. Onderdonk, S. Bengmark, and P. O. Hasselgren. 2007. Probiotics potentiate IL-6 production in IL-1beta-treated Caco-2 cells through a heat shock-dependent mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293:R1169-R1179. [DOI] [PubMed] [Google Scholar]
- 44.Rissoan, M. C., V. Soumelis, N. Kadowaki, G. Grouard, F. Briere, M. R. de Waal, and Y. J. Liu. 1999. Reciprocal control of T helper cell and dendritic cell differentiation. Science 283:1183-1186. [DOI] [PubMed] [Google Scholar]
- 45.Shanley, T. P., J. L. Foreback, D. G. Remick, T. R. Ulich, S. L. Kunkel, and P. A. Ward. 1997. Regulatory effects of interleukin-6 in immunoglobulin G immune-complex-induced lung injury. Am. J. Pathol. 151:193-203. [PMC free article] [PubMed] [Google Scholar]
- 46.Sokol, H., B. Pigneur, L. Watterlot, O. Lakhdari, L. G. Bermudez-Humaran, J. J. Gratadoux, S. Blugeon, C. Bridonneau, J. P. Furet, G. Corthier, C. Grangette, N. Vasquez, P. Pochart, G. Trugnan, G. Thomas, H. M. Blottiere, J. Dore, P. Marteau, P. Seksik, and P. Langella. 2008. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. U. S. A. 105:16731-16736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tagg, J., P. Wescombe, and J. Burton. 2006. Oral streptococcal BLIS: heterogeneity of the effector molecules and potential role in the prevention of streptococcal infections. Int. Congr. Ser. 1289:347-350. [Google Scholar]
- 48.Theodorakis, N. G., and R. I. Morimoto. 1987. Posttranscriptional regulation of hsp70 expression in human cells: effects of heat shock, inhibition of protein synthesis, and adenovirus infection on translation and mRNA stability. Mol. Cell. Biol. 7:4357-4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagenvoort, J. H., R. J. Penders, B. I. Davies, and R. Lutticken. 2005. Similar environmental survival patterns of Streptococcus pyogenes strains of different epidemiologic backgrounds and clinical severity. Eur. J. Clin. Microbiol. Infect. Dis. 24:65-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xing, Z., J. Gauldie, G. Cox, H. Baumann, M. Jordana, X. F. Lei, and M. K. Achong. 1998. IL-6 is an anti-inflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Invest. 101:311-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamoto, Y., and R. B. Gaynor. 2001. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J. Clin. Invest. 107:135-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zanoni, I., M. Foti, P. Ricciardi-Castagnoli, and F. Granucci. 2005. TLR-dependent activation stimuli associated with Th1 responses confer NK cell stimulatory capacity to mouse dendritic cells. J. Immunol. 175:286-292. [DOI] [PubMed] [Google Scholar]
- 53.Zeuthen, L. H., H. R. Christensen, and H. Frokiaer. 2006. Lactic acid bacteria inducing a weak interleukin-12 and tumor necrosis factor alpha response in human dendritic cells inhibit strongly stimulating lactic acid bacteria but act synergistically with gram-negative bacteria. Clin. Vaccine Immunol. 13:365-375. [DOI] [PMC free article] [PubMed] [Google Scholar]