Abstract
Pseudomonas aeruginosa can establish a niche within the plasma membrane of epithelial cells (bleb niches) within which bacteria can survive, replicate, and swim at speeds detectable by real-time phase-contrast imaging. This novel virulence strategy is dependent on the bacterial type three secretion system (T3SS), since mutants lacking the T3SS needle or known T3SS effectors localize to perinuclear vacuoles and fail to replicate. Here, we determined which of the three effectors (ExoS, ExoT, or ExoY) were required for bleb niche formation and intracellular replication. PAO1 strains with mutations in exoS, exoT, exoY, or combinations thereof were compared to wild-type and complemented strains. P. aeruginosa exoS mutants, but not exoT or exoY mutants, lost the capacity for bleb niche formation and intracellular replication. Complementation with exoS rescued both phenotypes, either in the background of an exoS mutant or in a mutant lacking all three known effectors. Complementation with activity domain mutants of exoS revealed that the ADP-ribosyltransferase (ADP-r) activity of ExoS, but not the Rho-GAP activity nor the membrane localization domain (MLD) of ExoS, was required to elicit this phenotype. Membrane bleb niches that contained P. aeruginosa also bound annexin V-enhanced green fluorescent protein (EGFP), a marker of early apoptosis. These data show that P. aeruginosa bleb niches and intracellular survival involve ExoS ADP-r activity and implicate a connection between bleb niche formation and the known role(s) of ExoS-mediated apoptosis and/or Rab GTPase inactivation.
Pseudomonas aeruginosa is an opportunistic bacterial pathogen that can cause serious infections of multiple tissue sites, including the airways, blood, skin, and cornea (27, 40). Interactions between P. aeruginosa and epithelial cells are thought to be pivotal in the development of most infections, where the interaction depends largely on the spectrum of effectors secreted by the bacterial type III secretion system (T3SS) (10, 12, 15, 16, 24-26). Individual P. aeruginosa strains carry genes encoding one or more of four known T3SS effectors (ExoU, ExoS, ExoT, and ExoY), each with the capacity to alter cell function, and therefore contribute to disease pathogenesis, when injected into the cytoplasm of a host cell (10, 24, 56). We, and others, have found that ExoS, ExoT, and ExoY share the capacity to inhibit P. aeruginosa internalization by epithelial cells (7, 8, 21). ExoY is an adenylate cyclase (55), while ExoS and ExoT each have both N-terminal GTPase-activating protein (GAP) activity and C-terminal ADP ribosyltransferase (ADP-r) activity (3). The GAP activities of both ExoS and ExoT can interfere with the actin cytoskeleton and phagocytosis by targeting Rho, Rac, and CDC42 (3, 21), while ExoS ADP-r activity targets multiple host cell substrates, including the actin cytoskeleton and associated proteins, Ras signal transduction, and endocytic pathways causing numerous toxic effects that do not necessarily compromise cell viability (3, 20, 35, 38, 39, 58). While the spectrum of host proteins that are ADP-ribosylated is more restricted than that in ExoS, ExoT efficiently ADP-ribosylates Crk proteins to uncouple integrin-mediated cell signaling (34), which is involved in the capacity to interfere with epithelial wound healing (22).
While some clinical isolates of P. aeruginosa carry genes encoding the cytotoxin ExoU, and are (therefore) acutely cytotoxic to epithelial and other cells, the majority of P. aeruginosa isolates (>70%) carry genes encoding ExoS, ExoT, and ExoY, but not ExoU. These “invasive” strains can invade and subsequently replicate within epithelial cells (11, 14-19).
Clearly, intracellular survival is not the sole virulence trait of P. aeruginosa. Indeed, P. aeruginosa is often referred to as an extracellular pathogen because it does not need to be intracellular to survive and grow, and it also possesses virulence factors that can target cells and/or tissue matrices when the bacteria are located extracellularly (23, 24, 52, 53). However, P. aeruginosa can become intracellular during infection in vivo (17, 57), and epithelial cell invasion has been shown to contribute to P. aeruginosa virulence in animal models (16, 17). Importantly, the establishment of an intracellular niche by invasive P. aeruginosa bacteria is likely to enable them to evade and/or modify host innate and acquired immune responses, thereby providing a survival advantage over bacteria equipped only for an extracellular lifestyle. Indeed, this may explain why the majority of P. aeruginosa clinical isolates are invasive (i.e., lack ExoU) rather than cytotoxic (encode ExoU) (14).
While other microbes that become intracellular localize to either membrane-bound vacuoles or cytoskeletal elements in the cytoplasm (6), we have found that invasive P. aeruginosa can create and occupy a novel intracellular niche within different types of epithelial cells (1). Several hours after cell entry, invasive P. aeruginosa induces the formation of, and traffic to, plasma membrane blebs. Within these “bleb niches,” bacteria display swimming motility that is easily detectable by real-time phase-contrast imaging (1). While infected cells eventually die, blebs containing bacteria can detach from live cells and travel significant distances with bacteria still swimming inside them. Active bacterial swimming inside detached blebs can even occur in the presence of a non-cell-permeable antibiotic (gentamicin) capable of killing extracellular bacteria, suggesting that the bleb membrane retains the capacity to exclude this antibiotic. Thus, bleb niche formation by P. aeruginosa could represent a novel dissemination strategy, in addition to being a mechanism for host defense evasion. Supporting membrane bleb niche formation as a survival strategy is the observation that the capacity to traffic to membrane blebs correlates with the ability of wild-type and mutant P. aeruginosa to replicate intracellularly (1).
Little is known about the mechanism for bleb niche formation by P. aeruginosa except that it requires the bacterial T3SS (1). Bacteria with mutations in the T3SS transcriptional regulator exsA or components of the T3SS needle (pscC or pscJ) or mutants lacking all known T3SS effectors (ΔexoS ΔexoT ΔexoY) do not form blebs, nor do those P. aeruginosa bacteria survive or replicate intracellularly. Instead, they traffic to perinuclear vacuoles that display the late endosomal marker LAMP-3, suggesting that T3SS mutant bacteria are targeted to lysosomes for degradation (1).
The goal of the current study was to determine the specific T3SS effector proteins that participate in bleb niche formation and in intracellular survival. This was done using an invasive strain (PAO1) (49) that carries genes encoding the three known effectors of invasive strains (ExoS, ExoT, and ExoY). We hypothesized that one or more of these effectors contributes to the phenotype, since each effector has biochemical activities with the potential to contribute to intracellular events.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The P. aeruginosa strains and mutants used in this study are listed in Table 1. Bacterial inocula were prepared from overnight cultures grown on Trypticase soy agar (TSA) plates (supplemented with carbenicillin at 400 μg/ml for plasmid-bearing strains) at 37°C for 14 to 16 h before suspension in serum-free cell culture medium without antibiotics (KGM-2 for human corneal epithelial cells) to a spectrophotometer optical density of 0.1 at 650 nm (∼1 × 108 CFU/ml). Inocula were then diluted in the same type of medium to ∼1 × 106 or ∼1 × 107 CFU/ml for intracellular survival or microscopy assays, respectively. We have previously found that this plasmid is stably retained by P. aeruginosa without antibiotic selection even after 48 h in vivo (31). Bacterial concentrations were confirmed by initial viable counts from inocula. Some strains were transformed with plasmid constructs by heat shock transformation, and plasmid-bearing transformants were selected by overnight growth on TSA with carbenicillin (400 μg/ml) as described above.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant description | Reference or source |
|---|---|---|
| PAO1 wild type | Complete type 3 secretion system (T3SS); expresses ExoS, ExoT, and ExoY | 50 |
| PAO1 ΔexoS ΔexoT ΔexoY | Deficient in known T3SS effectors | 50 |
| PAO1 ΔexoS | Deficient in ExoS | 50 |
| PAO1 ΔexoT | Deficient in ExoT | 50 |
| PAO1 ΔexoY | Deficient in ExoY | 50 |
| PAO1 ΔexoS ΔexoT | Deficient in ExoS and ExoT | 50 |
| PAO1 ΔexoS ΔexoY | Deficient in ExoS and ExoY | 50 |
| PAO1 ΔexoT ΔexoY | Deficient in ExoT and ExoY | 50 |
| PAO1 exsA::Ω | Cannot activate T3SS | 54 |
| pUCP18 | Vector control for complementation | 2, 41, 42, 44 |
| pUCP_exoS | Allows complementation with fully functional exoS | 2, 41, 42, 44 |
| pUCP_exoS(E381D) | Allows complementation of exoS with mutant ADP-r activity domain | 2, 41, 42, 44 |
| pUCP_exoS(R146K) | Allows complementation of exoS with mutant GAP activity domain | 2, 41, 42, 44 |
| pUCP_exoS(E379D/E381D/R146K) | Allows complementation of exoS with mutant ADP-r and GAP domains | 2, 41, 42, 44 |
| pUCP_exoS(Δ51-77) | Allows complementation of exoS with a mutant membrane localization domain | 2, 41, 42, 44 |
| PAO1 ΔexoS ΔexoT ΔexoY_pUCP18 | Deficient in all known T3SS effectors. Vector control for complementation | This study |
| PAO1 ΔexoS ΔexoT ΔexoY_pUCP_exoS | Deficient in all known T3SS effectors; complemented with fully functional version of exoS | This study |
| PAO1 ΔexoS ΔexoT ΔexoY_pUCP_exoS(E381D) | Deficient in all known T3SS effectors; complemented with exoS with mutant ADP-r activity domain | This study |
| PAO1 ΔexoS ΔexoT ΔexoY_pUCP_exoS(R146K) | Deficient in all known T3SS effectors; complemented with exoS with mutant Rho-GAP activity domain | This study |
| PAO1 ΔexoS ΔexoT ΔexoY_pUCP_exoS(E379D/E381D/R146K) | Deficient in all known T3SS effectors; complemented with exoS with mutant ADP-r and Rho-GAP domains | This study |
| PAO1 ΔexoS ΔexoT ΔexoY_pUCP_exoS(Δ51-77) | Deficient in all known T3SS effectors; complemented with exoS with mutant membrane localization domain | This study |
| PAO1 ΔexoS_pUCP18 | Deficient only in ExoS; empty vector control for complementation | This study |
| PAO1 ΔexoS_pUCP_exoS | Deficient only in ExoS; complemented with a wild-type version of exoS | This study |
| PAO1 ΔexoS_pUCP_exoS(E381D) | Deficient only in ExoS; complemented with exoS with mutant ADP-r activity domain | This study |
| PAO1 ΔexoS_pUCP_exoS(R146K) | Deficient only in ExoS; complemented with exoS with mutant Rho-GAP activity domain | This study |
| PAO1 ΔexoS_pUCP_exoS(E379D/E381D/R146K) | Deficient only in ExoS; complemented with exoS with mutant ADP-r and Rho-GAP domains | This study |
| PAO1 ΔexoS_pUCP_exoS(Δ51-77) | Deficient only in ExoS; complemented with exoS with mutant membrane localization domain | This study |
Cell culture.
Human telomerase-immortalized corneal epithelial cells (hTCEpi) were maintained in 75-mm vented flasks in serum-free KGM-2 medium (Lonza, Walkersville, MD) until confluent as previously described (45). Culture medium was replaced every 2 days after the cells had been washed with sterile phosphate-buffered saline (PBS; Sigma, St. Louis, MO). Approximately 2 days before each experiment, cells were seeded onto 22-mm glass coverslips placed in non-tissue-culture-treated 6-well plates (for microscopy) or 24-well tissue culture plates (for intracellular survival assays) and grown to ∼80% confluence. Cells were incubated at 37°C with 5% CO2 during culture and experiments.
Intracellular survival/replication assays.
Extended antibiotic survival assays were used to assess the intracellular viability of various P. aeruginosa strains after infection of hTCEpi cells. Cultured cells were grown as described earlier and then washed twice with sterile PBS (Sigma, St. Louis MO). Bacterial inocula were prepared as described above and then added to each well of a 24-well tissue culture plate (500 μl). Cells were incubated for 3 h at 37°C, and then the bacterial inoculum was removed and the cells were treated with tissue culture medium containing gentamicin (200 μg/ml) for 1 h at 37°C to kill extracellular bacteria. To quantify intracellular survival, select samples were incubated in gentamicin-containing solution for an extra 4-h period (a total of 5 h of gentamicin treatment). Inoculation of samples was timed so that gentamicin treatments concluded simultaneously to minimize sample disruption. To quantify intracellular bacteria, the gentamicin solution was removed; cells were washed twice with sterile PBS (500 μl) and then lysed with Triton X-100 (0.25%, vol/vol) in PBS (15 min). Cell lysates containing intracellular bacteria were quantified by viable counts using MacConkey agar (PML Microbiologicals, Wilsonville, OR). The difference in the numbers of viable bacteria recovered after 1 h of gentamicin treatment (invasion assay, 4-h time point) and 5 h of gentamicin treatment (survival/replication assay, 8-h time point) was used to determine the outcome of internalized bacteria over the extra 4-h time interval. Time intervals were chosen to allow adequate bacterial invasion and intracellular residence time to detect differences in survival, replication, and bleb formation between wild-type and mutant strains. At least three samples were used for each group in each experiment, and experiments were repeated at least twice. Data were expressed as a percent increase in viable bacteria for each group (comparing average numbers of CFU/ml from triplicate samples at 4 and 8 h) to clearly illustrate differences in intracellular replication between groups. Control experiments (not shown) confirmed that there were no significant differences in the growth rates of wild-type and mutant bacteria within the cell culture media used to prepare inocula over an 8-h time period and that strains were susceptible to killing by gentamicin.
Microscopy.
Experiments visualized by live video phase-contrast microscopy used the same experimental protocol described earlier for extended antibiotic survival assays, except that glass coverslips of cultured epithelial cells (contained within 6-well tissue culture plates) were inoculated with 2 ml of bacteria at ∼1 × 107 CFU/ml for 3 h prior to gentamicin treatment. In some experiments, glass-bottom tissue culture plates were used to streamline sample visualization. After 1 or 5 h of gentamicin treatment (4-h and 8-h time points, respectively), a coverslip was removed from the tissue culture well, washed once with PBS (1 ml), and then placed into an Attofluor cell chamber (Molecular Probes) and maintained at 37°C in fresh gentamicin solution (200 μg/ml). Phase-contrast videomicroscopy, where images were captured as real-time movies and as still images for subsequent analysis, was performed at a final magnification of ×1,000. For quantification of blebs (when needed), duplicate samples were counted for each group (20 fields per sample = 40 fields). Data were expressed as the mean (±standard deviation [SD]) number of blebbing cells per field, number of blebbing cells containing bacteria per field, and number of cells with vacuoles containing bacteria per field (using live cells in real time) relative to the total number of cells counted. For apoptosis assays using immunofluorescence microscopy, experiments were carried out as described above using annexin-enhanced green fluorescent protein (annexin-EGFP) (Abcam, Cambridge, MA) as a marker for visualization. Cells were treated and then incubated with annexin-EGFP (10 min). Fluorescent micrographs were captured at ×1,000 magnification and processed with Volocity software by Improvision (PerkinElmer).
Statistical analysis.
Intracellular survival/replication data and microscopy quantification data are presented as the mean ± SD for each bacterial strain. Differences between samples were evaluated for statistical significance using analysis of variance (ANOVA; with Fisher's protected least significant difference [PLSD] used for post hoc analysis) with data containing more than two groups. If two groups were being compared, Student's t test was used. P values of <0.05 were considered significant.
RESULTS
Mutation of exoS results in loss of bleb niche formation and lack of intracellular replicative capacity.
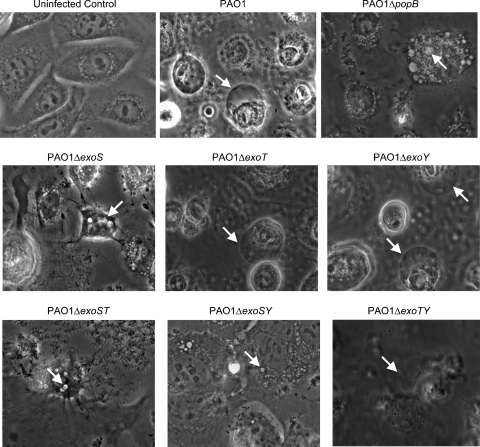
We previously established that a mutant of strain PAO1 lacking T3SS effectors (ΔexoS ΔexoT ΔexoY) lost bleb niche-forming capacity, localized to perinuclear vacuoles rather than membrane blebs, and did not replicate intracellularly. This implicated a role for one or more T3SS effectors to enable the phenotype that allowed P. aeruginosa to thrive intracellularly (1). P. aeruginosa deletion mutants with deletions in one or more T3SS effectors (Table 1) were used in human corneal epithelial cells to compare membrane blebbing with that of wild-type PAO1 (Fig. 1). Controls showed that uninfected cells remained as an intact monolayer during the 8-h time course and that wild-type PAO1 induced numerous membrane blebs (1), while a translocon mutant (PAO1 ΔpopB) did not form blebs and was trafficked to perinuclear vacuoles (Fig. 1, upper panels). P. aeruginosa mutants with mutations in either exoT or exoY behaved similarly to wild-type PAO1. However, P. aeruginosa mutants lacking exoS showed a reduced capacity for bleb niche formation and localized to perinuclear vacuoles (Fig. 1, middle panels). Quantification of blebs and P. aeruginosa localization within blebs or vacuoles confirmed reduction in the numbers of blebs formed (>3-fold) and of bacteria localized within blebs (>8-fold) for exoS mutants compared to that of wild-type PAO1. The data showed a corresponding increase (>3-fold) in vacuoles containing P. aeruginosa in cells infected with the exoS mutant compared to those infected with wild-type PAO1 (Table 2). Interestingly, the exoS mutant that expressed ExoT and ExoY retained a low-level capacity to form bleb niches.
FIG. 1.
Phase-contrast microscopy images of human corneal epithelial cells at 8 h postinoculation with P. aeruginosa strain PAO1 or different T3SS mutants (Table 1). The 8-h window consisted of 3 h of incubation with 2 × 107 CFU bacteria followed by 5 h of gentamicin treatment. Uninfected control cells are also shown. Arrows indicate the intracellular locations of P. aeruginosa within membrane bleb niches for strain PAO1 and other mutants which could express (and translocate) ExoS or within small intracellular vacuoles for strains with mutations in exoS (or which cannot translocate effectors, i.e., PAO1 ΔpopB). See Movies S1 to S3 in the supplemental material for a display of real-time microscopy images corresponding to wild-type PAO1 and effector mutants.
TABLE 2.
Quantification of bleb niches and bacterial localization in corneal epithelial cellsa
| Strain | No. of cells counted/fieldb | No. of blebbing cells/field (mean ± SD) | No. of blebbing cells containing bacteria/field (mean ± SD) | No. of cells with vacuoles containing bacteria/field (mean ± SD) |
|---|---|---|---|---|
| PAO1 (wild type) | 6.7 (268) | 3.4 ± 1.43 | 1.25 ± 1.16 | 0.4 ± 0.82 |
| PAO1 ΔexoS | 5.1 (204) | 0.95 ± 0.89 | 0.15 ± 0.37 | 1.25 ± 1.16 |
| Differencec | 3.6-fold (wild type > mutant) | 8.3-fold (wild type > mutant) | 3.1-fold (mutant > wild type) | |
| P value | 0.0001 | 0.0003 | 0.011 |
Quantification of hTCEpi was at 8 h postinoculation.
The total number of cells in all 40 fields is given in parentheses.
The difference in number of cells between the wild type and mutant is indicated.
Comparison of mutants that contained multiple-effector deletions revealed that the PAO1 ΔexoT ΔexoY mutant (expresses only ExoS) retained bleb niche-forming capacity, while the PAO1 ΔexoS ΔexoY mutant (expresses only ExoT) lost bleb niche-forming capacity. PAO1 ΔexoS ΔexoT mutants (which express only ExoY) showed an occasional capacity to form bleb niches (Fig. 1, lower panels). Thus, while ExoS had the ability to enable bleb niche formation, the bleb niche-forming efficiency of ExoY-expressing PAO1 was low and was not observed for ExoT-expressing PAO1.
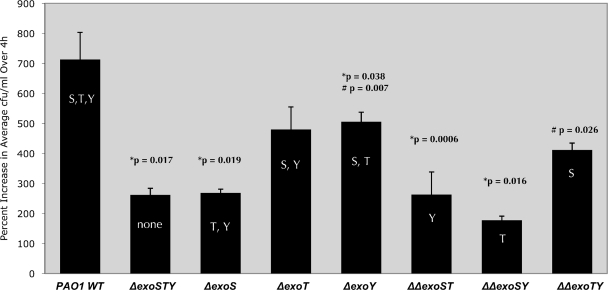
We then examined intracellular survival/replication levels for the single- and double-effector mutants using wild-type PAO1 and the PAO1 triple-effector mutant (ΔexoS ΔexoT ΔexoY) as positive and negative controls, respectively (Fig. 2). The data reconfirmed more efficient intracellular replication for wild-type PAO1 than for the PAO1 triple-effector mutant (P = 0.017). Mutation of exoS, either alone (ΔexoS) or in double-effector mutants (ΔexoS ΔexoT or ΔexoS ΔexoY), significantly reduced the capacity of the mutant PAO1 for intracellular growth compared to that of wild-type PAO1 (P = 0.019, 0.0006, and 0.016, respectively), with levels similar to the triple-effector-mutant negative control. Accordingly, PAO1 mutants expressing ExoS and ExoT (ΔexoY) or only ExoS (ΔexoT ΔexoY) showed higher intracellular replication levels than did the PAO1 triple-T3SS-effector mutant (P = 0.007 and P = 0.026, respectively). Replication levels for the ΔexoY mutant of PAO1 were somewhat reduced compared to that of wild-type PAO1 (P = 0.038) (Fig. 2).
FIG. 2.
Replication of P. aeruginosa or its T3SS mutants within human corneal epithelial cells. Data are shown as the percent increase in number of viable intracellular bacteria over a 4-h time window (i.e., from 4 to 8 h postinoculation with gentamicin in the extracellular medium). Corneal epithelial cells were exposed to 2 × 107 CFU of P. aeruginosa strain PAO1 or T3SS mutants for 3 h followed by a 1- or 5-h gentamicin treatment (4- and 8-h time points, respectively) before cell lysis and enumeration of viable intracellular bacteria. *, significantly different from results for PAO1 wild type; #, significantly different from results for ΔexoSTY mutant.
Mutations in the ADP-r domain of ExoS cause loss of bleb niche formation and intracellular replication.
The data above show that ExoS can enable the intracellular phenotype of P. aeruginosa. To identify which activity domain(s) of ExoS participates in bleb niche formation and in intracellular replication, plasmid constructs containing inactivated activity domains of exoS (Table 1) were transformed into both the exoS mutant PAO1 background and the triple-effector mutant PAO1 (PAO1 ΔexoS ΔexoT ΔexoY) background, and these mutants were compared to the corresponding PAO1 mutants complemented with either exoS or the vector (pUCP18) control.
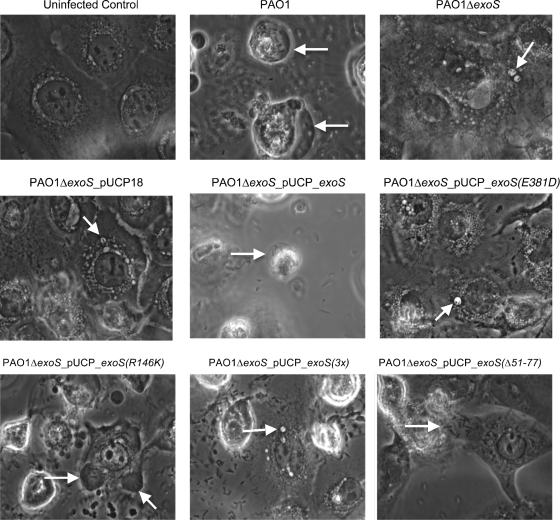
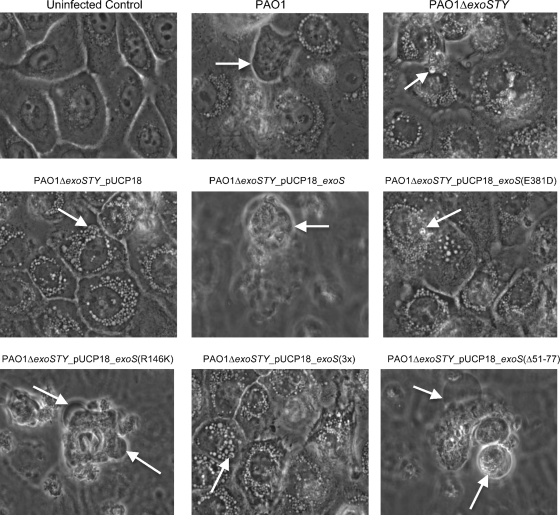
Wild-type PAO1, but not the PAO1 exoS mutant, induced efficient bleb niche formation in human corneal epithelial cells (Fig. 3, upper panels). Complementation of the PAO1 exoS mutant with exoS in trans (pUCP_exoS) restored bleb niche formation. In contrast, the phenotype was not restored by complementation with exoS containing an inactive ADP-r domain (pUCP_exoS_E381D) (Fig. 3, middle panels). Accordingly, complementation with a triple mutant form of exoS (pUCP_exoS_R146K/E379D/E381D) lacking ADP-r activity, Rho-GAP activity, and the membrane localization domain (MLD) [denoted pUCP_exoS(3x)] did not rescue the phenotype. These results showed that the ADP-r activity was required for ExoS to enable bleb niche formation. After confirmation that the GAP domain was not required for the phenotype, complementation with a mutation in only the GAP domain of exoS (pUCP_exoS_R146K) restored the phenotype. Surprisingly, the MLD was also found not to be required, since complementation with exoS containing a mutant MLD (pUCP_exoSΔ51-77) also restored the phenotype (Fig. 3, lower panels).
FIG. 3.
Phase-contrast microscopy images of human corneal epithelial cells at 8 h postinoculation with P. aeruginosa strain PAO1 or the ΔexoS mutant of PAO1 complemented with exoS or various domain mutant forms of exoS (Table 1). The 8-h window consisted of 3 h of incubation with 2 × 107 CFU bacteria followed by 5 h of gentamicin treatment. Uninfected control cells are also shown. Arrows indicate the intracellular locations of P. aeruginosa bacteria within membrane bleb niches for strain PAO1 or ΔexoS mutants of PAO1 complemented with exoS or other domain mutant forms of exoS which retained ADP-r activity.
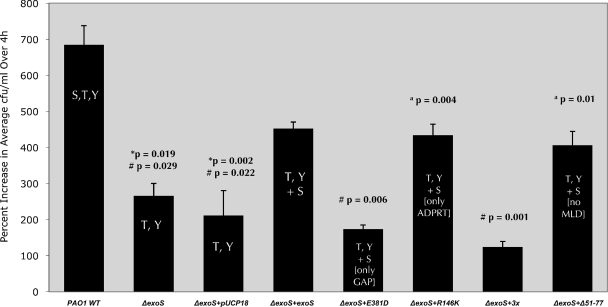
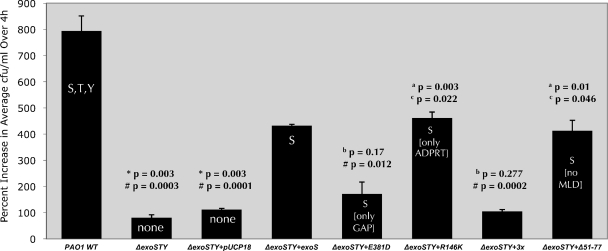
The PAO1 constructs were also used to determine if exoS ADP-r activity domain enabled intracellular replication (Fig. 4). Wild-type PAO1 replicated intracellularly, while the PAO1 exoS mutant (PAO1 ΔexoS) showed reduced intracellular replication (P = 0.019). Complementation of PAO1 with any form of exoS that possessed ADP-r activity restored the capacity for intracellular replication (versus pUCP18 alone: pUCP_exoS, P = 0.022; pUCP_exoS_R146K, P = 0.004; pUCP_exoSΔ51-77, P = 0.01) (Fig. 4). Plasmid complementation with ADP-r-active forms of exoS did not completely restore this phenotype, possibly reflecting differences between chromosome- and plasmid-encoded expression. However, intracellular replication of the PAO1 exoS mutant was not restored by complementation by exoS that lacked ADP-r activity, i.e., the ADP-r mutant form (pUCP_exoS_E381D) or the GAP/ADP-r triple mutant form (pUCP_exoS_R146K/E379D/E381D), neither of which were significantly different from the PAO1 exoS mutant complemented with the vector control (pUCP18) in the capacity to replicate intracellularly (Fig. 4). These data showed the importance of the ADP-r domain in intracellular replication and the lack of involvement of the Rho-GAP or MLD regions for this phenotype.
FIG. 4.
Replication of P. aeruginosa strain PAO1 or the ΔexoS mutant of PAO1 complemented with exoS or domain mutant forms of exoS within human corneal epithelial cells. Data are shown as the percent increase in number of viable intracellular bacteria over a 4-h time window (i.e., from 4 to 8 h postinoculation with gentamicin in the extracellular medium). Corneal epithelial cells were exposed to 2 × 107 CFU of P. aeruginosa strain PAO1 or plasmid-complemented strains for 3 h followed by a 1- or 5-h gentamicin treatment (4- and 8-h time points, respectively) before cell lysis and enumeration of viable intracellular bacteria. *, significantly different from results for PAO1 wild type; #, significantly different from results for ΔexoS mutant complemented with exoS (ΔexoS+exoS); a, significantly different from results for ΔexoS mutant complemented with the ADPRT domain mutant form of exoS (ΔexoS+E381D).
Complementation was also studied in the background of the triple-effector mutant PAO1 ΔexoS ΔexoT ΔexoY to rule out the possibility that interactions between the remaining effectors in the exoS mutant (which expresses ExoT and ExoY) might impact results obtained with the various ExoS constructs. The results showed that this was not the case; data obtained in the PAO1 triple mutant background were similar to those found for the PAO1 exoS mutant (Fig. 4, 5, 6, and 7).
FIG. 5.
Phase-contrast microscopy images of human corneal epithelial cells at 8 h postinoculation with P. aeruginosa strain PAO1 or the triple-effector mutant of PAO1 (ΔexoS ΔexoT ΔexoY) complemented with exoS or various domain mutant forms of exoS (Table 1). The 8-h window consisted of 3 h of incubation with 2 × 107 CFU bacteria followed by 5 h of gentamicin treatment. Uninfected control cells are also shown. Arrows indicate the intracellular locations of P. aeruginosa bacteria within membrane bleb niches for strain PAO1 or triple-effector mutants of PAO1 complemented with exoS or other domain mutant forms of exoS which retained ADP-r activity. Movies S4 to S7 in the supplemental material show real-time microscopy images of triple-effector mutants of strain PAO1 complemented with plasmids encoding ExoS or domain mutant forms of ExoS.
FIG. 6.
Replication of P. aeruginosa strain PAO1 or the triple-effector mutant of PAO1 (ΔexoS ΔexoT ΔexoY) complemented with exoS or domain mutant forms of exoS within human corneal epithelial cells. Data are shown as the percent increases in number of viable intracellular bacteria over a 4-h time window (i.e., from 4 to 8 h postinoculation with gentamicin in the extracellular medium). Corneal epithelial cells were exposed to 2 × 107 CFU of P. aeruginosa strain PAO1 or plasmid-complemented strains for 3 h followed by a 1- or 5-h gentamicin treatment (4- and 8-h time points, respectively) before cell lysis and enumeration of viable intracellular bacteria. *, significantly different from results for PAO1 wild type; #, significantly different from results for ΔexoSTY mutant complemented with exoS; a, significantly different from results for ΔexoSTY mutant complemented with pUCP18; b, not significantly different from results for ΔexoSTY mutant complemented with pUCP18; c, significantly different from results for ΔexoSTY mutant complemented with the ADPRT domain mutant form of exoS (E318D).
FIG. 7.
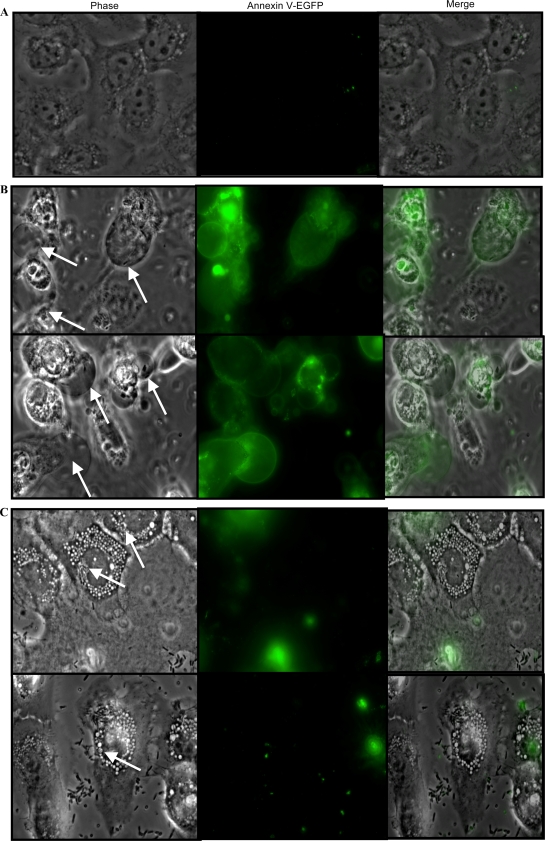
Fluorescence microscopy images of P. aeruginosa strains PAO1 and PAO1 ΔexoS in human corneal epithelial cells at 8 h postinoculation (3 h of incubation with 2 × 107 CFU bacteria followed by 5 h of gentamicin treatment) and subsequent labeling with annexin-EGFP. (A) Uninfected cells showed no annexin V-EGFP labeling. (B) Arrows indicate membrane bleb niches induced in cells infected with wild-type P. aeruginosa PAO1 in phase-contrast images which correspond to cells bearing annexin V-EGFP-labeled (apoptotic) blebs in fluorescence images. (C) Arrows indicate PAO1 ΔexoS mutant bacteria within intracellular vacuoles (phase-contrast images) in cells which did not become labeled with annexin V-EGFP.
The data show that the ADP-r activity of ExoS was responsible for bleb niche-forming capacity (Fig. 5) and the ability to enable intracellular replication (Fig. 6). The data also show that the Rho-GAP and MLD domains are dispensable for these phenotypes.
Bleb niches display apoptotic markers.
ADP-r activity of ExoS has previously been associated with apoptosis of mammalian cells (30). Plasma membrane modification, such as plasma membrane blebbing, can occur in the early states of apoptosis (46). Here, we examined whether ExoS-mediated bleb niches displayed apoptotic markers by using annexin V-EGFP, which recognizes externalized phosphatidylserine, an early apoptotic cell marker (51).
While uninfected cells were negative for annexin V-EGFP, membrane blebs induced by infection with wild-type P. aeruginosa PAO1 were found to be strongly labeled with the reagent (Fig. 7). In fact, only those cells that displayed bleb niches by phase-contrast microscopy were found positive for annexin V-EGFP. In contrast, cells infected with the PAO1 ΔexoS mutant (which did not enable bleb niche formation but displayed significant numbers of intracellular vacuoles containing bacteria) did not become labeled with annexin V-EGFP (Fig. 7 C). Thus, cells with bleb niches are apoptotic, and apoptosis is ExoS dependent.
DISCUSSION
We previously reported that P. aeruginosa utilizes a novel intracellular survival strategy within epithelial cells that involves the formation of, and trafficking of bacteria to, plasma membrane blebs. In that report, this phenotype, and the relative capacity of internalized bacteria to replicate intracellularly, depended on the P. aeruginosa T3SS (1). Here, we show that the T3SS effector ExoS, and specifically expression of ADP-r activity, enables this intracellular phenotype. The Rho-GAP activity domain and MLD of ExoS were not found to be required. The data obtained continue to support a correlation between bleb niche formation and the intracellular replication of internalized bacteria.
Some aspects of this intracellular phenotype might involve already described effects of ExoS ADP-r activity on cells. An earlier study showed that ExoS(E381D) had an ∼200-fold reduction in ADP-r activity with a primary defect in catalytic potential (33), showing that the phenotypic defect of ExoS(E381D) was attributable to the lack of ADP-r activity. ADP-r activity has been shown to induce actin cytoskeleton disruption and cell rounding by targeting Ras signaling (20, 39) and/or by targeting ERM (ezrin/radixin/moesin) family proteins, which mediate plasma membrane-cytoskeleton interactions in mammalian cells (35, 36). Dissociation of the plasma membrane from the actin cytoskeleton through ExoS ADP-ribosylation of ERM proteins (35, 36) provides a mechanism for ExoS-mediated bleb formation. Also, once injected into a host cell, ExoS has been found to localize to both the plasma membrane and perinuclear regions, and ADP-r activity can interfere with trafficking and maturation of intracellular vesicles/endosomes by colocalizing and ADP-ribosylating Rab5, which is critical for endosome maturation (9, 58). The uncoupling of Rab signaling may enable P. aeruginosa to evade trafficking to perinuclear vacuoles, which we previously showed display late endocytic markers when T3SS mutants traffic, while endowing P. aeruginosa with the capacity for intracellular replication. Finally, ExoS ADP-r activity has been shown to mediate P. aeruginosa-induced apoptosis in epithelial cells (29, 30). Cells with bleb niches not only tend to be rounded, which is characteristic of apoptosis, but also tend to be labeled with annexin V-EGFP, an early apoptotic marker (51). Plasma membrane blebbing can be a feature of early apoptosis (46). Whether or not bleb niches are actually apoptotic blebs, or whether apoptosis follows bacterial occupation of blebs (or is completely unrelated), is yet to be determined. Other types of membrane blebs have been described, such as those associated with cell division (cytokinesis) or cell migration (4, 5, 13) or those induced by specific types of cell insult (28, 37, 43).
The determination that the MLD of ExoS was not required for either bleb niche formation or intracellular replication was unexpected. The MLD is involved in targeting ExoS to the perinuclear region of host cells and transiently to the plasma membrane (58). Without the MLD, ExoS is found within the host cell cytosol (58), suggesting that the induction of bleb niche formation (and its associated intracellular survival) may be initiated from the cytosol. Further studies of the cell biology of P. aeruginosa-induced bleb niches, and the perinuclear trafficking of ADP-r deficient bacteria, are needed to understand the basis for lack of intracellular trafficking of P. aeruginosa to elicit these phenotypes.
The involvement of ExoS in intracellular survival is interesting given that ExoS inhibits initial P. aeruginosa internalization (21). This apparent dichotomy suggests that it is not the number of bacteria that initially invade that determines the significance of cell invasion by P. aeruginosa, but instead the fate of those bacteria which are internalized. Thus, when ExoS is expressed, a small number of bacteria invade, but those that do invade go on to thrive inside the cell, and the cell is eventually destroyed. In contrast, exoS mutants, and some other T3SS effector mutants (Fig. 2), which enter cells much more efficiently, do not replicate as well after invasion and traffic to perinuclear vacuoles, and the cell remains healthy (see movies in the supplemental material). Nevertheless, considering that P. aeruginosa has the capacity to regulate secretion of T3SS effectors, this may provide bacteria with significant flexibility in determining whether to establish an intracellular or extracellular niche based on prevailing conditions.
The two other known effectors expressed by P. aeruginosa strain PAO1 (ExoT and ExoY) did not show major roles in bleb niche formation and intracellular survival in these studies. The residual activity found for ExoY will require further investigation to determine its significance. Lack of ExoT participation might relate to more restricted host cellular targets that are ADP-ribosylated by ExoT, e.g., Crk proteins, and to the fact that these targets do not overlap with those of ExoS ADP-r activity (3). Interestingly, the observation of a relative reduction in intracellular replication of some effector mutants compared to that of the wild type, and the loss of intracellular replication of T3SS needle mutants observed previously (1), suggests that other components of the T3SS, which could include an unidentified effector(s), may contribute to this phenotype, at least in some P. aeruginosa strains.
The data presented in this report implicate a role for the T3SS in P. aeruginosa pathogenesis, allowing bacteria to traffic to epithelial cell membrane bleb niches and evade epithelial innate intracellular defenses that normally limit their capacity for intracellular replication. The data show that the ADP-r activity of ExoS is involved, which could relate to known cellular effects of ExoS in disrupting epithelial cytoskeleton function, endosome trafficking, signal transduction, and the induction of apoptosis, each of which involves ADP-ribosylation of various host cell targets (3, 9, 20, 29, 36, 39). Future studies will advance our understanding of the relationship between T3SS P. aeruginosa invasion bleb niche formation and intracellular survival within epithelial cells, the role of ExoS (and its ADP-r activity) in promoting P. aeruginosa survival within, and traversal of, epithelial barriers (48), and the contributions of ExoS to virulence in vivo (32, 47).
Supplementary Material
Acknowledgments
This work was supported by research grants from the National Institute of Allergy and Infectious Diseases to S.M.J.F. (AI079192) and J.T.B. (AI030162) and a National Science Foundation Graduate Research Program Fellowship Award to A.A.A.
We thank Arne Rietsch and Dara Frank for generously providing some of the mutant and parent strains used in this study.
Editor: A. Camilli
Footnotes
Published ahead of print on 23 August 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Angus, A. A., A. A. Lee, D. K. Augustin, E. J. Lee, D. J. Evans, and S. M. Fleiszig. 2008. Pseudomonas aeruginosa induces membrane blebs in epithelial cells, which are utilized as a niche for intracellular replication and motility. Infect. Immun. 76:1992-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbieri, J. T. 2000. Pseudomonas aeruginosa exoenzyme S, a bifunctional type-III secreted cytotoxin. Int. J. Med. Microbiol. 290:381-387. [DOI] [PubMed] [Google Scholar]
- 3.Barbieri, J. T., and J. Sun. 2004. Pseudomonas aeruginosa ExoS and ExoT. Rev. Physiol. Biochem. Pharmacol. 152:79-92. [DOI] [PubMed] [Google Scholar]
- 4.Charras, G., and E. Paluch. 2008. Blebs lead the way: how to migrate without lamellipodia. Nat. Rev. Mol. Cell Biol. 9:730-736. [DOI] [PubMed] [Google Scholar]
- 5.Charras, G. T. 2008. A short history of blebbing. J. Microsc. 231:466-478. [DOI] [PubMed] [Google Scholar]
- 6.Cossart, P., and P. J. Sansonetti. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304:242-248. [DOI] [PubMed] [Google Scholar]
- 7.Cowell, B. A., D. Y. Chen, D. W. Frank, A. J. Vallis, and S. M. Fleiszig. 2000. ExoT of cytotoxic Pseudomonas aeruginosa prevents uptake by corneal epithelial cells. Infect. Immun. 68:403-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowell, B. A., D. J. Evans, and S. M. Fleiszig. 2005. Actin cytoskeleton disruption by ExoY and its effects on Pseudomonas aeruginosa invasion. FEMS Microbiol. Lett. 250:71-76. [DOI] [PubMed] [Google Scholar]
- 9.Deng, Q., and J. T. Barbieri. 2008. Modulation of host cell endocytosis by the type III cytotoxin, Pseudomonas ExoS. Traffic 9:1948-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel, J., and P. Balachandran. 2009. Role of Pseudomonas aeruginosa type III effectors in disease. Curr. Opin. Microbiol. 12:61-66. [DOI] [PubMed] [Google Scholar]
- 11.Evans, D., T. Kuo, M. Kwong, R. Van, and S. Fleiszig. 2002. Pseudomonas aeruginosa strains with lipopolysaccharide defects exhibit reduced intracellular viability after invasion of corneal epithelial cells. Exp. Eye Res. 75:635-643. [DOI] [PubMed] [Google Scholar]
- 12.Evans, D. J., D. W. Frank, V. Finck-Barbancon, C. Wu, and S. M. Fleiszig. 1998. Pseudomonas aeruginosa invasion and cytotoxicity are independent events, both of which involve protein tyrosine kinase activity. Infect. Immun. 66:1453-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fackler, O. T., and R. Grosse. 2008. Cell motility through plasma membrane blebbing. J. Cell Biol. 181:879-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feltman, H., G. Schulert, S. Khan, M. Jain, L. Peterson, and A. R. Hauser. 2001. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 147:2659-2669. [DOI] [PubMed] [Google Scholar]
- 15.Finck-Barbançon, V., J. Goranson, L. Zhu, T. Sawa, J. P. Wiener-Kronish, S. M. Fleiszig, C. Wu, L. Mende-Mueller, and D. W. Frank. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 25:547-557. [DOI] [PubMed] [Google Scholar]
- 16.Fleiszig, S. M., J. P. Wiener-Kronish, H. Miyazaki, V. Vallas, K. E. Mostov, D. Kanada, T. Sawa, T. S. Yen, and D. W. Frank. 1997. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect. Immun. 65:579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleiszig, S. M., T. S. Zaidi, E. L. Fletcher, M. J. Preston, and G. B. Pier. 1994. Pseudomonas aeruginosa invades corneal epithelial cells during experimental infection. Infect. Immun. 62:3485-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleiszig, S. M., T. S. Zaidi, and G. B. Pier. 1995. Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect. Immun. 63:4072-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleiszig, S. M., T. S. Zaidi, M. J. Preston, M. Grout, D. J. Evans, and G. B. Pier. 1996. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect. Immun. 64:2288-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganesan, A. K., D. W. Frank, R. P. Misra, G. Schmidt, and J. T. Barbieri. 1998. Pseudomonas aeruginosa exoenzyme S ADP-ribosylates Ras at multiple sites. J. Biol. Chem. 273:7332-7337. [DOI] [PubMed] [Google Scholar]
- 21.Garrity-Ryan, L., B. Kazmierczak, R. Kowal, J. Comolli, A. Hauser, and J. N. Engel. 2000. The arginine finger domain of ExoT contributes to actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect. Immun. 68:7100-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrity-Ryan, L., S. Shafikhani, P. Balachandran, L. Nguyen, J. Oza, T. Jakobsen, J. Sargent, X. Fang, S. Cordwell, M. A. Matthay, and J. N. Engel. 2004. The ADP ribosyltransferase domain of Pseudomonas aeruginosa ExoT contributes to its biological activities. Infect. Immun. 72:546-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gooderham, W. J., and R. E. Hancock. 2009. Regulation of virulence and antibiotic resistance by two-component regulatory systems in Pseudomonas aeruginosa. FEMS Microbiol. Rev. 33:279-294. [DOI] [PubMed] [Google Scholar]
- 24.Hauser, A. R. 2009. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat. Rev. Microbiol. 7:654-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauser, A. R., and J. N. Engel. 1999. Pseudomonas aeruginosa induces type-III-secretion-mediated apoptosis of macrophages and epithelial cells. Infect. Immun. 67:5530-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauser, A. R., S. Fleiszig, P. J. Kang, K. Mostov, and J. N. Engel. 1998. Defects in type III secretion correlate with internalization of Pseudomonas aeruginosa by epithelial cells. Infect. Immun. 66:1413-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hazlett, L. D. 2007. Bacterial infections of the cornea (Pseudomonas aeruginosa). Chem. Immunol. Allergy 92:185-194. [DOI] [PubMed] [Google Scholar]
- 28.Herman, B., G. J. Gores, A. L. Nieminen, T. Kawanishi, A. Harman, and J. J. Lemasters. 1990. Calcium and pH in anoxic and toxic injury. Crit. Rev. Toxicol. 21:127-148. [DOI] [PubMed] [Google Scholar]
- 29.Jia, J., Y. Wang, L. Zhou, and S. Jin. 2006. Expression of Pseudomonas aeruginosa toxin ExoS effectively induces apoptosis in host cells. Infect. Immun. 74:6557-6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufman, M. R., J. Jia, L. Zeng, U. Ha, M. Chow, and S. Jin. 2000. Pseudomonas aeruginosa mediated apoptosis requires the ADP-ribosylating activity of exoS. Microbiology 146:2531-2541. [DOI] [PubMed] [Google Scholar]
- 31.Lee, E. J., B. A. Cowell, D. J. Evans, and S. M. Fleiszig. 2003. Contribution of ExsA-regulated factors to corneal infection by cytotoxic and invasive Pseudomonas aeruginosa in a murine scarification model. Invest. Ophthalmol. Vis. Sci. 44:3892-3898. [DOI] [PubMed] [Google Scholar]
- 32.Lee, V. T., R. S. Smith, B. Tummler, and S. Lory. 2005. Activities of Pseudomonas aeruginosa effectors secreted by the type III secretion system in vitro and during infection. Infect. Immun. 73:1695-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, S., S. M. Kulich, and J. T. Barbieri. 1996. Identification of glutamic acid 381 as a candidate active site residue of Pseudomonas aeruginosa exoenzyme S. Biochemistry 35:2754-2758. [DOI] [PubMed] [Google Scholar]
- 34.Liu, S., T. L. Yahr, D. W. Frank, and J. T. Barbieri. 1997. Biochemical relationships between the 53-kilodalton (Exo53) and 49-kilodalton (ExoS) forms of exoenzyme S of Pseudomonas aeruginosa. J. Bacteriol. 179:1609-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maresso, A. W., M. R. Baldwin, and J. T. Barbieri. 2004. Ezrin/radixin/moesin proteins are high affinity targets for ADP-ribosylation by Pseudomonas aeruginosa ExoS. J. Biol. Chem. 279:38402-38408. [DOI] [PubMed] [Google Scholar]
- 36.Maresso, A. W., Q. Deng, M. S. Pereckas, B. T. Wakim, and J. T. Barbieri. 2007. Pseudomonas aeruginosa ExoS ADP-ribosyltransferase inhibits ERM phosphorylation. Cell. Microbiol. 9:97-105. [DOI] [PubMed] [Google Scholar]
- 37.Marin-Castaño, M. E., K. G. Csaky, and S. W. Cousins. 2005. Nonlethal oxidant injury to human retinal pigment epithelium cells causes cell membrane blebbing but decreased MMP-2 activity. Invest. Ophthalmol. Vis. Sci. 46:3331-3340. [DOI] [PubMed] [Google Scholar]
- 38.Masters, S. C., K. J. Pederson, L. Zhang, J. T. Barbieri, and H. Fu. 1999. Interaction of 14-3-3 with a nonphosphorylated protein ligand, exoenzyme S of Pseudomonas aeruginosa. Biochemistry 38:5216-5221. [DOI] [PubMed] [Google Scholar]
- 39.Olson, J. C., J. E. Fraylick, E. M. McGuffie, K. M. Dolan, T. L. Yahr, D. W. Frank, and T. S. Vincent. 1999. Interruption of multiple cellular processes in HT-29 epithelial cells by Pseudomonas aeruginosa exoenzyme S. Infect. Immun. 67:2847-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Page, M. G., and J. Heim. 2009. Prospects for the next anti-Pseudomonas drug. Curr. Opin. Pharmacol. 9:558-565. [DOI] [PubMed] [Google Scholar]
- 41.Pederson, K. J., R. Krall, M. J. Riese, and J. T. Barbieri. 2002. Intracellular localization modulates targeting of ExoS, a type III cytotoxin, to eukaryotic signalling proteins. Mol. Microbiol. 46:1381-1390. [DOI] [PubMed] [Google Scholar]
- 42.Pederson, K. J., S. Pal, A. J. Vallis, D. W. Frank, and J. T. Barbieri. 2000. Intracellular localization and processing of Pseudomonas aeruginosa ExoS in eukaryotic cells. Mol. Microbiol. 37:287-299. [DOI] [PubMed] [Google Scholar]
- 43.Pérez, L. M., P. Milkiewicz, J. Ahmed-Choudhury, E. Elias, J. E. Ochoa, E. J. Sanchez Pozzi, R. Coleman, and M. G. Roma. 2006. Oxidative stress induces actin-cytoskeletal and tight-junctional alterations in hepatocytes by a Ca2+-dependent, PKC-mediated mechanism: protective effect of PKA. Free Radic. Biol. Med. 40:2005-2017. [DOI] [PubMed] [Google Scholar]
- 44.Radke, J., K. J. Pederson, and J. T. Barbieri. 1999. Pseudomonas aeruginosa exoenzyme S is a biglutamic acid ADP-ribosyltransferase. Infect. Immun. 67:1508-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robertson, D. M., L. Li, S. Fisher, V. P. Pearce, J. W. Shay, W. E. Wright, H. D. Cavanagh, and J. V. Jester. 2005. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Invest. Ophthalmol. Vis Sci. 46:470-478. [DOI] [PubMed] [Google Scholar]
- 46.Sen, S. 1992. Programmed cell death: concept, mechanism and control. Biol. Rev. Camb. Philos. Soc. 67:287-319. [DOI] [PubMed] [Google Scholar]
- 47.Shaver, C. M., and A. R. Hauser. 2004. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect. Immun. 72:6969-6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soong, G., D. Parker, M. Magargee, and A. S. Prince. 2008. The type III toxins of Pseudomonas aeruginosa disrupt epithelial barrier function. J. Bacteriol. 190:2814-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 50.Vance, R. E., A. Rietsch, and J. J. Mekalanos. 2005. Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo. Infect. Immun. 73:1706-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Heerde, W. L., S. Robert-Offerman, E. Dumont, L. Hofstra, P. A. Doevendans, J. F. Smits, M. J. Daemen, and C. P. Reutelingsperger. 2000. Markers of apoptosis in cardiovascular tissues: focus on Annexin V. Cardiovasc. Res. 45:549-559. [DOI] [PubMed] [Google Scholar]
- 52.Wagner, V. E., and B. H. Iglewski. 2008. P. aeruginosa biofilms in CF infection. Clin. Rev. Allergy Immunol. 35:124-134. [DOI] [PubMed] [Google Scholar]
- 53.Willcox, M. D. 2007. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom. Vis. Sci. 84:273-278. [DOI] [PubMed] [Google Scholar]
- 54.Yahr, T. L., and D. W. Frank. 1994. Transcriptional organization of the trans-regulatory locus which controls exoenzyme S synthesis in Pseudomonas aeruginosa. J. Bacteriol. 176:3832-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yahr, T. L., A. J. Vallis, M. K. Hancock, J. T. Barbieri, and D. W. Frank. 1998. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. U. S. A. 95:13899-13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yahr, T. L., and M. C. Wolfgang. 2006. Transcriptional regulation of the Pseudomonas aeruginosa type III secretion system. Mol. Microbiol. 62:631-640. [DOI] [PubMed] [Google Scholar]
- 57.Zaidi, T. S., J. Lyczak, M. Preston, and G. B. Pier. 1999. Cystic fibrosis transmembrane conductance regulator-mediated corneal epithelial cell ingestion of Pseudomonas aeruginosa is a key component in the pathogenesis of experimental murine keratitis. Infect. Immun. 67:1481-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, Y., Q. Deng, and J. T. Barbieri. 2007. Intracellular localization of type III-delivered Pseudomonas ExoS with endosome vesicles. J. Biol. Chem. 282:13022-13032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.