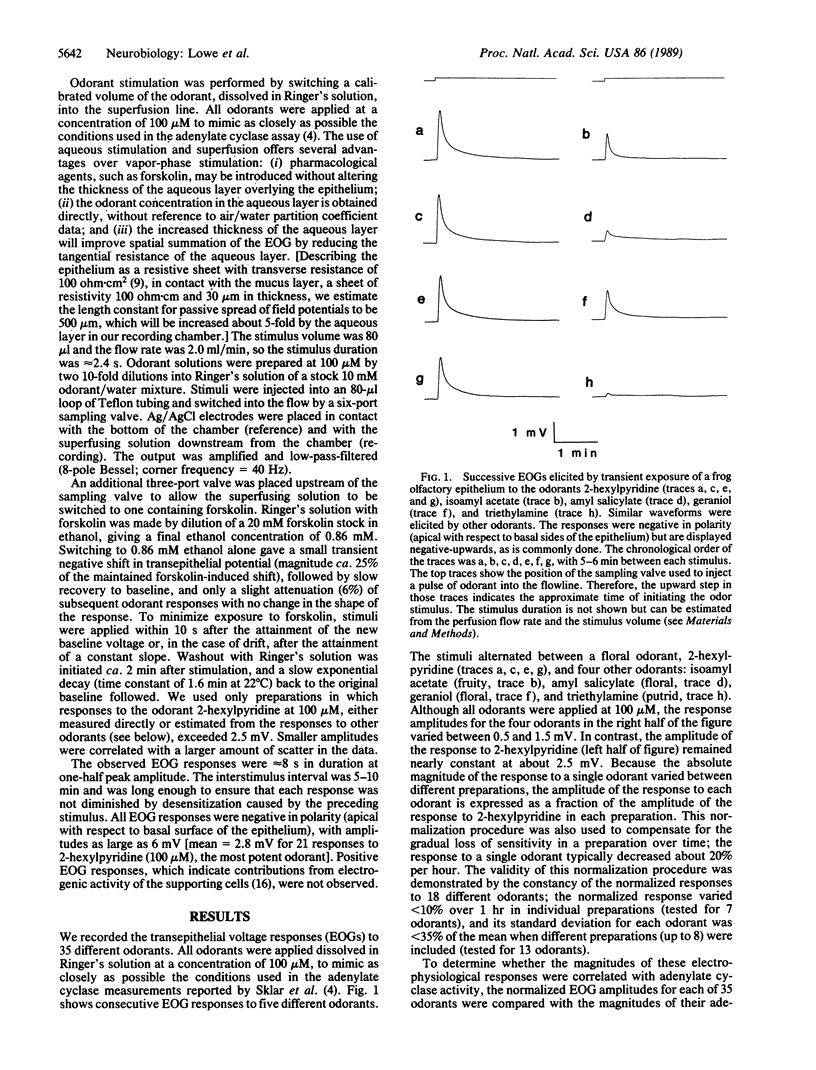
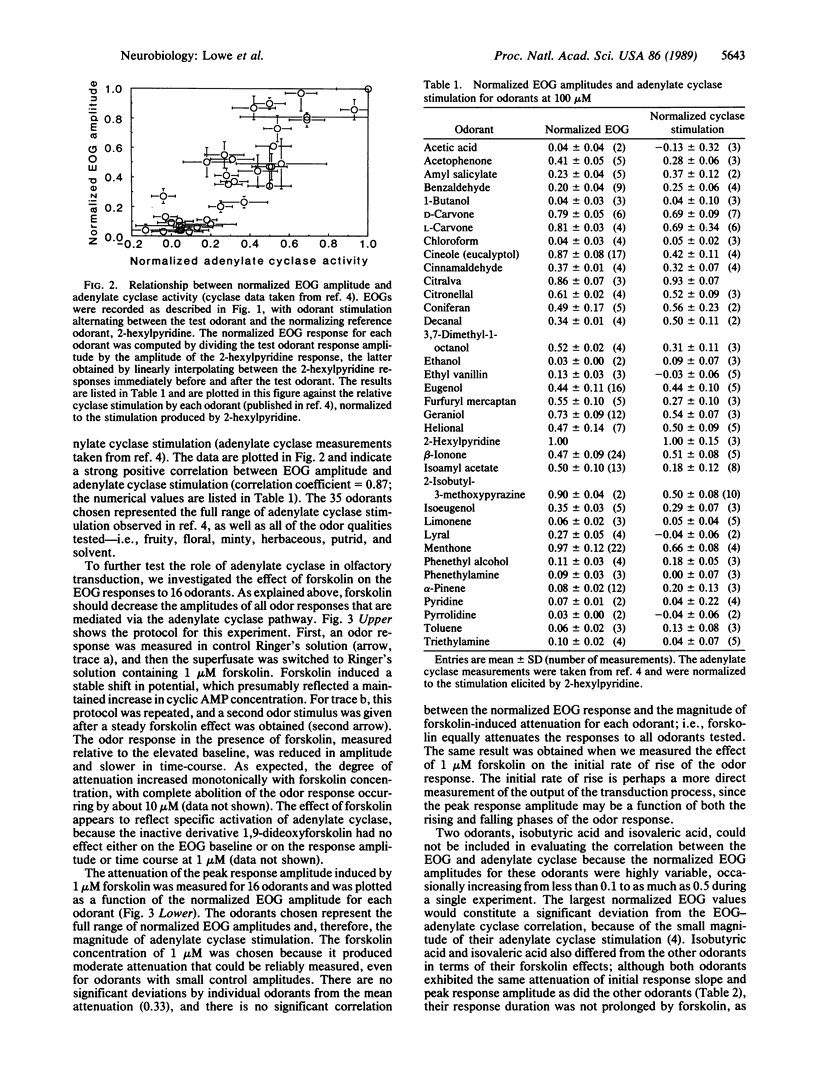
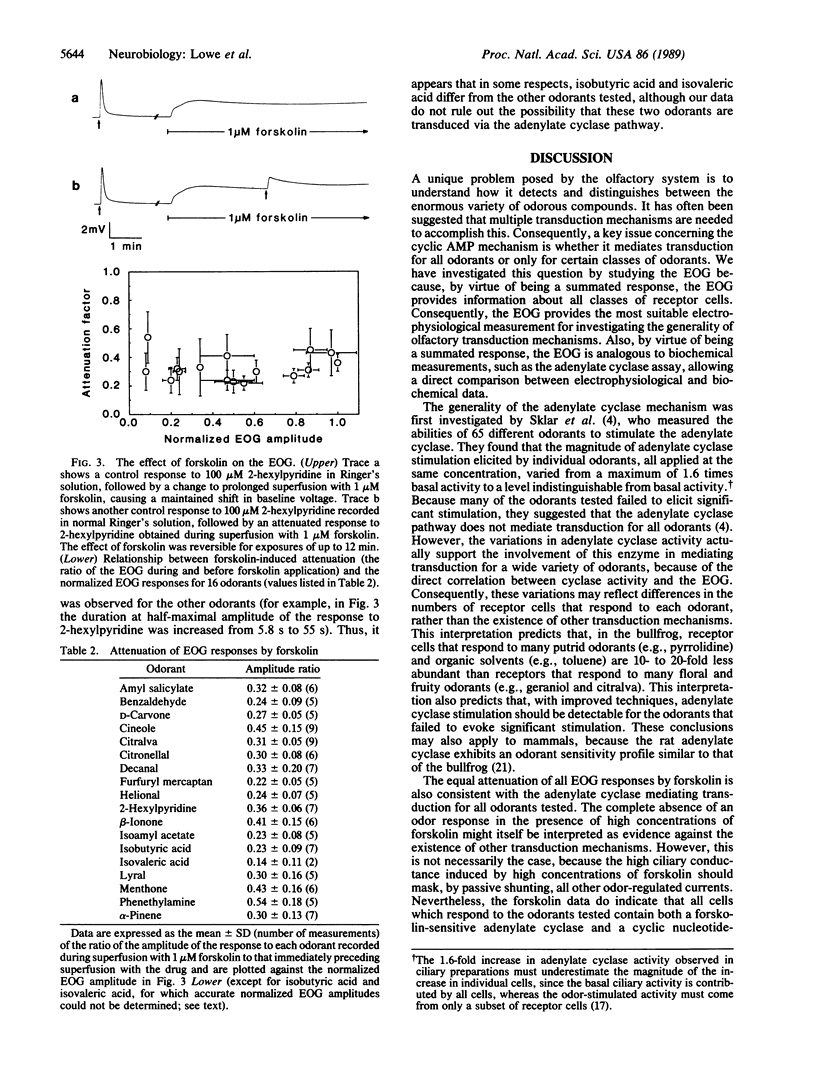
Abstract
An odor-stimulated adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] is thought to mediate olfactory transduction in vertebrates. However, it is not known whether the adenylate cyclase serves this function for all odorants or for only certain classes of odorants. To investigate this question, we have compared the abilities of 35 odorants to stimulate the adenylate cyclase and to elicit an electrophysiological response. We report a strong positive correlation between the magnitude of adenylate cyclase stimulation and the summated electrical response of the olfactory epithelium (electro-olfactogram) evoked by individual odorants. We also show that the adenylate cyclase stimulator forskolin equally attenuates the electro-olfactogram response for all odorants tested. These data provide evidence that the adenylate cyclase mediates transduction for a wide variety of odorants.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Getchell T. V. Electrogenic sources of slow voltage transients recorded from frog olfactory epithelium. J Neurophysiol. 1974 Nov;37(6):1115–1130. doi: 10.1152/jn.1974.37.6.1115. [DOI] [PubMed] [Google Scholar]
- Getchell T. V. Functional properties of vertebrate olfactory receptor neurons. Physiol Rev. 1986 Jul;66(3):772–818. doi: 10.1152/physrev.1986.66.3.772. [DOI] [PubMed] [Google Scholar]
- Gold G. H. Plasma membrane calcium fluxes in intact rods are inconsistent with the "calcium hypothesis". Proc Natl Acad Sci U S A. 1986 Feb;83(4):1150–1154. doi: 10.1073/pnas.83.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huque T., Bruch R. C. Odorant- and guanine nucleotide-stimulated phosphoinositide turnover in olfactory cilia. Biochem Biophys Res Commun. 1986 May 29;137(1):36–42. doi: 10.1016/0006-291x(86)91172-1. [DOI] [PubMed] [Google Scholar]
- Kinnamon S. C. Taste transduction: a diversity of mechanisms. Trends Neurosci. 1988 Nov;11(11):491–496. doi: 10.1016/0166-2236(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Lancet D. Vertebrate olfactory reception. Annu Rev Neurosci. 1986;9:329–355. doi: 10.1146/annurev.ne.09.030186.001553. [DOI] [PubMed] [Google Scholar]
- Menevse A., Dodd G., Poynder T. M. Evidence for the specific involvement of cyclic AMP in the olfactory transduction mechanism. Biochem Biophys Res Commun. 1977 Jul 25;77(2):671–677. doi: 10.1016/s0006-291x(77)80031-4. [DOI] [PubMed] [Google Scholar]
- Minor A. V., Sakina N. L. Rol' tsiklicheskogo adenozin-3,5'-monofosfata v oboniatel'noi retseptsii. Neirofiziologiia. 1973 Jul-Aug;5(4):415–422. [PubMed] [Google Scholar]
- Nakamura T., Gold G. H. A cyclic nucleotide-gated conductance in olfactory receptor cilia. 1987 Jan 29-Feb 4Nature. 325(6103):442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- OTTOSON D. Studies on the relationship between olfactory stimulating effectiveness and physico-chemical properties of odorous compounds. Acta Physiol Scand. 1958 Aug 25;43(2):167–181. doi: 10.1111/j.1748-1716.1958.tb01585.x. [DOI] [PubMed] [Google Scholar]
- Pace U., Hanski E., Salomon Y., Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985 Jul 18;316(6025):255–258. doi: 10.1038/316255a0. [DOI] [PubMed] [Google Scholar]
- Persaud K. C., Heck G. L., DeSimone S. K., Getchell T. V., DeSimone J. A. Ion transport across the frog olfactory mucosa: the action of cyclic nucleotides on the basal and odorant-stimulated states. Biochim Biophys Acta. 1988 Sep 15;944(1):49–62. doi: 10.1016/0005-2736(88)90315-x. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- Shirley S. G., Robinson C. J., Dickinson K., Aujla R., Dodd G. H. Olfactory adenylate cyclase of the rat. Stimulation by odorants and inhibition by Ca2+. Biochem J. 1986 Dec 1;240(2):605–607. doi: 10.1042/bj2400605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar P. B., Anholt R. R., Snyder S. H. The odorant-sensitive adenylate cyclase of olfactory receptor cells. Differential stimulation by distinct classes of odorants. J Biol Chem. 1986 Nov 25;261(33):15538–15543. [PubMed] [Google Scholar]
- Teeter J., Gold G. H. Sensory transduction. A taste of things to come. Nature. 1988 Jan 28;331(6154):298–299. doi: 10.1038/331298a0. [DOI] [PubMed] [Google Scholar]


