Abstract
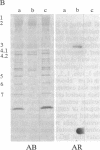
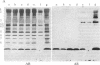
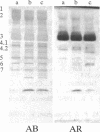
We report a human band 3 alteration that is associated with anemia as determined by a reticulocyte count of 20%. Erythrocyte defects included increased IgG binding, increased breakdown products of band 3, and altered anion- and glucose-transport activity in middle-aged cells. These changes were observed during normal erythrocyte aging in situ. Binding of ankyrin to band 3 was normal. Serum/cell crossover studies indicated that a neoantigen appears on the propositus' erythrocytes to which IgG from both propositus and control serum binds as measured with a protein A binding assay. IgG eluted from the propositus' erythrocytes appeared to have a specificity for senescent cell antigen as determined by a phagocytosis inhibition assay. Immunoelectron microscopy showed that antibodies to band 3, which do not normally bind to intact erythrocytes, bound to the propositus' erythrocytes. Antibody 980 binds to normal old cells but not young or middle-aged cells. It also binds to a distinct region of band 3 in immunoblots of membranes from the propositus' middle-aged cells. Cells from both of the propositus' parents exhibited increased IgG binding and altered anion and glucose transport. The results of these studies suggest that (i) band 3 is aging prematurely in erythrocytes from the propositus, (ii) senescent cell antigen appears on the propositus' middle-aged red cells, and (iii) band 3 alterations observed in the propositus may have a genetic component.
Full text
PDF

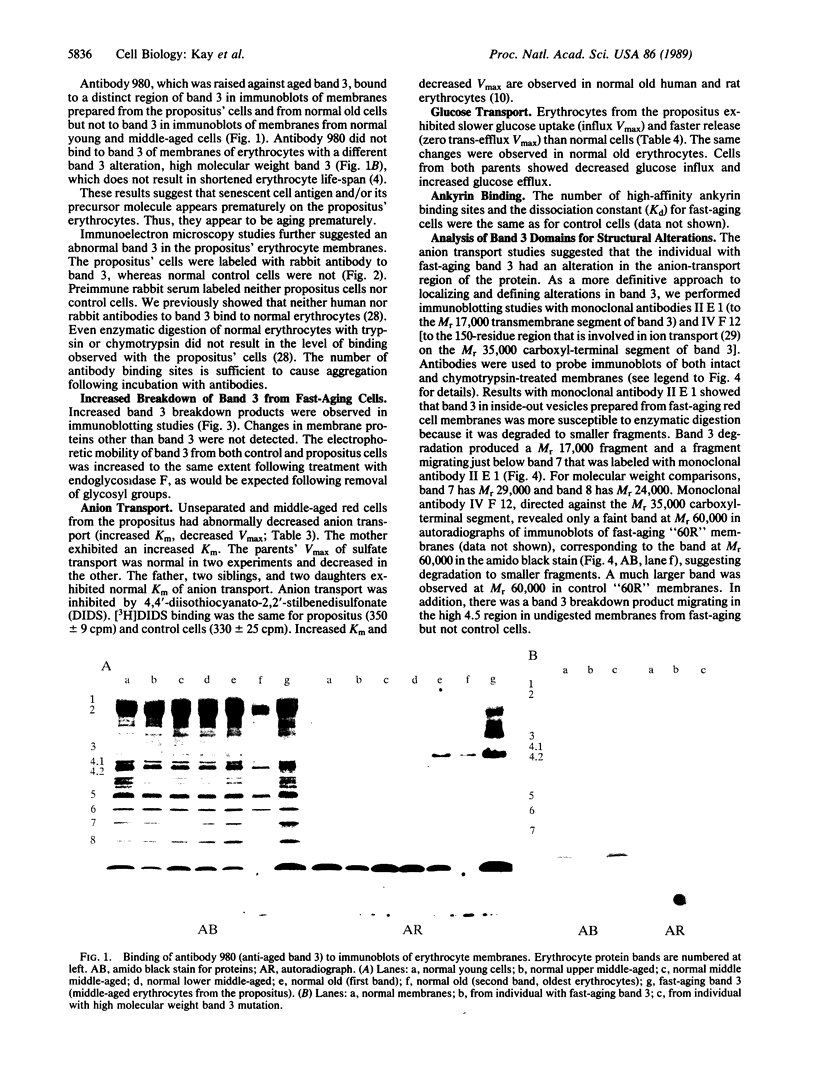
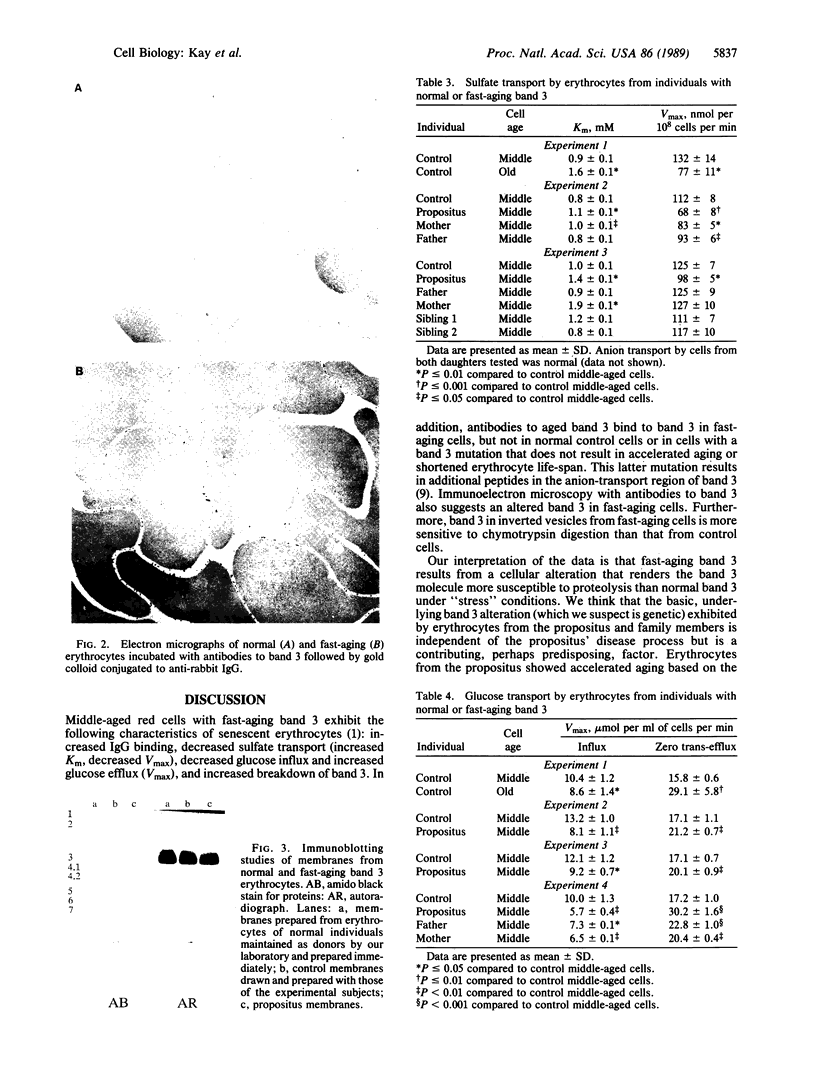

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett G. D., Kay M. M. Homeostatic removal of senescent murine erythrocytes by splenic macrophages. Exp Hematol. 1981 Mar;9(3):297–307. [PubMed] [Google Scholar]
- Bosman G. J., Kay M. M. Erythrocyte aging: a comparison of model systems for simulating cellular aging in vitro. Blood Cells. 1988;14(1):19–46. [PubMed] [Google Scholar]
- Goodman S. R., Shiffer K. A., Casoria L. A., Eyster M. E. Identification of the molecular defect in the erythrocyte membrane skeleton of some kindreds with hereditary spherocytosis. Blood. 1982 Sep;60(3):772–784. [PubMed] [Google Scholar]
- Hargreaves W. R., Giedd K. N., Verkleij A., Branton D. Reassociation of ankyrin with band 3 in erythrocyte membranes and in lipid vesicles. J Biol Chem. 1980 Dec 25;255(24):11965–11972. [PubMed] [Google Scholar]
- Jennings M. L., Anderson M. P., Monaghan R. Monoclonal antibodies against human erythrocyte band 3 protein. Localization of proteolytic cleavage sites and stilbenedisulfonate-binding lysine residues. J Biol Chem. 1986 Jul 5;261(19):9002–9010. [PubMed] [Google Scholar]
- Johnson G. J., Allen D. W., Cadman S., Fairbanks V. F., White J. G., Lampkin B. C., Kaplan M. E. Red-cell-membrane polypeptide aggregates in glucose-6-phosphate dehydrogenase mutants with chronic hemolytic disease. A clue to the mechanism of hemolysis. N Engl J Med. 1979 Sep 6;301(10):522–527. doi: 10.1056/NEJM197909063011004. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Bosman G. J., Johnson G. J., Beth A. H. Band-3 polymers and aggregates, and hemoglobin precipitates in red cell aging. Blood Cells. 1988;14(1):275–295. [PubMed] [Google Scholar]
- Kay M. M., Bosman G. J., Lawrence C. Functional topography of band 3: specific structural alteration linked to functional aberrations in human erythrocytes. Proc Natl Acad Sci U S A. 1988 Jan;85(2):492–496. doi: 10.1073/pnas.85.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M., Bosman G. J., Shapiro S. S., Bendich A., Bassel P. S. Oxidation as a possible mechanism of cellular aging: vitamin E deficiency causes premature aging and IgG binding to erythrocytes. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2463–2467. doi: 10.1073/pnas.83.8.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M., Goodman J. R. IgG antibodies do not bind to band 3 in intact erythrocytes; enzymatic treatment of cells is required for IgG binding. Biomed Biochim Acta. 1984;43(6):841–846. [PubMed] [Google Scholar]
- Kay M. M., Goodman S. R., Sorensen K., Whitfield C. F., Wong P., Zaki L., Rudloff V. Senescent cell antigen is immunologically related to band 3. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1631–1635. doi: 10.1073/pnas.80.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Isolation of the phagocytosis-inducing IgG-binding antigen on senescent somatic cells. Nature. 1981 Feb 5;289(5797):491–494. doi: 10.1038/289491a0. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Localization of senescent cell antigen on band 3. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5753–5757. doi: 10.1073/pnas.81.18.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Mechanism of removal of senescent cells by human macrophages in situ. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3521–3525. doi: 10.1073/pnas.72.9.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Role of physiologic autoantibody in the removal of senescent human red cells. J Supramol Struct. 1978;9(4):555–567. doi: 10.1002/jss.400090409. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Sorensen K., Wong P., Bolton P. Antigenicity, storage, and aging: physiologic autoantibodies to cell membrane and serum proteins and the senescent cell antigen. Mol Cell Biochem. 1982 Nov 26;49(2):65–85. doi: 10.1007/BF00242486. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Tracey C. M., Goodman J. R., Cone J. C., Bassel P. S. Polypeptides immunologically related to band 3 are present in nucleated somatic cells. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6882–6886. doi: 10.1073/pnas.80.22.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Naftalin R. J., Smith P. M., Roselaar S. E. Evidence for non-uniform distribution of D-glucose within human red cells during net exit and counterflow. Biochim Biophys Acta. 1985 Nov 7;820(2):235–249. doi: 10.1016/0005-2736(85)90117-8. [DOI] [PubMed] [Google Scholar]
- Schnell K. F., Gerhardt S., Schöppe-Fredenburg A. Kinetic characteristics of the sulfate self-exchange in human red blood cells and red blood cell ghosts. J Membr Biol. 1977 Jan 28;30(4):319–350. doi: 10.1007/BF01869675. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Koziarz J. J., Singh M. K., Reddy G., Köhler H. Preparation and analysis of seven major, topographically defined fragments of band 3, the predominant transmembrane polypeptide of human erythrocyte membranes. Biochemistry. 1978 Apr 4;17(7):1216–1222. doi: 10.1021/bi00600a013. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler T. J. Kinetics of glucose transport in human erythrocytes: zero-trans efflux and infinite-trans efflux at 0 degree C. Biochim Biophys Acta. 1986 Nov 17;862(2):387–398. doi: 10.1016/0005-2736(86)90242-7. [DOI] [PubMed] [Google Scholar]
- Yam P., Petz L. D., Spath P. Detection of IgG sensitization of red cells with 125I staphylococcal protein A. Am J Hematol. 1982 Jun;12(4):337–346. doi: 10.1002/ajh.2830120405. [DOI] [PubMed] [Google Scholar]